Abstract
Given the complex and multidimensional nature of human evolution, we need to develop theoretical and methodological frameworks to account for and model the dynamic feedbacks between co-operational biological and cultural evolutionary systems to better understand the processes that produced modern human behavior. Equally important is the generation of explicit theory-based models that can be tested against the empirical paleoanthropological record. We present a case study that examines evidence for culturally-driven behavioral change among Late Pleistocene hominins that altered the social niche occupied by hominins in western Eurasia, with consequences for subsequent biological and cultural evolution. We draw on a large sample of 167 Pleistocene assemblages across western Eurasia and employ mathematical and computational modeling to explore the feedbacks between cultural and biological inheritance. Shifts in land-use strategies changed the opportunities for social and biological interaction among Late Pleistocene hominins in western Eurasia with a cascade of consequences for cultural and biological evolution, including the disappearance of Neanderthals from the fossil and archaeological records, and the acceleration of cultural evolution among ancestors of modern humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Late Pleistocene (ca. 128,000–11,500 years ago), spanning the last Interglacial/Glacial cycle, was a time of dramatic and often rapid global environmental change. The study of human ecological dynamics during the Late Pleistocene is critical to understanding our origins and resilience to global environmental change. Moreover, human biogeographical responses to shifting Late Pleistocene environments underlie the spread of the form of humans that inhabits the earth today—those that we call “modern.” Important to understanding the dynamics of Upper Pleistocene human ecology is a broad and rapidly growing consensus that a large portion of human behavior is the phenotypic expression of a cumulative body of information transmitted between individuals by social learning rather than genes—often glossed as “culture” (Alvard 2003; Boyd and Richerson 2005; Boyd and Richerson 1985; Henrich 2004; Henrich and McElreath 2003; Hill et al. 2009; Powell et al. 2009; Richerson and Boyd 2005; Shennan 2001). Culture and its transmission constitute an inheritance system that functions independently from biological evolution; these two inheritance systems can operate in parallel, coevolve, or even act in opposition to one another (Barton 2008; Laland et al. 2010; Richerson and Boyd 2005; Richerson et al. 2010).
Given the complex and multidimensional nature of human evolution, we need theoretical and methodological frameworks in which to combine increasingly sophisticated interdisciplinary research on the biological, cultural and environmental dimensions of human evolution. Importantly, such frameworks must be able to account for and model the dynamic feedbacks between these co-operational evolutionary systems if we are to understand the processes that produced the behaviors that characterize humans today. While several recent studies exemplify the value of taking a broader, interdisciplinary view of human evolution during the Late Pleistocene (e.g., d’Errico et al. 2003a; Finlayson and Carrión 2007; Laland and Brown 2006; Powell et al. 2009; Premo and Hublin 2009; Stiner and Kuhn 2006), there is little research that attempts to incorporate the feedbacks across these three evolutionary systems remains (see discussion in Riel-Salvatore 2010).
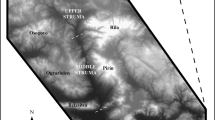
To illustrate the potential of a multi-dimensional approach to human biocultural evolution, we present a case study of culturally and environmentally driven changes in land-use behaviors among Late Pleistocene hominins in western Eurasia. We use a model that links the characteristics of hunter-gatherer stone artifact assemblages to land-use practices to track long-term shifts in human ecology, and then apply computational modeling to explore potential biological consequences of these behavioral changes. We employ data from 167 archaeological assemblages spanning the Late Pleistocene and derived from 31 localities across western Eurasia, from southern Iberia to the Near East (Fig. 1, Table 1). While we recognize the geographical bias of current paleoanthropological data and are certain that much will be learned from the great expanses of the world that remain minimally studied or unstudied (c.f., Glantz et al. 2009; Serangeli and Bolus 2008), western Eurasia presents the most comprehensive and regionally extensive database of hominin biological, cultural, and environmental variation currently available for the Late Pleistocene.
Locations of archaeological sites for assemblages used in analyses. Map shows Late Pleistocene coastlines (−100 m bmsl) in dark grey with modern coastlines as white line. 1: Gibraltar, 2: Spanish sites (3 sites), 3: Riparo Bombrini; 4 Castelcivita; 5: other Italian sites (6 sites); 6: Romanian/Carpathian sites (14 sites); 7: Jordanian sites (6 sites)
Importantly, rather than using this large database to make inferences about the human past, we emphasize the generation of explicit models—narrative, mathematical, and computational—that we test against this record to examine feedbacks between cultural and biological inheritance. While models are widely found in paleoanthropology, the great majority is inferential—created inductively to fit some body of empirical data. There is recursive interplay of induction and deduction in scientific explanation, of course. While induction (and inference) is especially important in the construction of explanatory models, deductive protocols generally provide the most robust means of evaluating the explanatory capabilities of models. Because inferential models are empirically derived, they inherently fit the data on which they are based. Moreover, the highly incomplete nature of the paleoanthropological record means that multiple models often can be inferred from the same data. This makes systematic, comparative evaluation of competing models very difficult, leading to irresolvable debates over different interpretations of the same record. Hence, it is important to balance traditional inference with theory-based deductive models that can be tested against (rather inferred from) the paleoanthropological record. We emphasize the latter approach here, beginning with a model for stone technology grounded in human behavioral ecology that provides a means to track prehistoric land-use dynamics. We follow this with a model of biobehavioral feedbacks and evolutionary consequences of behavioral change.
A Model for Lithic Technology in Ecological Context
At the scale of human lifetimes, the ecology of hunter-gatherer land-use is complex and conditioned by a changing suite of environmental and social parameters (Bettinger 1991; Binford 2001; Brantingham 2006; Eder 1984; Foley 1985; Grove 2009, 2010; Kelly 1983, 1995; Potts 1994). Most hunter-gatherers shift their residences with some regularity, and there is variability in the frequency and distance of movement that is linked to the spatial and temporal distribution of important resources (especially food resources) and the ways in which these resources are harvested. Residential and logistical mobility strategies (RMS and LMS) are concepts that characterize ethnographically observed variation in hunter-gather mobility, representing endpoints on a continuum of hunter-gatherer land-use (Binford 1979; Grove 2010; Nelson 1991). Residential mobility refers to a land-use strategy in which hunter-gatherers tend to move their residential camps to exploit resources available at different times and places; logistical mobility (similar to central-place foraging) is a strategy in which camps are moved less often and groups of hunter-gatherers make targeted forays to acquire resources and return them to these residential base camps (Grove 2010; Kim 2003). While these concepts sometimes have been applied to contemporary hunter-gatherers, the RMS-LMS continuum is perhaps most useful as a framework for expressing variability in this important dimension of hunter-gatherer ecology at the multigenerational scale that characterizes the archaeological record (Bamforth 1986; Binford 1980; Kelly 1983; Kuhn 1992; Riel-Salvatore and Barton 2004). RMS and LMS tend to predominate under different environmental conditions and there is a general, though not exclusive, tendency for logistical resource forays to cover greater distances than residentially mobile hunter-gatherers moving from one resource patch to the next (Binford 1980, 2001; Grove 2009, 2010; Kelly 1983, 1995). This means that RMS foragers tend to move more often, but within an area of more limited geographic extent; LMS foragers, on the other hand, may travel less often, but they tend to do so within a region of much greater geographic extent on an annual and lifetime basis. These strategies respond to the ways resources are structured spatially and temporally and affect the ways in which resources are procured, processed, and consumed (Ibid.). Variation in the spatial extent over which members of a forager group regularly range also affects the size of their potential mating network (sensu Wobst 1974; see Amick 1996 for an extreme case) with implications for human biocultural evolution (see below).
Lithic technology operates within the context of hunter-gatherer land-use strategies and is affected by it in distinctive ways. Stone technology is the most ancient and consistently visible example of the extended human phenotype, and was an essential part of human life since long before the origin of the genus Homo. Moreover, since hunter-gatherers leave little material evidence of their activities, and even less survives after tens of millennia, durable lithic artifacts (and sometimes animal bones) are the primary proxy data for Late Pleistocene human ecology.
A quarter-century of diverse research has produced a coherent and comprehensive body of theory for lithic technology and its role in hunter-gatherer ecology (see reviews in Bleed 2001; Hiscock 2007; Holdaway and Douglass 2011; Riel-Salvatore and Barton 2004). The behaviors that create variability in the morphology of lithic artifacts are very different from the industrial technological processes that produce variability in the manufactured objects that pervade modern life and materials culture (Barton 1991). Especially relevant for hominin paleoecology, most variation in the forms of lithic tools recovered from archaeological assemblages deposited by hunter-gatherers (and, consequently, the frequencies of these artifacts within assemblages) is largely a result of variation in the life histories of morphologically dynamic artifacts, human discard behaviors, and sometimes post-depositional taphonomy rather than variation in the makers’ intent and design (Barton 1997; Barton 1991; Bleed 2001; Dibble et al. 1997; Hiscock 2007; Holdaway and Douglass 2011; Riel-Salvatore and Barton 2004; Rolland and Dibble 1990; Shott and Weedman 2007). From this life history perspective, most retouch (i.e., removing flakes from an edge)—especially in Paleolithic assemblages—is to resharpen or rejuvenate dulled edges of lithic artifacts and extend their use-lives, rather than a way to shape a predetermined implement. Analytically, most retouch serves as a measure of stone artifact curation rather than an expression of the maker’s intent. It also has been long recognized that curation and discard behaviors, responsible for the accumulation of artifact assemblages found by archaeologists, are closely tied to ecological contexts of hunter-gather land-use and mobility strategies (Bamforth 1986; Binford 1979; Kelly 1983, 1992; Kuhn 1989, 1991, 1992; Nelson 1991; Rolland and Dibble 1990; Shott 1996). The reasons for this association are clear when lithic technology is viewed from the perspective of human ecology.
