Abstract
In the context of a main project that aims to recover modern data on diatom distribution applicable to paleosalinity reconstructions in coastal areas of Southern South America, the composition and distribution of dead diatom assemblages in the littoral zone of the Quequén Salado estuary (Argentina) were studied. Diatom zones were defined along the estuarine gradient by cluster analysis and related to the salinity range and sediment composition by Canonical Correspondence Analysis. Four diatom zones were identified. A mixture of marine, brackish and freshwater diatoms, probably allochthonous, characterized the inlet (zone I). Marine/brackish taxa, represented mainly by Paralia sulcata dominated zone II, characterized by polyhaline conditions and sandy sediments. Zone III was characterized by mesohaline conditions, muddy sediments and the dominance of the estuarine diatom Amphora helenensis. Brackish/freshwater and freshwater diatoms dominated the headwaters (zone IV), where salinity was always below 5‰. The comparison of Quequén Salado diatom assemblages with previous results from the Quequén Grande estuary showed a similar taxonomic composition between both estuaries. However, differences in the salinity ranges of the estuaries (related to differences in the degree of human impact and tidal range) lead to a displacement in their spatial distribution along the longitudinal estuarine axis. This paper contributes to the knowledge of the ecological requirements of South American estuarine diatoms and provides useful data for paleosalinity reconstructions in the region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diatoms have been successfully used to infer Holocene sea level changes in many areas of the world (Ng & Sin, 2003). In Southern South America, they have been widely employed to reconstruct changes in salinity, depth and trophic status related to fluctuations in shoreline positions (e.g. Espinosa et al., 2003; García-Rodríguez et al., 2004; Inda et al., 2006). These paleoecological reconstructions were based only on autoecological data gathered from European authors (e.g. De Wolf, 1982; Vos & De Wolf, 1988; Denys, 1991/1992) because the information about the ecology and distribution of modern diatoms in estuarine and coastal systems of Southern South America is scarce and fragmentary. However, there are unsolved paleoecological problems related to the application of European ecological codes and keys in non-european diatom studies (Denys, 1991/1992). It is well known that ecological differences may exist between morphologically identical strains from the same species with different geographic distribution (Denys, 1991/1992; Round et al., 1990). These geographical variations may lead to a loss of indicative value for certain taxa and faulty interpretations based on autoecological codes (Denys, 1991/1992). Subsequently, only through the detailed study on the ecology and distribution of diatom communities in modern local settings can the full variability of environmental tolerance within diatom taxa be assessed.
In order to achieve quantitative ecological information useful as modern analogous in diatom-based paleoecological reconstructions in Southern South America, the composition and spatial distribution of modern diatom assemblages in a partially mixed estuary (Quequén Grande river; 38°30′ S, 58°45′ W) and a coastal lagoon (Mar Chiquita; 37°40′ S, 57°20′ W) from the northeastern coast of Argentina have been recently assessed (Hassan et al., 2006). The usefulness of the modern data set was successfully tested in the paleoenvironmental reconstruction of a late Holocene sequence from Mar Chiquita coastal lagoon. However, it is necessary to add new estuarine systems to the model in order to increase the scope and precision of the diatom-based paleoenvironmental reconstructions in the region.
The Quequén Salado river lies in the northeastern coast of Argentina (38°50′ S, 60°30′ W), and is located 100 km southwest of the Quequén Grande river. This estuary has not been as widely impacted by human activity as the Quequén Grande estuary, where large human settlements, bridges, jetties or harbors have been set in its mouth. Therefore, the Quequén Salado River provides a useful source of information on diatom assemblage composition in pristine estuarine environments.
The aims of the present study were: (1) to assess the composition and spatial distribution of modern diatom assemblages in the Quequén Salado estuary; and (2) to compare diatom assemblages from two estuarine systems with different degrees of human impact. Results of this contribution will increase our knowledge on estuarine diatom communities in Southern South America, allowing for the future development of accurate diatom-based environmental reconstructions in coastal settings of the region.