A mobile hunter-gatherer needs to use more energy to carry lithic artifacts (composed primarily of SiO2 at 2.2 g/cm³) than equivalent volumes of food, water, or infants. Yet chipped stone’s ubiquity in the archaeological record for over 2,500,000 years attests to its importance for human survival—for procuring and processing resources, and for crafting other items. Several inherent characteristics of chipped stone technology are key to its role in hunter-gather ecology and the formation of archaeological assemblages. Stone’s durability is the reason it dominates the archaeological record for Pleistocene hominins. However, stone tools commonly have use-lives on the order of hours or days because their brittle edges dull rapidly, and resharpening those edges through retouch soon reduces them to an unusable size (Bamforth 1986; Barton 1990; Dibble 1995; Frison 1968; Gould et al. 1971; Holdaway and Douglass 2011). On the other hand, making new lithic artifacts with sharp, useable edges can be done easily and quickly if appropriate stone is available, and rapidly produces a large quantity of flakes, fragments, and debris, only some of which are useful (Ahler 1989; Andrefsky 2001; Holdaway and Douglass 2011; Magne 1989; Magne and Pokotylo 1981; Mauldin and Amick 1989; Newcomer 1971; Shott 1994).
Because stone is heavy but also essential, hunter-gatherers with land-use dominated by RMS tend to transport and discard relatively few stone artifacts, and also tend to extend the short use-lives of those that they do carry by repeatedly retouching dulled edges. Regular movement of residential camps puts a premium on portability and can create an ‘effective scarcity’ of lithic materials (Riel-Salvatore and Barton 2007). Such conditions, in turn, encourage the curation of stone artifacts to ensure that they are available for critical uses, while avoiding the need to carry any more heavy stone than absolutely necessary. Portability is also important to LMS-organized hunter-gatherers on targeted resource forays. However, they can stockpile stone at base camps where they are transiently sedentary between resource forays (Kuhn 1992), creating an ‘effective abundance’ of useable stone and offering less incentive for curation. Thus, the tendency for curation of lithic artifacts in land-use emphasizing RMS means that relatively few lithics are discarded at residential sites, and that curated (i.e., retouched) pieces will be comparatively common in discard assemblages. On the other hand, when LMS dominate, hunter-gatherers can more readily create new artifacts from stockpiled raw material at base camps and have less need to extend artifact use-life through retouch. With less curation, more artifacts are made and discarded in base camps, and because lithic technology can rapidly generate large quantities of byproducts, these camps should be characterized by abundant lithic assemblages with lower frequencies of retouched artifacts. This theoretical model of the way in which lithic technology interacts with human ecological behaviors offers several clear expectations that can be tested with the empirical paleoanthropological record.
Model Testing
At most Pleistocene hominin sites, artifact assemblages are time-averaged palimpsests of trash from repeated occupations by hunter-gatherer groups that accumulated over generations, rather than residues of discrete encampments (Barton and Clark 1993; Colcutt 1979; Farrand 2001; Holdaway and Douglass 2011; Riel-Salvatore and Barton 2004), incorporating variable mixtures of lithics produced and discarded under different land-use strategies. The interactions between hunter-gatherer land-use and lithic technology outlined above suggest the following expectations when applied to cumulative assemblages in archaeological sites. The more that refuse from LMS base camps contributed to an assemblage, the lower the frequency of retouched artifacts and the greater the volumetric density of all lithic material. Conversely, the more that land-use emphasizing RMS contributed lithic trash to an assemblage, the higher the retouch frequency and the lower the total lithic density. In other words, given the model of lithic technology and hunter-gatherer land-use strategies proposed, there should be a negative relationship between retouch frequency and artifact density (i.e., total artifacts per cubic meter of excavated sediment) across multiple lithic assemblages. In fact, we have repeatedly identified such negative correlations between retouch frequency and artifact densities in assemblages across southern and central Europe (Fig. 2) (Barton 1998; Riel-Salvatore 2007; Riel-Salvatore and Barton 2004, 2007; Riel-Salvatore et al. 2008), and others have replicated these results at additional localities (Clark 2008; Kuhn 2004; Sandgathe 2006).
Retouch frequency vs. total lithic density for Late Pleistocene assemblages from sites across western Eurasia. Solid black symbols are assemblages classified as middle paleolithic, solid grey symbols are classified as ‘transitional industries’, and open symbols are classified as upper paleolithic. a Gorham’s Cave, Gibraltar; b sites in eastern Spain (squares are Cova Negra, diamonds Cova del Salt, triangle Cova Pastor, and circles Cova Beneito); c Grotto Mario Bernardini; d Grotto di Uluzzo C; e 13 caves/shelter sites from the Carpathian Mountains in western Romania (Barton 1998; Riel-Salvatore and Barton 2004, 2007; Riel-Salvatore et al. 2008; Villaverde et al. 1998)
Of course numerous other factors can potentially contribute to the morphology of individual artifacts or characteristics of assemblages discarded during a single occupation of hunter-gather campsites. However, the fact that empirical data at the scale of multi-generational palimpsests commonly found in the archaeological record consistently conform to the predictions of the ecologically-based lithic technology model outlined above indicates its robustness as a proxy for prehistoric forager land-use across multiple assemblages recovered in many different research projects. Other kinds of assemblage configurations can be imagined—assemblages with high artifact densities and high retouch frequencies (i.e., not a result of very slow natural deposition processes) that could be generated by intensive processing of other materials, for example—but are rare in the western Eurasian sites we and others have studied. By far the most common kinds of assemblages found are those that conform to the predictions of our model.
The reasons these deceptively simple measures can serve as a robust proxy for the complex of hunter-gatherer behaviors we gloss as mobility strategies are probably in large part because most Paleolithic assemblages are time-averaged across multiple occupations and that the most visible (and hence most likely excavated) Paleolithic sites were locales at which high densities of behavioral residues accumulated due to repeated occupations and/or longer occupations. Under these kinds of conditions, the lithic signal for long-term mobility strategies remains apparent while signals for other kinds of short-term behaviors become increasingly blurred. If we actually could recover a representative sample of assemblages from the variety of individual occupations by Paleolithic hunter-gatherers, it might be more difficult to distinguish general mobility strategies from the many other factors that affect technological behaviors on a day-to-day basis.
Additionally important from a pragmatic standpoint, these simple measures of retouch frequency and artifact density can be obtained in a consistent manner from lithic assemblages regardless of their assignment to one of the many named (and sometimes poorly defined) Paleolithic industries that have been created by archaeologists across this continental region. This industry-independent measure allows us to assess changes in human ecology across the broad geographic and temporal span of the Upper Pleistocene of western Eurasia.
Nevertheless, while both retouch frequency and artifact density can serve as covarying proxies for human land-use strategies, lithic density can be affected by variation in deposition rates at different sites and even over time within individual sites (Barton 1998; Riel-Salvatore 2007; Riel-Salvatore and Barton 2004; Riel-Salvatore et al. 2008). Retouch frequency, in contrast, is a normalized measure much less affected by differences in depositional environments or assemblage size; the palimpsest nature of most hominin assemblages further ensures that it reflects general behavioral trends rather than short-term variations. Given the ability of our model to predict assemblage-level variability in the empirical paleoanthropological record—and the absence of competing models that can better predict this variability—we use assemblage-level retouch frequencies as a proxy for general trends in prehistoric land-use across western Eurasia.
Late Pleistocene Trends in Land-Use Strategies
As can be seen in Fig. 3, there are clear time trends for a decrease over time in both the frequency of retouch in assemblages and in the variability in retouch frequency expressed during a time interval. In terms of standard archaeological classification, these assemblages represent lithic meta-industries labeled Middle Paleolithic (commonly thought to be made by Neanderthals), Upper Paleolithic (commonly thought to be made by morphologically modern humans [MMH]), and some considered ‘transitional’ (whose makers are generally thought to be Neanderthals) (Riel-Salvatore and Barton 2007; Riel-Salvatore and Clark 2007; Zilhão and d’Errico 2003). Although only a few of the assemblages analyzed have been dated radiometrically (unfortunately still the norm for sites of this age), we can assign them to a Marine Isotope Stage from relevant published reports. Applying the lithic technology/land-use model in prior work suggested time-transgressive reduction in the variability in land-use strategies humans practiced, with increasing predominance of LMS at local and regional scales (Barton 1998; Riel-Salvatore 2007; Riel-Salvatore and Barton 2004, 2007; Riel-Salvatore et al. 2008; Villaverde et al. 1998). Applying this model to a much larger group of assemblages (Fig. 3) shows that these same trends are repeated on a continental scale for all of western Eurasia; land-use strategies varied widely from RMS to LMS in MIS 5. Subsequently, assemblages show a decreasing contribution from RMS and are dominated by LMS by MIS 2. The distribution of retouch frequency values in Fig. 3 also make it clear that diverse land-use strategies, ranging from RMS to LMS, have been practiced since MIS 5. Moreover, the time trends for land-use strategies we document began prior to sustained evidence for MMH presence in this region (i.e., by MIS 4) and continue through MIS 2, when the fossil record indicates that the region was occupied only by MMH. This suggests that all hominins in western Eurasia—Neanderthals and MMH alike—were able to utilize a wide range of land-use strategies and responded to conditions that increasingly favored LMS over RMS strategies over the course Late Pleistocene.