Materials and methods
Study area
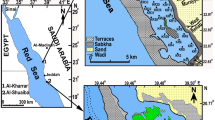
The Quequén Salado river (Fig. 1a), flows across the region known as the Humid Pampas. It has a mean discharge of 10.76 m3/seg (min: 28.8 m3/seg, max: 4.6 m3/seg; Marini & Piccolo, 2000). The estuary is located in a mesotidal coast (2–3 m tidal range). The zone has a temperate climate (annual mean temperature of 14°C) and an annual mean rainfall of 800 mm (Marini & Piccolo, 2004). The river runs along the rounded plain (so called “Undulated Pampa”) between the Tandilia and Ventania mountains. The coastal cliffs of this undulated plain appear saltuary at the coast, and in this sense they are assumed to record the tectonic component of the area during the Quaternary. The lower valley is oriented NNW–SSE, with step walls of 8–15 m high. This portion of the river is characterized by rapids caused by resistant caliche levels at the bottom. In the last 5 km the river runs across a sandy barrier composed of vegetated dunes (Marini & Piccolo, 2000).
Location of the study area and detail of the sampling stations at (a) Quequén Salado estuary (present study) and (b) Quequén Grande estuary (Hassan et al., 2006)
The Quequén Grande river is located 100 km northeast of the Quequén Salado river (Fig. 1b), in a microtidal coast (1–2 m tidal range). It has a mean discharge of 8.1 m3/seg (min: 5.3 m3/seg, max: 11.4 m3/seg; Perillo et al., 2005). Given its economic and strategic importance, the estuary has been the focus of many man-made modifications (i.e., dredging, jetty and harbour construction, etc., see Fig. 1b) that have reduced water circulation producing strong reductive and even anoxic conditions (Perillo et al., 2005).
Perillo et al. (2005) studied the effects of anthropogenic modifications upon the estuary through hydrological analysis including longitudinal salinity profiles, vertical salinity, density and temperature profiles, and current velocity measurements. Results of hydrologic analyses showed that, although the general salinity structure of the estuary resembles that of a typical partially mixed estuary, a strong stratification occurs in the harbour area, because of the continuous dredging that generates an 8 m step in the thalweg. The presence of the step induces an area of quiet, saltier water, over which the fresh water input from the river generates moderate stratification, much more similar to a fjord-like circulation pattern than a salt wedge estuary. Additionally, the construction of two jetties has modified the littoral circulation and sediment transport pattern. Originally, the mouth of the estuary was deflected to the north by a spit that was cut periodically by seasonal peak runoff. The presence of the N jetty reduces the width of the mouth to 165 m developing a basin with low circulation (Perillo et al., 2005).
Field and laboratory methods
Ten sampling stations representative of the salinity gradient from the inlet to the inner reaches of the estuary were selected (Fig. 1a). In sites under tide action (sites 1–7), a transect from the top of the foreshore to low water was paced out and divided into three sections. In October 2005, sediment samples were taken from each section by triplicate with a 20 mm diameter × 100 mm length plastic tube and plotted.
To avoid contamination with living individuals, the top 10 mm were removed from the core, and a 10 mm subsample was taken from the “cleaned” surface. Since most areas of the foreshore support a surface diatom flora and these diatoms are not part of the dead assemblage, it is important that they are not included with the sediment sample (Juggins, 1992). Samples were preserved with 4% formalin. 5 g of dry sediment were oxidised with hydrogen peroxyde (30%) and hydrochloric acid (10%), washed 3 or 4 times with distilled water, and diluted to a total volume of 50 ml. After complete homogenization, a subsample of 20 μl was transferred to a coverslip and air-dried. Permanent drop slides were mounted with Naphrax®. On each slide at least 300 diatom valves were counted in random transects at 1000× magnification.
Diatom species were identified according to Hustedt (1930, 1937–1938, 1959–1966), Germain (1981), Archibald (1983), Krammer & Lange-Bertalot (1986, 1988), Hartley (1996), Rumrich et al. (2000) and Lange-Bertalot (2001). Ecological classifications of De Wolf (1982), Denys (1991/1992) and Vos & De Wolf (1988, 1993) were used to classify diatom taxa according to their salinity and habitat preferences in order to relate diatom ecological groups with the measured environmental conditions.
Dead diatom assemblages from surface sediments are assumed to contain a time integrated flora. Whilst the collection of surface sediment samples usually only requires a single visit to the field, samples for water chemistry need to be taken several times to generate a mean or median value for each site (Battarbee, 1986). Therefore, in October 2005 and January 2006 three replicate measurements of salinity were taken at low and high tide in each sampling station with a Horiba-U10 water quality analyzer and mean salinity values were used in this work. In order to characterize the diatom substrate, sediment grain size was analyzed by dry sieving (Folk, 1968). Categories of grain size included the coarse fraction (>500 μm), medium sand (250–499 μm), fine sand (125–249 μm), very fine sand (63–124 μm) and mud (silt and clay, <63 μm). Additionally, the organic content of each sample was estimated using the loss on ignition method (LOI, 4 h at 550°C) and water content was calculated by drying the sediment 24 h at ca. 105°C (Heiri et al., 2001).
Data analysis
Constrained incremental sum of squares cluster analysis was performed on the data matrix using the computer program TILIA CONISS (Grimm, 1991). Q-mode constrained and R-mode unconstrained analyses were generated for the sampling sites and diatom taxa, respectively.
In order to allow a clearer definition of the distribution of diatom taxa along estuarine salinity gradients, diatom assemblage compositions obtained in this study were compared with those published by Hassan et al. (2006) for the Quequén Grande river by means of MDS (Multidimensional Scaling), a statistical technique that allows one to map the similarities between points in a high dimensional space into a lower dimensional space. For a given set of observed similarities between every pair of N items, MDS finds a representation of the items in as few dimensions as possible, such that the similarities in the lower dimension match as closely as possible the original similarities (Johnson, 1998). A numerical measure of the closeness between the similarities in the lower dimensional and the original spaces is called stress. The stress has a value between 0 and 1, with 0 indicating perfect fit and 1 indicating worst possible fit. The MDS plot was constructed based on similarities of correlation matrices using the computer program STATISTICA (Statsoft, 1998).
In order to relate species data from both estuaries to the measured environmental variables, a Canonical Correspondence Analysis (CCA; ter Braak, 1986) was computed over the combined data set using the computer program MSVP (Kovach Computing Services, 2002).
Results
Diatom assemblages in the Quequén Salado estuary
A total of 115 diatom species were identified in sediments of Quequén Salado river, of which only 32 reached relative abundances >2% in at least one sample and were included in multivariate analyses.
Four diatom zones were defined according to the Q-mode cluster analysis (Fig. 2), whereas R-mode cluster analysis allowed the identification of five diatom groups with different salinity preferences (Fig. 3). Each diatom zone was characterized by different taxonomic composition, salinity ranges and sediment characteristics as follows:
Zone I
Only one sample (station 1), located 200 m from the inlet, comprises this zone. Salinity ranged from 20 to 37‰ (see Fig. 2). The substratum was mainly composed of sandy sediments (particularly medium to fine sands, Fig. 4). LOI was low (1%) and the water content of sediments was 15% (Fig. 4).
Diatom assemblages were dominated by a mixture of marine, brackish and freswater taxa, such as Nitzschia cf. perminuta (Grunow) Peragallo, Navicula gregaria Donking, Amphora helenensis Giffen, Cocconeis placentula var. euglypta (Ehrenberg) Grunow and Cyclotella meneghiniana Kützing (see Fig. 2).
Zone II
This zone clustered three sampling stations located between 0.5 and 3 km from the estuary mouth. Salinity ranged between 13 and 37‰ (Fig. 2). Similarly to zone I, sandy sediments dominated this zone, LOI values were higher (1–8%) than those of zone I and water content ranged between 12 and 50% (Fig. 4).
Diatom taxa from groups B and E dominated this zone (Fig. 3). Diatom assemblages were represented by marine/brackish episammic, planktonic and tychoplanktonic taxa such as Paralia sulcata (Ehrenberg) Cleve, Cymatosira belgica Grunow, Raphoneis amphiceros Ehrenberg, Thalassiosira eccentrica (Ehrenberg) Fryxell, Hasle and T. decipiens (Grunow) Jorgensen.
Zone III
This zone included sampling stations 5 and 6, located between 4.5 and 5.5 km from the estuary mouth. The recorded salinity values ranged between 3 and 13‰ (Fig. 2). The substratum was mainly composed of fine grained sediments, particularly very fine sand and mud. LOI ranged between 2.5 and 10%, and water content was 20–60% (Fig. 4).
Amphora helenensis was the dominant species, associated to taxa from the group C, which clustered epipelic and planktonic taxa from marine/brackish and brackish/freshwater affinities (Fig. 3).