Top retouch frequency for all sites, by Marine Isotope Stage. Box plots show median and mid-spread; whiskers extend a second midspread beyond the median. Middle Paleolithic assemblages are solid circles, Transitional assemblages are asterisks, and Upper Paleolithic assemblages are open circles. Data points are randomly jittered horizontally within each stage to improve visibility. N = 167. Bottom time trend analysis of retouch frequency. X-axis of both plots is the mid-point in calendar years of each Marine Isotope Stages 5–2. R2 and p shown for least squares regressions of time trends. Equations for the fit lines of the time trends are: \( {{ \log }\left( {variance} \right) = - {4}{.910558} + \left( {age \times {2}{.483}{e^{{ - {5}}}}} \right)} \); \( {retouch = {0}{.2833127} - \left( {{3013}{.5417}/age} \right)} \). These graphs do not imply any causality, but simply serve to distill the non-random temporal trends seen in the upper box plots
These results are consistent with Féblot-Augustins’ (1993) study of the distance lithic raw materials were transported during the Late Pleistocene Middle Paleolithic in France and Central Europe, independent of measures of artifact curation we use here, to the extent that LMS tends to involve longer distance movements than RMS land-use. Figure 4 presents Féblot-Augustins’ results in a format comparable with our results (Fig. 3). There seems to be a slight trend toward increasingly longer distances of stone transport from MIS 5 to MIS 4/3 for Middle Paleolithic assemblages (presumably made by Neanderthals), although the small sample size and chronological uncertainty precludes statistical assessment. Additionally, among the retouched tool components of the Transitional and Upper Paleolithic assemblages that appear in MIS 3 and 2, small “backed” artifacts are increasingly common (Bar-Yosef and Kuhn 1999; Riel-Salvatore 2007, 2009). It is widely thought that these backed artifacts formed the cutting edges of compound tools whose portability and maintainability in the field (sensu Bleed 1986; Myers 1989; Torrence 1989) made them especially useful for long-distance logistical resource forays.
Given the relatively short time-span of this shift in land-use behavior (i.e., mostly taking place in <50 ka from MIS 4–2) and the fact that the large-scale patterns that we document cross-cut assemblages made by Neanderthals and MMH, it likely that culture and social learning were primary drivers of these behavioral dynamics. Other studies suggest that both Neanderthal and MMH ecological behavior responded to Late Pleistocene environmental shifts (Finlayson and Carrión 2007; Stiner and Kuhn 2006), and trends toward reduced variability in land-use strategies and increasing reliance on LMS-type foraging noted above coincide with global climate changes from the last Interglacial to the Last Glacial Maximum (LGM). MIS 5–2 is characterized by climate fluctuations that are increasingly abrupt and (through MIS 3) increasingly intense with regard to inferred temperature changes (Fig. 5). It is possible that increasing environmental unpredictability and consequent risk of resource shortfalls in MIS 3 may have favored LMS foraging because it allowed humans to target a wider array of resources over a larger spatial extent, although we do not test such an association here. Indeed, studies of recent hunter-gatherers show that LMS are more common at high latitudes, with low mean temperatures and high spatial/temporal variance in resource distribution and abundance, while RMS predominate at low latitudes (Binford 1980, 2001; Grove 2010; Kelly 1983, 1995; Kuhn 1992).
Delta 18O/16O values from the GISP2 Greenland Ice Core (Meese et al. 1997). Marine isotope state (MIS) boundaries indicated
Biological and Social Consequences of Land-Use Change
Hominin behavioral responses to changing Late Pleistocene climates and landscapes, particularly an increase in long distance forays to collect resources for provisioning base camps associated with LMS, would have altered the biological and social environment of Eurasian hominins by increasing opportunities for social and biological interactions among hominins across broader geographic regions. This would have had consequences for human biocultural variation and change.
The continental geography of Europe, as a long peninsula extending westward from Asia, left western European hominins geographically semi-isolated from other hominin populations—especially during periods of continental and Alpine ice sheets—allowing Neanderthals to emerge during the Middle and early Late Pleistocene as a regional population, morphologically and genetically distinct from other contemporaneous hominins outside of western Eurasia (Harvati 2007; Hublin 2009; Klein 2003; Wolpoff et al. 2004). Of course, it is impossible to know whether Neanderthals and MMH (or other Late Pleistocene hominins) were able to interbreed and produce viable offspring. Moreover, there is no clear standard for the minimum genetic or macromorphological skeletal differences needed to clearly identify species from the standpoint of the biological species concept of reproductive isolation (see Harvati 2007 for a review of relevant issues). However, comparisons with mammalian speciation rates can shed light on this issue.
DNA sequencing of a relatively recent Neanderthal specimen reveals slight differences from a sample of modern human DNA, and has suggested that the last common ancestor of the sampled modern humans and Neanderthal lived between 670,000 and 120,000 years ago (95% confidence interval), with a mean at 370 ka (Noonan et al. 2006). Other recent genetic studies similarly suggest 300,000 ka as a mean age of divergence (Garrigan and Kingan 2007; Green et al. 2006; Krau et al. 2007), while Harvati (2007) interprets these data as suggesting a somewhat older time of divergence at around 500 ka. Mammalian rates for the evolution of hybrid non-viability are considerably faster than those of other vertebrates, but still average 2–4 million years (Fitzpatrick 2004; Garrigan and Kingan 2007; Holliday 2006), much longer than any estimate for Neanderthal/modern human divergence. Hence, lacking evidence that hominins are significantly different from other mammals in this regard, it seems parsimonious to accept that Neanderthals and MMH probably could have produced viable offspring if they had an opportunity to mate. Even when viable, however, offspring of genetically distinct populations often exhibit differential fertility with respect to one or both of the ancestral populations (Demuth and Wade 2007; Holliday 2006).
In other organisms, biogeographical changes that increase interactions among members of different populations or even sister species (e.g., removal of a geographic barrier or transportation by humans of one taxon into the range of another), commonly increase hybridization rates, leading to a rapid disappearance of the less numerous group as a recognizably distinct variant or species (Garrigan and Kingan 2007; Wolf et al. 2001). It should be noted that following the usage of these researchers, we employ the term “hybridization” here in a broad sense to refer to offspring of parents from genetically distinct populations, whether or not those populations would be considered different species with regard to biological, morphological, or other criteria. Such extinction through hybridization is sufficiently common to be an important concern in conservation biology, with significant impacts on rare and endangered species (Ibid.). Given that Neanderthals represented a biologically distinct hominin population, geographically isolated from other hominins, but who probably could have interbred and produced viable offspring given the opportunity to do so, they would have been potential candidates for this kind of extinction under conditions of increased interaction with other hominin populations—conditions provided by the land-use changes we describe above for Late Pleistocene western Eurasia. The possibility of variable amounts of genetic exchange between Neanderthals of western Eurasia and populations of other hominins—particularly MMH—has long been a topic of discussion in paleoanthropology (e.g., Harvati 2007; Trinkaus 2005, 2007; Wolpoff et al. 2004). It also has recently found support in analyses of new genomic data (Eswaran et al. 2005; Garrigan and Kingan 2007; Green et al. 2010; Hawks and Cochran 2006). However, these comparative analyses generally do not address the dynamics of hunter-gatherer interactions that could have led to such genetic exchange.
Formal Modeling of Bio-Behavioral Interactions
We use computational and mathematical modeling to examine the potential effects of the kinds of behavioral shifts documented above on biological and social interactions between Neanderthals and other hominins beyond western Eurasia. A model is any abstract representation of real-world phenomena. Models in science are commonly created to simplify very complex reality so that we can better identify and comprehend critical relationships among entities and key processes that drive the operation of real-world systems. Scientific models are generally evaluated by their ability to account for a constrained set of relevant empirical observations. In the great majority of cases, paleoanthropological models take the form of narrative prose that may or may not be supported by statistics or graphs. We use a narrative format for our model of lithic technology and land-use. Narratives are a format that is intuitively easy to understand because they can help us to mentally envision long dead hominins and the world they inhabited, and they share with all models a goal of abstracting the real world in order to better understand it.
To examine the bio-behavioral consequences of Late Pleistocene trends in land-use strategies, however, we develop formal models in algorithmic formats that are beginning to see wider use in other natural sciences but have not been applied in paleoanthropology until very recently (e.g., Christiansen and Kirby 2003; Janssen et al. 2007; Powell et al. 2009; Premo and Hublin 2009). Mathematical and algorithmic models are explicit in that they express and abstract real-world phenomena in terms of a limited set of carefully defined mathematical or algorithmic functions, whose meaning has been agreed on by scientists around the world (Christiansen and Kirby 2003; van der Leeuw 2004). They do not use potentially ambiguous natural language and are thus highly transparent: all who view them understand them in the same way. Because of these characteristics of explicitness and transparency, formal models are much more easily falsified than narratives. This does not mean that formal models are more often incorrect, but that they can be evaluated in a systematic way to distinguish those that better account for the empirical record from those that do so less well. Important for the cumulative nature of scientific knowledge, it is a straightforward task to modify a formal model to improve its ability to represent the world and account for our observations, which is less often the case with narrative prose.