Zone IV
This zone comprised sites 7, 8, 9 and 10, located at the headwaters of the estuary, where salinity values were lower than 5‰, (in most sites average salinities were 2–3‰). This zone was characterized by fine grained sediments; LOI values were lower than 5% and sediment water content ranged between 20 and 30%.
Diatom assemblages were dominated by brackish/freshwater and freshwater epipelic, epiphytic and planktonic taxa (groups A and D, Fig. 3), such as Cocconeis placentula, Cyclotella meneghiniana, Nitzschia cf. perminuta, Hippodonta hungarica (Grunow) Lange-Bertalot, Metzeltin & Witkowski, Nitzschia denticula Grunow and Amphora veneta Kützing.
Comparison between Quequén Salado and Quequén Grande estuaries
Diatom zones defined in Quequén Grande river (QG, see Fig. 4 in Hassan et al., 2006) and Quequén Salado river (QS, present paper) were plotted in the MDS. A summary of the main characteristics of each diatom zone in both estuaries is listed in Table 1.
A stress of 0.098 was obtained when the correlation coefficients were mapped into a two-dimensional space in the MDS (Fig. 5). This small value of stress indicated that the data indeed resided in a two-dimensional space, and therefore no additional dimensions were needed.
The MDS ordination plot of QG and QS diatom assemblages revealed considerable differences in the structure of assemblages among zones defined by cluster analysis. In both estuaries, samples showed a distributional pattern along axis 1 clearly related to the estuarine gradient: sites located at the estuary mouth were plotted on the right side of the diagram, while sites located at the headwaters were plotted on the left side. The only exception was QS zone I that was located between zones III and IV.
Although there was a correspondence between QS zone III and QG zone 2, and between QS zone IV and QG zone 3, QS zone II, corresponding to the outer part of the Quequén Salado estuary, was not comparable to any of the Quequén Grande zones. The most external sample from QG, located at the estuary mouth, was equivalent to that of QS zone III, located in an inner portion of the QS estuary (4.5–5.5 km from the estuary mouth). This correspondence of QS and QG diatom zones may be related to the presence of similar diatom salinity groups (Table 1). However, relative frequencies of diatom taxa varied between both estuaries, and the slight displacement observed along axis 2 may be related to the differences in diatom percentages between equivalent zones of both estuaries.
The displacement of the main diatom zones to the inner reaches of the QG can be related to the differences in environmental characteristics of each zone when compared with QS (Table 1). The saltier condition of QS becomes evident when salinity is plotted against distance from the estuary mouth in both estuaries (Fig. 6). Polyhaline conditions and sandy sediments are recorded in the first 3 km of the QS estuary, whereas than in the QG they are present only in the mouth. According to its salinity regimes and sediment composition, QS zone I is not comparable with any of the QG zones, whereas QS zone II is only partially comparable with QG site 1 (zone 1). Marine/brackish diatom assemblages were more widely distributed in QS than in QG, since they dominated in a more extensive portion of the inlet. So, the absence in QG of a diatom assemblage analog to the one which dominates the first kms of the QS may be related to their differences in salinity regimes (Fig. 6, Table 1).
Plot of salinity ranges versus distance from estuary mouth for Quequén Salado (empty quadrates) and Quequén Grande (filled circles) estuaries. Salinity terminology according to Day (1981)
Relationship between diatom assemblages and environmental parameters
The CCA allowed the relation of the samples with the measured environmental parameters in both estuaries (Fig. 7). The first two axes explained the 64% of the variance of the combined data set. The first axis (40% of the variance explained) showed a strongly positive correlation with salinity and medium sediment fractions (medium and fine sand), and a negative correlation with finer sediment fractions (very fine sand and mud). Accordingly, sites were ordinated along this axis following a salinity and grain size gradient (Fig. 7a). The second axis (24% of the variance explained) was correlated with coarse and fine sand.
Ordination plots of dead diatom assemblages with respect to environmental variables based on Canonical Correspondence Analysis (CCA) for Quequén Salado (black circles) and Quequén Grande (empty circles). Sampling sites (a) and diatom taxa (b) (abbreviation codes are given in electronic supplementary material) are displayed on CCA axis in separated plots. S: salinity; CS: coarse sand; MS: medium sand; FS: fine sand; VFS: very fine sand; M: mud; LOI: loss on ignition; WC: water content
Discussion
Dead diatom assemblages showed a spatial zonation in the Quequén Salado estuary, which was characterized by a marine assemblage in the inlet, a brackish/freshwater assemblage in the middle estuary and brackish/freshwater and freshwater assemblages in the upper estuary. Similar diatom zonations were recorded in estuaries characterized by salinity gradients (Moore & McIntire, 1977; Ampsoker & McIntire, 1978; Juggins, 1992; Debenay et al., 2003; Resende et al., 2005). As salinity is one of the main environmental factors controlling diatom distribution in estuaries (Cooper, 1999), the diatom zonation observed in the present study can be related to the existence of a salinity gradient in the Quequén Salado estuary. The importance of the salinity gradient on diatom distribution was supported by CCA results, which identified salinity as one of the most important of the measured environmental variables in explaining diatom variation in both estuaries.
According to CCA, grain size was almost as important as salinity in determining diatom assemblage composition along the estuarine gradient of both estuaries. The dominance of epipsammic, planktonic and tychoplanktonic diatoms in Quequén Salado zones I and II and Quequén Grande zone I may be explained by the instability of its sandy sediments, whereas the dominance of epipelic diatoms in the upper reaches of both estuaries may be related to the presence of finer sediments. It is well known that physical properties of sediments play a fundamental role in the nature of the microphytobenthos assemblage that colonizes sediments (Trites et al., 2005). The settlement of large benthic diatom biomasses is prevented in sandier, unstable sediments, where the development of colonies on the open surface of sand grains is inhibited by agitation (McIntire & Ampsoker, 1986). Moreover, the species composition of estuarine diatom assemblages exhibits a distributional continuum of epipsammic and epipelic taxa, which are closely related to a gradient of sediment particle sizes (Ampsoker, 1977; Ampsoker & McIntire, 1978; Whiting & McIntire, 1985).
The dominance of a mixture of diatom assemblages with different salinity preferences at zone I and its proximity to assemblages from zones III and IV in Fig. 5, suggest that this site was dominated by allochthonous valves, probably transported from the headwaters of the estuary. This zone was dominated by similar proportions of the freshwater taxa, which dominated zone III (e.g., Nitzschia cf. perminuta and Amphora helenensis, see Fig. 2), mixed with marine and brackish taxa. The composition of different dominant ecological groups has been proposed as a criterion for the detection of allochthonous components in a diatom assemblage: if the habitat of two ecological groups do not overlap (e.g. freshwater and marine groups) at least one of them must be allochthonous (Vos & De Wolf, 1993). Therefore, the presence of freshwater and brackish/freshwater diatom taxa in this zone, characterized by polyhaline conditions, can be regarded as a consequence of taphonomic mixing of transported floras.
The high proportions of marine plankton and tychoplankton that dominate zone II are characteristic of tidal channels and tidal inlets. In these environments, the conditions (high current velocities and subsequent low irradiance at the bottom) are permanently unfavorable for the development of benthic and epiphytic diatom populations (Vos & De Wolf, 1993). The dominant taxon in this zone, P. sulcata, is a tychoplanktonic diatom characteristic of temperate coastal waters and very abundant in coastal sediment records (McQuoid & Nordberg, 2003). P. sulcata is generally thought to be an indicator of coastal conditions (Cooper, 1999; McQuoid & Nordberg, 2003). Yet, in this contribution it was found at salinities ranging from 15 to 37‰. Consequently, we point out that the diatom assemblage found in zone II (and particularly P. sulcata) should be classed equally as a marine/brackish assemblage. Thus, it constitutes a useful analog for marine/brackish tidal environments in the Argentinean coast.
Zone III was dominated by A. helenensis, an estuarine diatom that was also found in the lower course and estuarine zones of the Sunday river (South Africa; Archibald, 1983) and the Quequén Grande river (Hassan et al., 2006). This is a transitional zone between marine/brackish and brackish/freshwater conditions, and marks a discontinuity in the distribution of the main diatom salinity groups. This discontinuity in the diatom flora related to the salinity range has been previously reported and attributed to differences in osmotic regulation between fresh- and brackish water diatoms (Ampsoker & McIntire, 1978; Snoeijs, 1999). The transition from 5 to 7‰ represents the lowest limit of salinity tolerance for many marine taxa, resulting in the absence or very low abundance of these taxa below 5‰ (Snoeijs, 1999). Accordingly, oligohalobous taxa dominated the non-tidal environments of zone IV, where salinity was always under this limit.