Agent-Based Model
We use a computational agent-based model and a numerical model (ABM—also called an individual-based model in ecology) and a numerical model to explore the biological and social consequences of shifting land-use strategies in the Late Pleistocene of western Eurasia. An ABM allows us to conduct replicable experiments in hominin biogeography otherwise unavailable to historical sciences like paleoanthropology (Bankes et al. 2002; Brantingham 2006; Powell et al. 2009; Premo and Hublin 2009). We used NetLogo (Wilensky 1999) to create an ABM laboratory with two interacting populations who possess a simple two-allele genome, focusing especially on the effects of varying the geographic extent of land-use. This is an abstract model whose goal is to shed light on a limited set of dynamic biocultural interactions, not to recreate the past in a computer. Hence, we have kept our initial assumptions about the populations we model to a minimum. In all cases we start with two different populations and assume that they are initially geographically segregated. For most model runs, we further assume that there are fewer individuals in one population than the other, since there were probably fewer Neanderthals within the geographically constrained area of western Eurasia than there were hominins throughout the rest of the Old World. The potential for mating between individuals is a function of the chance that individuals have an opportunity to physically interact (i.e., by occupying the same geographic locale); this in turn is in part a function of varying land-use strategies and especially the distance each agent moves on a regular basis. We do not make initial assumptions about characteristics such as selective advantage or assortative mating in either population for which there is no independent evidence in the paleoanthroplogical record—although the experimental design of the ABM permits testing the effects of such characteristics (see below). An important feature of this kind of simple but explicit computational model is that we or other researchers can subsequently develop this model to investigate the consequences of other forms of agent behavior.
In the ABM, each agent is treated as an individual, assigned initially to one of two distinct populations (e.g., Neanderthals and MMH), whose initial size (i.e., number of agents) can be varied systematically. All agents ‘forage’ (i.e., move) only within a geographic territory that is established when the agent is created—during initialization or when an agent is ‘born’ after two agents reproduce—and whose radius is the maximum possible distance an agent can move from the center of its territory (e.g., for foraging or other activities). This maximum movement or foraging distance also can be varied systematically for each population. As indicated above, this computational laboratory is not designed to simulate realistic movement patterns of hunter-gatherers on a realistic landscape, but to explore some biocultural consequences of shifting land-use strategies, particularly variations in regular movement distances associated with RMS and LMS. So, we do not simulate different detailed movement patterns (e.g., circulating or radiating) for RMS and LMS, nor do agents migrate or engage in any other goal-directed movement. Rather, the agents simply move random distances from a ‘home base’ at the center of their territory, within a maximum movement radius that is specified for each population and each experimental run.
The fitness of the agents is set directly as birth rates and death rates that can be varied for each original population and for any hybrid agents. These rates directly determine the probability that each agent will reproduce or die in each model cycle; agents do not consume resources, gain or lose energy, or have a predetermined life span. When an agent does reproduce, it mates with another agent within its territory, as defined by the radius of its maximum movement distance. We use a realistic map of western Eurasia for the ‘world’ in which our ABM laboratory is set (Fig. 6) to help readers visualize the spatial dynamics of MMH and Neanderthal interactions at a continental scale. However, confining agents to a real-world geography is not necessary to carry out experiments on the effects of hunter-gatherer mobility on biogeographical change, and has no bearing on the working of the model or the resulting patterns. Also, the details of agent distributions on the landscape should not be taken as predicting the locations of hominin sites in the fossil record. Initially all agents of population 1 are confined to western Eurasia (Neanderthals) while all individuals of population 2 are initially placed in the ‘rest of the world’ (MMH) (Fig. 7a). The fact the agents are sometimes initialized at different densities (i.e., because we create different sized populations on opposite sides of the virtual world) has no impact on the model results and has no bearing on the relative fitness of the agents.
Example visual output from the agent-based model. Distribution of agents of population 1 (black dots), population 2 (black triangles), and hybrids (grey “x”) at initialization and after 1,500 cycles of the simulation with small (2 patches) and large (16 patches) maximum foraging distances. One patch (grid square) is approximately 0.46° longitude x 0.46° latitude; in Central Europe (12° E, 50° N) this is approximately 32.7 × 32.7 km. The model constrains agents to the white patches that represent the land area of western Eurasia and adjacent parts of the world during the Late Pleistocene (glacial ice is not represented). Population 1 was initialized with 100 agents only within western Eurasia; population 2 was initialized with 500 agents outside of western Eurasia; there were no hybrids initially. Note the clumping that often emerges (e.g., Fig. 7b) in agent spatial distributions at small foraging radii distances—even though there are no rules in the simulation that encourage or discourage such spatial aggregation
Population trends from the agent-based model. Plots of total agents in population 1, population 2, and hybrids for 1,500 cycles with different foraging distances. The model was initialized with a large population of 500 agents (schematically representing MMH), distributed randomly across the eastern half of western Eurasia, and a smaller population of 100 agents (representing Neanderthals), distributed randomly across western Eurasia. Note the decline in population 1 and rise in hybrids in Fig. 7b
Our ABM uses a very simplified form of genetic algorithm (Holland 1992; Mitchell 1998; Whitley 1994) in that agents have a genome and pass on their genes to offspring according to rules of independent assortment. However, agent fitness is set at the population level by the researcher (i.e., birth rates and death rates) rather than being an outcome of selection (i.e., differential responses of different genomes to environmental conditions). Agent genomes are a simple two allele system; initially all members of each population are homozygous for one of the alleles. Although a complex of interacting genes probably differentiated Neanderthals from MMH (Currat and Excoffier 2004; Eswaran et al. 2005; Garrigan and Kingan 2007) our goal here is to examine the effects of mobility on biogeography. This is best done with a simple case where effects of changing input parameters are easier to identify. Simulating a suite of interacting genes, while potentially more realistic, would be less informative at this point because of the increased difficulty of controlling for the combined effects of mobility with gene interaction. Once the simple case is well understood, greater complexity can be added in future studies (Barton and Riel-Salvatore 2011).
Any individual experimental run may not reach a permanently stable equilibrium state because each agent moves, reproduces, and dies individually according to rules that include stochasticity—rather than an aggregate function that describes all agents as a group (as is the case with a Population Dynamics Model, described below, and with diffusion models). For example, if the birth rate is set at 0.06, any individual agent has a 6% chance of reproducing in any one model cycle; it could live for 100 cycles without reproducing or reproduce in the first cycle. For this reason, we ran each simulation for a fixed number of iterations. Following empirical testing, we found that 1,500 iterations were sufficient to clearly show trends in the data. Moreover, while each run is unique, multiple model runs with the same parameter settings can produce different results, and may or may not cluster around a particular outcome. Hence, we carried out 10 replications of a 1,500-iteration run for each configuration of maximum movement distance and fitness values assess. There are not yet generally accepted standards for evaluating the number of replications needed for this kind of modeling (Bankes et al. 2002), but 10 replications of each configuration provided clear trends for the study we present here. Figure 7 exemplifies the simulation histories that produced the graphs in Figs. 8 and 9. Note that with small territories (i.e., small maximum agent movement radii), populations 1 and 2 vary little from their initial values across 1,500 iterations, and there are very few hybrids. With large territories, population 1 declines over time as hybrids become more numerous.
Agent-based model experiments of biobehavioral interaction for two populations of varying sizes and maximum movement (foraging) distances. Maximum movement distance varied from 2 to 32 patches around the placement of each agent. Initial population sizes varied from 400 and 400 (initial population size ratio = 1.0) to 100 and 700 (initial population size ratio = 0.14). Fitness is the same for both populations and for any hybrids produced (birth rate = death rate = 0.006). There were 10 replicate experiments for each combination of initial population size and movement distance. Each data point represents the total numbers of agents remaining after runs of 1,500 cycles for each model run. The trend surface is for the mean of each set of replicates. Note that the number of agents remaining after 1,500 cycles declines with increasing movement distance and with increasing disparity (smaller initial population size ratio) in the initial sizes of each population
Agent-based model experiments of biobehavioral interaction with varying movement distances and hybrid fitness for two populations of unequal size. Plots show the effects for the smaller initial population. In the experiments shown, fitness of populations 1 and 2 was represented by birth rate = death rate = 0.006; hybrid birthrate was varied from 0.005 to 0.007 and death rate was kept at 0.006. Each data point represents the total numbers of agents remaining after runs of 1,500 cycles. As in Fig. 7, these simulation experiments were initialized with a large population of 500 agents, distributed randomly across the eastern half of western Eurasia, and a smaller population of 100 agents distributed randomly across western Eurasia. Maximum foraging distance varied from 2 to 32 patches around the placement of each agent. Box plots show median, mid-spreads, and range of the total number of individuals after 1,500 for 10 replicate experiments for each foraging radius; solid line connects the means of the 10 replicates; dashed grey lines show initial population sizes for reference
The modeling experiments in which we varied the maximum movement distance, relative population sizes, and agent fitness illustrate potential biogeographical consequences of the land-use dynamics we document on the paleoanthropological and genomic records (Figs. 8 and 9). When movement distances are small, simulating movement associated with RMS, both populations show minimal demographic effects. With greater potential distances that agents can move, simulating effects of a shift towards increasing LMS, either of the two populations can tend toward extinction. The greater the disparities population size, the more likely it is that the smaller of the two populations becomes extinct—even if the fitness of all agents are identical (Fig. 9). Additional experiments that further clarify the effects of these ecological behaviors on hominin biogeography can be found in the Supplementary Material. In summary, either population size disparities or increased opportunities for interaction can lead to extinction through hybridization, but the combination makes this much more likely for the smaller of two interacting populations. Additionally, even prior to complete extinction, a shift toward LMS with associated opportunities to interact biologically with other hominins over a larger geographical range can result in morphologically recognizable ‘Neanderthal’ agents becoming so dispersed among the more numerous other agents that it is unlikely that any sample of agents (i.e., a fossil hominid site) would recover a Neanderthal specimen, even if a few remained (Fig. 6).