The comparison of diatom groups between the Quequén Salado and Quequén Grande estuaries showed a distribution according to their ecological preferences of salinity and sediment properties. The marine/brackish diatom assemblage was more widely distributed in Quequén Salado river and had no analogues when compared with Quequén Grande river assemblages. This difference between both estuaries may be related to their differences in salinity and grain size distribution. In fact, the range of salinities and the sediment grain sizes in the first kilometers of Quequén Salado estuary were higher than those recorded at Quequén Grande estuary, where polyhaline conditions and sandy sediments were recorded only in the first meters of the inlet.
The differences in salinity ranges between both estuaries may be attributed to the tidal range and the grade of human impact in each estuary. It has been pointed out that, beside their differences in tidal range (1 m mean tidal difference), the Quequén Salado estuary presently represents the exact conditions that the Quequén Grande estuary had before the anthropogenic influence (Perillo et al., 2005). Whereas many modifications have produced major consequences altering the original geomorphology and circulation in the Quequén Grande estuary in the last 100 years, particularly the obstruction of the incoming tidal wave (Perillo et al., 2005), the Quequén Salado mouth dynamics has remained almost unaltered. Since diatom distribution is influenced mainly by the salinity range and sediment type in these estuaries, their morphological differences originated by human modification constitute a key factor in explaining the observed differences in diatom distribution.
Conclusion
This contribution shows that a clear diatom zonation is present along the longitudinal axis of the Quequén Salado estuary and provides diatom assemblages indicators of (1) marine/brackish, (2) brackish/freshwater and (3) brackish/freshwater to freshwater conditions. Some of these diatom assemblages were also found in the Quequén Grande estuary, showing a regional distributional pattern. The comparison between the two estuaries under different grades of anthropogenic modification allowed the identification of baseline conditions for these environments and showed a displacement of diatom salinity groups along the estuarine gradient as a possible consequence of the presence of obstacles to the incoming tidal wave. Moreover, diatom distribution in both estuaries were closely related to sediment properties, particularly grain size, and reflected the sedimentological differences between both estuaries.
Given the scarcity of local ecological information, it becomes clear that more studies regarding South American estuarine diatoms need to be conducted in order to increase the accuracy of diatom based paleoenvironmental reconstructions. The identification of useful modern analogs in the Quequén Salado river constitutes a step in that direction. Moreover, the integration of the present data set with data from previously studied estuarine systems from the region, such as the Mar Chiquita coastal lagoon and the Quequén Grande river, may lead to the future development of a diatom-based calibration model, allowing reliable quantitative reconstructions of Holocene paleosalinity fluctuations in Southern South America.
References
Ampsoker, M. C., 1977. The distribution of intertidal epipsammic diatoms on Scripps Beach, La Jolla, California, U.S.A. Botanica Marina 20: 227–232.
Ampsoker, M. C. & C. D. McIntire, 1978. Distribution of intertidal diatoms associated with sediments in Yanquina Estuary, Oregon. Journal of Phycology 14: 387–395.
Archibald, R. E. M., 1983. The diatoms of the Sundays and Great Fish Rivers in the eastern Cape Province of South Africa. In Cramer J. (ed.), Bibliotheca Diatomologica, Vol. 1. A.R. Gantner Verlag, Vaduz.
Battarbee, R. W., 1986. Diatoms as indicators of surface water acidity. In Stoermer E. F. & J. P. Smol (eds), The Diatoms: Applications for the Environmental and Earth Sciences. Cambridge University Press, London, 85–123.
Cooper, S. R., 1999. Estuarine paleoenvironmental reconstructions using diatoms. In Stoermer E. F. & J. P. Smol (eds), The Diatoms: Applications for the Environmental and Earth Sciences. Cambridge University Press, London, 352–373.
Day, J. H. (ed), 1981. Estuarine Ecology, with Particular Reference to Southern Africa. A.A. Balkema, Rotterdam.
De Wolf, H., 1982. Method of coding of ecological data from diatoms for computer utilization. Med Rijks Geol Dienst 36: 95–99.
Debenay, J. P., P. Carbonel, M.-T. Morzadec-Kerfourn, A. Cazaboun, M. Denèfle & A. -M. Lézine, 2003. Multi-bioindicator study of a small estuary in Vendée (France). Estuarine Coastal and Shelf Science 58: 843–860.