Population Dynamics Model
A more traditional way of formally modeling population dynamics is by using ordinary differential equations (ODE). ODE models characterize the effects of differential mobility in a simple way at the aggregate level of populations, while ABM takes the perspective of simple rules for discrete agents. When both approaches produce similar results it lends confidence that the underlying concepts have been correctly translated into a formal model so that the outcomes consistently result from those concepts applied to input parameters and boundary conditions rather than a fluke of a particular computation. We briefly review an example of an ODE model to represent the effects of mixing two biologically distinct populations (see Supplementary Material for more details). Assuming two initial populations (types 1 and 2), which may interbreed to produce hybrids (type 3), there are 6 possible ways individuals of each type could mate (1–1, 2–2, 3–3, 1–2, 1–3, and 2–3). If we denote N i (t) as the number of type i individuals at time t, and P ij (t) as the net reproduction resulting from interactions of type j, j = {1,2,…,6}, we can express the population dynamics as
If we make the fewest assumptions, all individuals have the same chance of mating when they have an opportunity to interact and can produce 3 possible phenotypes defined by a simple, single locus, two-allele genetic model. This provides the simplest case to more clearly examine the effects of increasing opportunities for genetic interaction due to changing land-use strategies. Defining f i = N i /N as the frequency of individuals of type i in the population at time t and M as the total number of potential mating events (i.e., meetings between individuals) per unit time, then, for reasonably large populations we can write the number of mating events of type j as \( {\left( {{f_{{{l^j}}}}{f_{{{m^j}}}}} \right){p_j}M} \) where l j and m j are the genotype indexes for potential mating events of type j (e.g., l 1 = 1, m 1 = 1, l 4 = 1, m 4 = 2, etc.) and p j is the probability of a mating event of type j occuring (i.e., \( {{f_{{{l^j}}}}{f_{{{m^j}}}}} \)is the probability of a type j meeting, while p j is the probability this meeting will lead to a mating event). If the number of type i offspring resulting from type j mating events is r ij then \( {{P_{{ij}}} = {r_{{ij}}}\left( {{f_{{{l^j}}}}{f_{{{m^j}}}}} \right){p_j}M} \) for like pairings (i.e. j = 1, 2, 3), and \( {{P_{{ij}}} = {2}{{\text{r}}_{{ij}}}\left( {{f_{{{l^j}}}}{f_{{{m^j}}}}} \right){p_j}M} \) for unlike pairings (i.e. j = 4, 5, 6). If we choose our time scale so that M = N and assume that all mating types have the same reproductive potential so that r ij = r we can write the population dynamics as:
For random mating, these probabilities are all the same and in this case, the model will converge to the Hardy-Weinberg equilibrium (see Supplementary Material). More importantly, however, this model also can represent population dynamics in the real world context of limited, spatially dispersed hominin populations and limited time. Changing the reproductive potential (r) is equivalent to altering mating opportunities. When r is low for a given time interval, Neanderthals persist at near their initial frequency (Fig. 10a), but their population drops rapidly over the same period (Fig. 10b) when r is increased (i.e., due to greater opportunities for interaction). The ODE results closely match those of the ABM, lending support to the algorithmic and mathematical expression of these population dynamics.
Discussion
Taken together, the model of lithic technology and formal models of biobehavioral interaction comprise a simple ‘neutral model’ (sensu Brantingham 2003) for Neanderthal extinction that requires minimal assumptions about Pleistocene hominin behavior or cognitive capacities beyond information well documented in the paleoanthropological record. That is, it does not require assumptions of selective differences, assortative mating, or language ability to account for the disappearance of Neanderthals. Nevertheless, such behavioral and cognitive variation is of considerable interest (and of course debate), and the methods we illustrate here offer a robust, new framework in which researchers can begin to examine the effects that such invisible characteristics could have on the observable record.
Our primary focus here, however, is on the evolutionary impacts of shifts in land-use behaviors for Late Pleistocene hominins in western Eurasia. Archaeological evidence indicates that LMS land-use became increasingly prevalent among Late Pleistocene hominins in western Eurasia, possibly in response to environmental stresses brought on by changing glacial climates. Changing land-use strategies provided previously isolated Neanderthals greater opportunities to interact biologically and culturally with MMH at the margins of Europe (Hawks and Cochran 2006). Computational and population dynamics modeling show how the demographic consequences of this increased level of interaction this can lead to the extinction of recognizably distinct Neanderthals.
Given the geography of western Eurasia, the spatially explicit ABM also predicts that Neanderthal disappearance should assume an east-to-west gradient. This spatial dynamic is seen empirically in the distribution of the earliest MMH specimens in eastern Europe and the youngest Neanderthal remains in the Iberian Peninsula (Bolus and Conard 2001; Conard and Bolus 2003; Finlayson and Carrión 2007; Finlayson et al. 2006; Trinkaus 2005, 2007; Trinkaus et al. 2003) and is consistent with recent proposals to characterize Neanderthals and MMH as syngameons (Holliday 2006) or as a ring species (Voisin 2006).
The models presented here also suggest the potential for varying amounts of interbreeding between Neanderthals and MMH during at least part of MIS 3, although the simple two-allele system we use should not be taken as indicative of even the relative numbers of recognizable hybrid individuals. Other experiments modeling a more complex multi-allele system suggest that the processes that lead to Neanderthal extinction here would also result in the disappearance of most hybrids except those with small levels of Neanderthal introgression (Barton and Riel-Salvatore 2011). These results are consistent with recent genetic and culture/genetic models of potential MMH/Neanderthal interbreeding if strong selective advantage on the part of MMH is not assumed (Eswaran et al. 2005; Garrigan and Kingan 2007). Diffusion models that suggest little or no Neanderthal introgression require these assumptions of strong selective advantage and significant demographic expansion by MMH to explain Neanderthal disappearance from the fossil record (Currat and Excoffier 2004; Hawks 2006). When such mechanisms are not invoked, these models permit Neanderthal contribution to the MMH genome. Indeed, recent sequencing of Neanderthal DNA suggests a small, but significant signal of Neanderthal introgression into modern the European genome (Green et al. 2010; see also Hammer et al. 2011)—still apparent even after millennia of large scale Holocene population movements and gene flow into this region.
If the evidence for Neanderthal introgression continues to stand up to scrutiny, Neanderthal-MMH hybrids must have existed, but there is no agreement on how to recognize them in skeletal morphology—as evidenced by the ongoing debates over the taxonomic status of the Lagar Velho child (e.g., Duarte et al. 1999; Tattersall and Schwartz 1999; Trinkaus 2007). Systematic metric comparisons of the same features across multiple specimens of Late Pleistocene Neanderthals and MMH would be helpful in this regard, but are yet few. However, the comparative studies that have been done report considerable variation among specimens classified as Neanderthals (and also among Late Pleistocene MMH), and also varying degrees of continuous variation between Neanderthal and MMH specimens—especially when Neanderthal are compared with contemporaneous MMH specimens, as opposed to modern humans who often vary in many respects from Late Pleistocene MMH as well as from Neanderthals (Bailey 2004; Harvati 2003; Trinkaus 2005; Trinkaus et al. 2007; Willermet 2001; Willermet and Clark 1995; Willermet and Hill 1997). This is not to say that Neanderthals are not morphologically distinctive from MMH, but simply that observed morphometric continuity and overlap among Late Pleistocene hominin specimens classified as Neanderthals or MMH could encompass possible hybrids, especially where the most numerous hybrids would likely be ones with minimal Neanderthal contribution to their genome (Glantz et al. 2009; Willermet 2001). However, prevailing systematics and typological practices also make the recognition of any hybrids difficult. Late Pleistocene hominin specimens in western Eurasia typically are classified as either Neanderthal or MMH; no intermediate classifications are recognized (see discussions in Glantz et al. 2009; Willermet 2001; Willermet and Hill 1997), leaving potential hybrids typologically invisible.