Denys, L., (1991/1992). A check-list of the diatoms in the Holocene deposits of the Western Belgian coastal plain with the survey of their apparent ecological requirements, I. Introduction, ecological code and complete list. Professional Paper 246, Belgian Geological Survey, Belgium.
Espinosa, M. A., C. G. De Francesco & F. I. Isla, 2003. Paleoenvironmental reconstruction of Holocene coastal deposits from the southeastern Buenos Aires province, Argentina. Journal of Paleolimnology 29: 49–60.
Folk, R. L., 1968. Petrology of Sedimentary Rocks. University of Texas at Hemphills, Austin.
García-Rodríguez, F., P. Sprechmann, D. Metzeltin, L. Scafati, D. L. Melendi, W. Volkheimer, N. Mazzeo, A. Hiller, W. Von Tümpling Jr. & F. Scasso, 2004. Holocene trophic state changes in relation to sea level variation in Lake Blanca, SE Uruguay. Journal of Paleolimnology 31: 99–115.
Germain, H., 1981. Flore des Diatomées. Eaux douces et saumâtres du massif Armoricain et des contrées voisines d́Europe Occidentale. Societé nouvelle des Editions Boubée, Paris.
Grimm, E. C., 1991. TILIA Software. Illinois State Museum. Research and Collection Center. Springfield, IL, USA.
Hartley, B., 1996. An Atlas of British Diatoms. In Sims P. A. (ed.), Biopress Ltd., Bristol.
Hassan, G., M. A. Espinosa & F. I. Isla, 2006. Modern diatom assemblages in surface sediments from estuarine systems in the southeastern Buenos Aires Province, Argentina. Journal of Paleolimnology 35: 39–53.
Heiri, O., A. F. Lotter & G. Lemcke, 2001. Loss on ignition as a method for estimating organic and carbonate content in sediments: reproductibility and comparability of results. Journal of Paleolimnology 25: 101–110.
Hustedt, F., 1930. Bacillariophyta (Diatomae). In Pascher A. (ed.), Die Süsswasserflora Mitteleuropas, G. Fisher Verlag, Jena.
Hustedt, F., 1937–1938. Systematische und ökologische Untersuchungen über die Diatomeen. “Flora von Java, Bali und Sumatra nach dem Material der Deutschen Limnologischen Sunda-Expedition”. Archive von Hydrobiologie supplement, Band 15: 131–506.
Hustedt, F., 1959–1966. Die Kieselalgen. In Rabenhorst L. (ed.), Kryptogamen flora von Deutschland, Österreich und der Schweiz 7(2)6: 737–845 1959; 7(3)2: 161–348 1962; 7(3)4: 557–816 1966.
Inda, H., F. García-Rodríguez, I. Del Puerto, V. Acevedo, D. Metzeltin, C. Castiñeira, R. Bracco & J. B. Adams, 2006. Relationships between trophic state, paleosalinity and climate changes during the first Holocene marine transgression in Rocha Lagoon, southern Uruguay. Journal of Paleolimnology 35: 699–713.
Johnson, D. E., 1998. Applied Multivariate Methods for Data Analysts. Duxbury Press, Belmont, CA.
Juggins, S., 1992. Diatoms in the Thames estuary, England: ecology, paleoecology, and salinity transfer function. In Lange-Bertalot H. (ed.), Bibliotheca Diatomologica. J. Cramer, Berlin 25: 1–216.
Kovach Computing Services, 2002. Multivariate Statistical Package version 3.13 b. Anglesey, Wales.
Krammer, K. & H. Lange-Bertalot, 1986. Bacillariophyceae. 1. Teil: Naviculaceae. In Ettl H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Band 2/1. VEB Gustav Fisher Verlag, Jena.
Krammer, K. & H. Lange-Bertalot, 1988. Bacillariophyceae. 2. Teil: Bacillariophyceae, Epithemiaceae, Surirellaceae. In Ettl H., J. Gerloff, H. Heynig & D. Mollenhauer (eds), Süsswasserflora von Mitteleuropa, Band 2/2. VEB Gustav Fisher Verlag. Jena.
Lange-Bertalot, H., 2001. Navicula sensu stricto, 10 genera separated from Navicula sensu lato Frustulia. In Lange-Bertalot H. (eds), Diatoms of Europe, Vol. 2. Koeltz Scientific Books, Koenigstein.
Marini, M. F. & M. C. Piccolo, 2000. El balance hídrico en la cuenca del río Quequén Salado, Argentina. Papeles de Geografía 31: 39–53.
Marini, M. F. & M. C. Piccolo, 2004. Water quality for supplementary irrigation in the Quequén Salado River basin (Argentina). Papeles de Geografía 39: 157–172.
McIntire, C. D. & M. C. Ampsoker, 1986. Effects of sediment properties on benthic primary production in the Columbia River Estuary. Aquatic Botany 24: 249–267.
McQuoid, M. R. & K. Nordberg, 2003. The diatom Paralia sulcata as an environmental indicator species in coastal sediments. Estuarine, Coastal and Shelf Science 56: 339–354.
Moore, W. W. & C. D. McIntire, 1977. Spatial and seasonal distribution of littoral diatoms in Yanquina estuary, Oregon (USA). Botanica Marina 20: 99–109.
Ng, S. L. & F. S. Sin, 2003. A diatom model for inferring sea level change in the coastal waters of Hong Kong. Journal of Paleolimnology 30: 427–440.
Perillo, G. M. E., D. E. Pérez, M. C. Piccolo, E. D. Palma & D. G. Cuadrado, 2005. Geomorphologic and physical characteristics of a human impacted estuary: Quequén Grande River Estuary, Argentina. Estuarine Coastal and Shelf Science 62: 301–312.
Resende, P., U. Azeiteiro & M. J. Pereira, 2005. Diatom ecological preferences in a shallow temperate estuary (Ria de Aveiro, Western Portugal). Hydrobiologia 544: 77–88.
Round, F. E., R. M. Crawford & D. G. Mann, 1990. The Diatoms—Biology and Morphology of the Genera. Cambridge University Press, Cambridge London.
Rumrich, U., H. Lange-Bertalot & M. Rumrich, 2000. Diatoms of the Andes. From Venezuela to Patagonia/Tierra del Fuego. In Lange-Bertalot H. (eds), Iconographia Diatomologica vol. 9, A.R.G. Gantner Verlag, distributed by Koeltz Scientific Books, Königstein.
Snoeijs, P., 1999. Diatoms and environmental change in brackish waters. In Stoermer E. F. & J. P. Smol (eds), The Diatoms: Applications for the Environmental and Earth Sciences. Cambridge University Press, London, 298–333.
Statsoft, 1998. STATISTICA for Windows. Rel. 5.1. Statsoft Inc., Tulsa, OK.
teer Braak, C. J. F., 1986. Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67: 1167–1179.
Trites, M., I. Kaczamarska, J. M. Ehrman, P. W. Hicklin & J. Ollerhead, 2005. Diatoms from two macro-tidal mudflats in Chignecto Bay, Upper Bay of Fundy, New Brunswick, Canada. Hydrobiologia 544: 299–319.
Vos, P. C. & H. De Wolf, 1988. Methodological aspects of paleo-ecological diatom research in coastal areas of the Netherlands. Geologie en Mijnbouw 67: 31–40.
Vos, P. C. & H. De Wolf, 1993. Diatoms as a tool for reconstructing sedimentary environments in coastal wetlands; methodological aspects. Hydrobiologia 269: 285–296.
Whiting, M. C. & C. D. McIntire, 1985. An investigation of distributional patterns in the diatom flora of Netarts Bay, Oregon, by correspondence analysis. Journal of Phycology 21: 655–661.
Acknowledgements
This research is part of the doctoral thesis of G. S. Hassan at the University of Mar del Plata. Financial support for this work was provided by the project 15/E125 from Universidad Nacional de Mar del Plata. We are indebted to C. De Francesco for fieldwork assistance and for his valuable comments and suggestions on earlier versions of the manuscript. Judit Padisák and two anonymous reviewers provided helpful comments improving this paper. G. S. Hassan was funded by the National Council of Scientific and Technical Research of Argentina (CONICET) through a fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: J. Padisak
Electronic supplementary material
Below are the electronic supplementary materials.
Rights and permissions
About this article
Cite this article
Hassan, G.S., Espinosa, M.A. & Isla, F.I. Dead diatom assemblages in surface sediments from a low impacted estuary: the Quequén Salado river, Argentina. Hydrobiologia 579, 257–270 (2007). https://doi.org/10.1007/s10750-006-0407-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-006-0407-6