The shift in land-use strategies described above equally may have had consequences for hominin socioecological niches in western Eurasia, including both Neanderthals and (by mid-MIS 3) MMH. Increasing foraging distances and accompanying opportunities for social interaction with more individuals and more diverse cultural practices would have altered the environment of cultural selection, ultimately shaping long-term biological and cultural evolutionary trajectories of humans at the end of the Pleistocene (Riel-Salvatore 2010). We do not model these consequences here, but other modeling work is potentially relevant (Kline and Boyd 2010; Powell et al. 2009; Premo and Hublin 2009; Shennan 2001); the results presented here offer additional reasons to continue and expand on these pioneering studies. Moreover, there is growing empirical evidence for increases in the diversity, complexity, and rates of change in socially-transmitted behaviors that span the Late Pleistocene in this region (Davies and Underdown 2006; d’Errico et al. 2003a,b; Hill 2009; Langley et al. 2008; Soffer 2009; Vanhaeren 2005; Zilhão 2007; Zilhão et al. 2010). In an increasingly diverse and dynamic biocultural landscape, there is no reason to assume a priori that social groups with Neanderthal biological affinities would necessarily be only recipients of culturally generated, maintained, and transmitted behaviors and material culture.Footnote 1 In such a social context, all hominins, including Neanderthals, would have become increasingly encultured.
The End of the Neanderthals
The approach we illustrate here of testing models against the paleoanthropological record rather than inferring scenarios of the past from that record offers new insights into the processes that drove human biocultural evolution. Computational modeling especially is a valuable tool for studying highly complex and sometimes counterintuitive interactions of diverse environmental, behavioral, and biological phenomena and the cascade of consequences that can result. The kind of protocols that we use here offer an avenue toward rigorous empirical testing and evaluation of alternative, inferentially-generated scenarios that may finally begin to resolve long-running debates about human evolution.
Among the results of the complex feedbacks between ecological, biological, and cultural dynamics that we model for hominin populations of Late Pleistocene western Eurasia were the disappearance of recognizable Neanderthals from the fossil record and the disappearance of distinctive Middle Paleolithic artifact assemblages from the archaeological record. In one sense we could say that their extinction was the result of Late Pleistocene globalization as Neanderthals were biologically and culturally absorbed into a pan-Eurasian genome and cultural sphere. But in another sense, they disappeared because of their ultimate success in adapting to rigorous, rapidly changing glacial environment through culturally driven behavioral change.
Notes
The oft-repeated assertion that Neanderthal must have learned to make transitional industries from MMH also implies 1) that Neanderthals were fully capable of socially learning complex behaviors from other hominins, including manufacture and use of compound implement technologies; 2) Neanderthals spent sufficient time in physically close, regular, peaceful contact with MMH individuals to socially learn how to make and use these technologies (learning to make and use complex tools like this cannot done through observation from a distance alone); and 3) Neanderthals were able to subsequently transmit these technological behaviors socially to other Neanderthals or we would not find these industries distributed across multiple locales and long temporal spans.
References
Ahler, S. A. (1989). Mass analysis of flaking debris: studying the forest rather than the tree. In Henry, D. O., Odell, G. H. (eds.), Alternative approaches to lithic analysis. Archaeological Papers of the American Anthropological Association Number 1, pp. 85–118
Alvard, M. S. (2003). The Adaptive Nature of Culture. Evolutionary Anthropology 12: 136–149.
Amick, D. (1996). Regional Patterns of Folsom Mobility and Land Use in the American Southwest. World Archaeology 27: 411–426.
Andrefsky, W. (2001). Lithic Debitage: Context, Form, Meaning. University of Utah Press, Salt Lake City.
Bailey, S. E. (2004). A Morphometric Analysis of Maxillary Molar Crowns of Middle-Late Pleistocene Hominins. Journal of Human Evolution 47: 183–198 doi:10.1016/j.jhevol.2004.07.001.
Bamforth, D. B. (1986). Technological Efficiency and Tool Curation. American Antiquity 51: 38–50.
Bankes, S. C., Lempert, R., and Popper, S. (2002). Making Computational Social Science Effective: Epistemology, Methodology, and Technology. Social Science Computer Review 20: 377–388.
Barton, C. M. (1990). Beyond Style and Function: A View from the Middle Paleolithic. American Anthropologist 92: 57–72.
Barton, C. M. (1991). Retouched tools: fact or fiction? Paradigms for interpreting chipped stone. In Clark, G. A. (ed.), Perspectives in Prehistory Paradigmatic Biases in Circum-Mediterranean Hunter-Gatherer Research. University of Pennsylvania Press, Philidelphia, pp. 143–163.
Barton, C. M. (1997). Stone tools, style, and social identity: an evolutionary perspective on the archaeological record. In Barton, C. M., and Clark, G. A. (eds.), Rediscovering Darwin: Evolutionary Theory in Archaeological Explanation. American Anthropological Association, Washington DC, pp. 141–156.
Barton, C. M. (1998). Looking back from the world’s end: paleolithic settlement and mobility at Gibraltar. In Sanchidrián Torti, J. L., and Simón Vallejo, M. D. (eds.), Las culturas del Pleistoceno superior en Andalucía. Patronato de la Cueva de Nerja, Nerja, pp. 13–23.
Barton, C. M. (2008). General fitness, transmission, and human behavioral systems. In O’Brien, M. J. (ed.), Cultural Transmission. American Archaeology Press, Washingtion DC, pp. 112–119.
Barton, C. M., and Clark, G. A. (1993). Cultural and natural formation processes in late quaternary cave and rockshelter sites of Western Europe and the Near East. In Goldberg, P., Nash, D. T., and Petraglia, M. D. (eds.), Formation Processes in Archaeological Context. Prehistory Press, Madison, pp. 33–52.
Barton CM, Riel-Salvatore J (2011). Agents of Change: Modeling Biocultural Evolution in Upper Pleistocene Western Eurasia. Advances in Complex Systems. doi:10.1142/S0219525911003359
Bar-Yosef, O., and Kuhn, S. L. (1999). The Big Deal About Blades: Laminar Technologies and Human Evolution. American Anthropologist 101: 322–338.
Bettinger, R. L. (1991). Hunter-Gatherers: Archaeological and Evolutionary Theory. Plenum Press, New York.
Binford, L. R. (1979). Organization and Formation Processes: Looking at Curated Technologies. Journal of Anthropological Research 35: 255–273.
Binford, L. R. (1980). Willow Smoke and Dogs’ Tails: Hunter-Gatherer Settlement Systems and Archaeological Site Formation. American Antiquity 45: 4–20.
Binford, L. R. (2001). Constructing Frames of Reference: An Analytical Method for Archaeological Theory Building Using Hunter-Gatherer and Environmental Data Sets, 1st ed. University of California Press, Berkeley.
Bleed, P. (1986). The Optimal Design of Hunting Weapons: Maintainability or Reliability. American Antiquity 51: 737–747.
Bleed, P. (2001). Trees or Chains, Links or Branches: Conceptual Alternative for Consideration of Stone Tool Production and Other Sequential Activities. Journal of Archaeological Method and Theory 8: 101–127.
Bolus, M., and Conard, N. J. (2001). The Late Middle Paleolithic and Earliest Upper Paleolithic in Central Europe and Their Relevance for the Out of Africa Hypothesis. Quaternary International 75: 29–40.
Boyd, R., and Richerson, P. J. (1985). Culture and the Evolutionary Process. The University of Chicago Press, Chicago.
Boyd, R., and Richerson, P. J. (2005). The Origin and Evolution of Cultures. Oxford University Press, Oxford.
Brantingham, P. J. (2003). A Neutral Model of Stone Raw Material Procurement. American Antiquity 68: 487–509.
Brantingham, P. J. (2006). Measuring Forager Mobility. Current Anthropology 47: 435–459.
Christiansen, M. H., and Kirby, S. (2003). Language Evolution: Consensus and Controversies. Trends in Cognitive Sciences 7: 300–307 doi:10.1016/S1364-6613(03)00136-0.
Clark, A. E. (2008). Changes in Occupation Intensity During the Lower and Middle Paleolithic at Tabun Cave. Master’s Paper, University of Arizona, Israel.
Coinman, N. R. (2000). The Archaeology of the Wadi al-Hasa, West-Central Jordan, Vol. 2: Excavations at Middle, Upper, and Epipaleolithic Sites in the Hasa. Arizona State University Anthropological Research Papers, Tempe.
Colcutt, S. N. (1979). The Analysis of Quaternary Cave Sediments. World Archaeology 10: 290–301.
Conard, N. J., and Bolus, M. (2003). Radiocarbon Dating the Appearance of Modern Humans and Timing of Cultural Innovations in Europe: new Results and New Challenges. Journal of Human Evolution 44: 331–371 doi:10.1016/S0047-2484(02)00202-6.
Currat, M., and Excoffier, L. (2004). Modern Humans Did Not Admix with Neanderthals During Their Range Expansion into Europe. PLoS Biology 2: e421 doi:10.1371/journal.pbio.0020421.
d’Errico, F., Henshilwood, C., Lawson, G., et al. (2003a). Archaeological Evidence for the Emergence of Language, Symbolism, and Music–An Alternative Multidisciplinary Perspective. Journal of World Prehistory 17: 1–70 doi:10.1023/A:1023980201043.
d’Errico, F., Julien, M., Liolios, D., et al. (2003b). Many awls in our argument. Bone tool manufacture and use in the Châtelperronian and Aurignacian levels of the Grotte du Renne at Arcy-sur-Cure. In Zilhão, J., and d’Errico, F. (eds.), The Chronology of the Aurignacian and of the Transitional Technocomplexes: Dating, Stratigraphies, Cultural Implications. Instituto Portugués de Arqueología, Lisbon, pp. 247–270.
Davies, R., and Underdown, S. (2006). The Neanderthals: A Social Synthesis. Cambridge Archaeological Journal 16: 145–164 doi:10.1017/S0959774306000096.
Demuth, J. P., and Wade, M. J. (2007). Population Differentiation in the Beetle Tribollium Castaneum. I. Genetic Architecture. Evolution 61: 494–509.
Dibble, H. L. (1995). Middle Paleolithic Scraper Reduction: Background, Clarification, and Review of the Evidence to Date. Journal of archaeological method and theory 2: 299–368.
Dibble, H. L., Chase, P. G., McPherron, S. P., and Tuffreau, A. (1997). Testing the Reality of a “Living Floor” with Archaeological Data. American Antiquity 62: 629–651.
Duarte, C., Mauricio, J., Pettitt, P. B., et al. (1999). The Early Upper Paleolithic Human Skeleton from the Abrigo do Lagar Velho (Portugal) and Modern Human Emergence in Iberia. Proceedings of the National Academy of Sciences 96: 7604–7609.
Eder, J. F. (1984). The Impact of Subsistence Change on Mobility and Settlement Pattern in a Tropical Forest Foraging Economy: Some Implications for Archeology. American Anthropologist 86: 837–853.
Eswaran, V., Harpending, H., and Rogers, A. R. (2005). Genomics Refutes an Exclusively African Origin of Humans. Journal of Human Evolution 49: 1–18.
Farrand, W. R. (2001). Archaeological sediments in caves and rockshelters. In Stein, J. K., and Farrand, W. R. (eds.), Sediments in Archaelogical Context. University of Utah Press, Salt Lake City, pp. 29–66.
Féblot-Augustins, J. (1993). Mobility Strategies in the Late Middle Paleolithic of Central Europe and Western Europe: Elements of Stability and Variability. Journal of Anthropological Archaeology 12: 211–265.
Finlayson, C., and Carrión, J. S. (2007). Rapid Ecological Turnover and its Impact on Neanderthal and Other Human Populations. Trends in Ecology & Evolution 22: 213–222.
Finlayson, C., Giles Pacheco, F., Rodriguez-Vidal, J., et al. (2006). Late Survival of Neanderthals at the Southernmost Extreme of Europe. Nature 443: 850–853.
Fitzpatrick, B. M. (2004). Rates of Evolution of Hybrid Inviability in Birds and Mammals. Evolution 58: 1865–1870.
Foley, R. (1985). Optimality Theory in Anthropology. Man 20: 222–242.
Frison, G. C. (1968). A Functional Analysis of Certain Chipped Stone Tools. American Antiquity 33: 149–155.
Garrigan, D., and Kingan, S. B. (2007). Archaic Human Admixture: A View from the Genome. Current Anthropology 48: 895–902.
Glantz, M., Athreya, S., and Ritzman, T. (2009). Is Central Asia the Eastern Outpost of the Neandertal Range? A Reassessment of the Teshik-Tash Child. American Journal of Physical Anthropology 138: 45–61 doi:10.1002/ajpa.20897.
Gould, R. A. K., Koster, D. A., and Sontz, A. H. L. (1971). The Lithic Assemblage of the Western Desert Aborigines of Australia. American Antiquity 36: 149–168.
Green, R. E., Krause, J., Ptak, S. E., et al. (2006). Analysis of One Million Base Pairs of Neanderthal DNA. Nature 444: 330–336.
Green, R. E., Krause, J., Briggs, A. W., et al. (2010). A Draft Sequence of the Neandertal Genome. Science 328: 710–722 doi:10.1126/science.1188021.
Grove, M. (2009). Hunter-Gatherer Movement Patterns: Causes and Constraints. Journal of Anthropological Archaeology 28: 222–233 doi:10.1016/j.jaa.2009.01.003.
Grove, M. (2010). Logistical Mobility Reduces Subsistence Risk in Hunting Economies. Journal of Archaeological Science 37: 1913–1921 doi:10.1016/j.jas.2010.02.017.
Hammer, M. F., Woerner, A. E., Mendez, F. L., et al. (2011). Genetic Evidence for Archaic Admixture in Africa. Proceedings of the National Academy of Sciences 108: 15123–15128 doi:10.1073/pnas.1109300108.
Harvati, K. (2003). The Neanderthal Taxonomic Position: Models of Intra- and Inter-Specific Craniofacial Variation. Journal of Human Evolution 44: 107–132 doi:10.1016/S0047-2484(02)00208-7.
Harvati K (2007) Neanderthals and Their Contemporaries. Handbook of Paleoanthropology. pp. 1717–1748
Hawks J (2006) Selection Selection on Mitochondrial DNA and the Neanderthal Problem. In: Harvati K, Harrison T (eds) Neanderthals Revisited: New Approaches and Perspectives. pp. 221–238
Hawks, J., and Cochran, G. (2006). Dynamics of Adaptive Introgression from Archaic to Modern Humans. PaleoAnthropology 2006: 101–115.
Henrich, J. (2004). Demography and Cultural Evolution: How Adaptive Cultural Processes can Produce Maladaptive Losses: The Tasmanian Case. American Antiquity 69: 197–214.
Henrich, J., and McElreath, R. (2003). The Evolution of Cultural Evolution. Evolutionary Anthropology: Issues, News, and Reviews 12: 123–135 doi:10.1002/evan.10110.
Hill, K., Barton, C. M., and Hurtado, A. M. (2009). The Emergence of Human Uniqueness: Characters Underlying Behavioral Modernity. Evolutionary Anthropology: Issues, News, and Reviews 18: 187–200.
Hiscock, P. (2007). Looking the Other way: A Materialist/Technological Approach to Classifying Tools and Implements, Cores and Retouched Flakes. In McPherron, S. P. (ed.), Tools Versus Cores. Alternative Approaches to Stone Tool Analysis. Cambridge Scholars Publishing, Newcastle, pp. 198–222.
Holdaway, S., and Douglass, M. (2011). A Twenty-First Century Archaeology of Stone Artifacts. Journal of Archaeological Method and Theory doi:10.1007/s10816-011-9103-6.
Holland, J. D. (1992). Genetic Algorithms. Scientific American 267: 44–50.
Holliday, T. (2006). Neanderthals and Modern Humans: An Example of a Mammalian Syngameon? New Approaches and Perspectives, Neanderthals Revisited, pp. 281–297.
Hublin, J.-J. (2009). The Origin of Neandertals. Proceedings of the National Academy of Sciences 106: 16022–16027 doi:10.1073/pnas.0904119106.
Janssen, M. A., Sept, J. M., Griffith, C. S. (2007). Hominids Foraging in a Complex Landscape: Could Homo ergaster and Australopithecus boisei Meet Their Calories Requirements? In Takahashi, S., Salach, D., Rouchier, J. (eds.), Advancing Social Simulation: The First World Congress. Springer Japan, pp. 307–318
Kelly, R. L. (1983). Hunter-Gatherer Mobility Strategies. Journal Anthropology Research 39: 277–306.
Kelly, R. L. (1992). Mobility/Sedentism: Concepts, Archaeological Measures, and Effects. Annual Review of Anthropology 21: 43–66 doi:10.1146/annurev.an.21.100192.000355.
Kelly, R. L. (1995). The Foraging Spectrum: Diversity in Hunter-Gatherer Lifeways. Smithsonian Institution Press, Washington, DC.
Kim, J. (2003). Land-Use Conflict and the Rate of the Transition to Agricultural Economy: A Comparative Study of Southern Scandinavia and Central-Western Korea. Journal of Archaeological Method and Theory 10: 277–321 doi:10.1023/A:1026087723164.
Klein, R. G. (2003). PALEOANTHROPOLOGY: Whither the Neanderthals? Science 299: 1525–1527.
Kline, M. A., and Boyd, R. (2010). Population Size Predicts Technological Complexity in Oceania. Proceedings of the Royal Society B: Biological Sciences 277: 2559–2564 doi:10.1098/rspb.2010.0452.
Krau, J., Lalueza-Fox, C., Orlando, L., et al. (2007). The Derived FOXP2 Variant of Modern Humans was Shared with Neandertals. Current Biology 17: 1908–1912.
Kuhn, S. L. (1989). Hunter-getherer foraging organization and strategies of artifact replacement and discard. In Amick, D. S., and Mauldin, R. P. (eds.), Experiments in Lithic Technology. BAR, Oxford, pp. 33–47.
Kuhn, S. L. (1991). Unpacking Reduction: Lithic raw-Material Economy in the Mousterian of West-Central Italy. Journal of Anthropological Archaeology 10: 76–106.
Kuhn, S. L. (1992). On Planning and Curated Technologies in the Middle Paleolithic. Journal of Anthropological Research 48: 185–214.
Kuhn SL (2004). Middle Paleolithic Assemblage Formation at Riparo Mochi. In: Johnson AL (ed) Processual Archaeology: Exploring Analytical Strategies, Frames of Reference and Culture Process. Greenwood Publishing Group, Westport, CT, pp 31–60.
Laland, K. N., and Brown, G. R. (2006). Niche Construction, Human Behavior, and the Adaptive-lag Hypothesis. Evolutionary Anthropology: Issues, News, and Reviews 15: 95–104 doi:10.1002/evan.20093.
Laland, K. N., Odling-Smee, F. J., and Myles, S. (2010). How Culture Shaped the Human Genome: Bringing Genetics and the Human Sciences Together. Nature Reviews Genetics 11: 137–148 doi:10.1038/nrg2734.
Langley, M. C., Clarkson, C., and Ulm, S. (2008). Behavioural Complexity in Eurasian Neanderthal Populations: A Chronological Examination of the Archaeological Evidence. Cambridge Archaeological Journal 18: 289–307 doi:10.1017/S0959774308000371.
Magne, M. P. (1989). Lithic reduction stages and assemblage formation processes. In Amick, D. S., and Mauldin, R. P. (eds.), Experiments in Lithic Technology. BAR, Oxford, pp. 15–31.
Magne, M. P., and Pokotylo, D. (1981). A Pilot Study in Bifacial Lithic Reduction Sequences. Lithic Technology 10: 34–47.
Mauldin, R. P., and Amick, D. S. (1989). Investigating patterning in debitage from experimental bifacial core reduction. In Amick, D. S., and Mauldin, R. P. (eds.), Experiments in Lithic Technology. BAR, Oxford., pp. 67–88.
Meese, D. A., Gow, A. J., Alley, R. B., et al. (1997). The Greenland Ice Sheet Project 2 Depth-age Scale: Methods and Results. Journal of Geophysical Research 102: 26411–26423.
Mitchell, M. (1998). An Introduction to Genetic Algorithms, 1st ed. MIT Press, Cambridge.
Myers, A. (1989). Reliable and maintainable technological strategies in the mesolithic of mainland Britain. In Torrence, R. (ed.), Time Energy and Stone Tools. Cambridge University Press, Cambridge, pp. 78–91.
Nelson, M. C. (1991). The Study of Technological Organization. Archaeological Method and Theory 3: 57–100.
Newcomer, M. H. (1971). Some Quantitative Experiments in Handaxe Manufacture. World Archaeology 3: 85–94.
Noonan, J. P., Coop, G., Kudaravalli, S., et al. (2006). Sequencing and Analysis of Neanderthal Genomic DNA. Science 314: 1113–1118.
Popescu, G., Riel-Salvatore, J., and Barton, C. M. (2007). Biogeographie umană şi organizare tehnologică în Pleistocenul Superior în regiunea Carpaţilor Meridionali. Materiale şi Cercetări Arheologice Serie Noua III: 19–42.
Potts, R. (1994). Variables Versus Models of Early Pleistocene Hominid Land-use. Journal of Human Evolution 27: 7–24.
Powell, A., Shennan, S., and Thomas, M. G. (2009). Late Pleistocene Demography and the Appearance of Modern Human Behavior. Science 324: 1298–1301 doi:10.1126/science.1170165.
Premo, L. S., and Hublin, J.-J. (2009). Culture, Population Structure, and low Genetic Diversity in Pleistocene Hominins. Proceedings of the National Academy of Sciences 106: 33–37 doi:10.1073/pnas.0809194105.
Richerson, P. J., and Boyd, R. (2005). Not by Genes Alone: How Culture Transformed Human Evolution. University of Chicago Press, Chicago.
Richerson, P. J., Boyd, R., and Henrich, J. (2010). Colloquium Paper: Gene-Culture Coevolution in the Age of Genomics. Proceedings of the National Academy of Sciences 107: 8985–8992 doi:10.1073/pnas.0914631107.
Riel-Salvatore, J. (2007). The Uluzzian and the Middle-Upper Paleolithic Transition in Southern Italy. Arizona State University, PhD Dissertation.
Riel-Salvatore, J. (2009). What is a “transitional” industry? The Uluzzian of southern Italy as a case study. In Camps, M., and Chauhan, P. (eds.), Sourcebook of Paleolithic Transitions: Methods, Theories, and Interpretations. Springer, New York, pp. 377–396.
Riel-Salvatore, J. (2010). A Niche Construction Perspective on the Middle–Upper Paleolithic Transition in Italy. Journal of Archaeological Method and Theory 17: 323–355 doi:10.1007/s10816-010-9093-9.
Riel-Salvatore, J., and Barton, C. M. (2004). Late Pleistocene Technology, Economic Behavior, and Land-use Dynamics in Southern Italy. American Antiquity 69: 273–290.
Riel-Salvatore, J., Barton, C. M. (2007). New Quantitative Perspectives on the Middle-Upper Paleolithic Transition: The View from the Northern Mediterranean. Early Upper Paleolithic “Transitional” Industries: New Questions, New Methods
Riel-Salvatore, J., and Clark, G. A. (2007). New Approaches to the Study of Early Upper Paleolithic “Transitional” Industries in Western Eurasia. Transitions Great and Small. Archaeopress, Oxford.
Riel-Salvatore, J., Popescu, G., and Barton, C. M. (2008). Standing at the Gates of Europe: Human Behavior and Biogeography in the Southern Carpathians During the Late Pleistocene. Journal of Anthropological Archaeology 27: 399–417.
Rolland, N., and Dibble, H. L. (1990). A new Synthesis of Middle Paleolithic Variability. American Antiquity 55: 480–499.
Sandgathe, D. M. (2006). Examining the Levallois Reduction Strategy from a Design Theory Point of View. Archaeopress, Oxford.
Serangeli, J., and Bolus, M. (2008). Out of Europe - The Dispersal of a Successful European Hominin Form. Quartär 55: 83–98.
Shennan, S. (2001). Demography and Cultural Innovation: A Model and Its Implications for the Emergence of Modern Human Culture. Cambridge Archaeological Journal 11: 5–16 doi:10.1017/S0959774301000014.
Shott, M. J. (1994). Size and Form in the Analysis of Flake Debris: Review and Recent Approaches. Journal of Archaeological Method and Theory 1: 69–110 doi:10.1007/BF02229424.
Shott, M. J. (1996). An Exegesis of the Curation Concept. Journal of Anthropological Research 52: 259–280.
Shott, M. J., and Weedman, K. J. (2007). Measuring Reduction in Stone Tools: An Ethnoarchaeological Study of Gamo Hidescrapers from Ethiopia. Journal of Archaeological Science 34: 1016–1035 doi:10.1016/j.jas.2006.09.009.
Soffer O (2009). Defining Modernity, Establishing Rubicons, Imagining the Other—and the Neanderthal Enigma. In: Camps M, Chauhan P (eds) Sourcebook of Paleolithic Transitions. Springer New York, New York, NY, pp 43–64.
Stiner, M. C., and Kuhn, S. L. (2006). Changes in the “Connectedness” and Resilience of Paleolithic Societies in Mediterranean Ecosystems. Human Ecology 34: 693–712.
Tattersall, I., and Schwartz, J. H. (1999). Hominids and Hybrids: The Place of Neanderthals in Human Evolution. Proceedings of the National Academy of Sciences of the United States of America 96: 7117–7119.
Torrence, R. (1989). Retooling: Towards a Behavioral Theory of Stone Tools. In Torrence, R. (ed.), Time Energy and Stone Tools. Cambridge University Press, Cambridge, pp. 57–66.
Trinkaus, E. (2005). EARLY MODERN HUMANS. Annual Review of Anthropology 34: 207–230 doi:10.1146/annurev.anthro.34.030905.154913.
Trinkaus, E. (2007). European Early Modern Humans and the Fate of the Neandertals. Proceedings of the National Academy of Sciences 104: 7367–7372.
Trinkaus, E., Moldovan, O., Milota stefan, et al. (2003). An Early Modern Human from the Pestera cu Oase, Romania. Proceedings of the National Academy of Sciences 100: 11231–11236.
Trinkaus, E., Maki, J., and Zilhão, J. (2007). Middle Paleolithic Human Remains from the Gruta da Oliveira (Torres Novas), Portugal. American Journal of Physical Anthropology 134: 263–273.
van der Leeuw, S. E. (2004). Why Model? Cybernetics and Systems: An International Journal 35: 117 doi:10.1080/01969720490426803.
Vanhaeren, M. (2005). The evolutionary significance of beadmaking and use. In d’Errico, F., and Blackwell, L. (eds.), From Tools to Symbols: From Early Hominids to Modern Humans. Witwatersrand University Press, Johannesburg, pp. 525–535.
Villaverde, V., Aura Tortosa, J. E., and Barton, C. M. (1998). The Upper Paleolithic in Mediterranean Spain: A Review of Current Evidence. Journal of World Prehistory 12: 121–198.
Voisin, J.-L. (2006). Speciation by Distance and Temporal Overlap: A new way of Understanding Neanderthal Evolution. In Harisson, T., and Harvati, K. (eds.), Neanderthals Revisited: New Approaches and Perspectives. Springer, New York, pp. 299–314.
Whitley, D. (1994). A Genetic Algorithm Tutorial. Statistics and Computing 4: 65–85.
Wilensky, U. (1999). NetLogo. Center for Connected Learning and Computer-Based Modeling. Northwestern University
Willermet, C. M. (2001). Fuzzy Logic as a Classification Tool: A Case Study Using Levantine Archaic Hominids. Arizona State University, PhD dissertation.
Willermet, C. M., and Clark, G. A. (1995). Paradigm Crisis in Modern Human Origins Research. Journal of Human Evolution 29: 487–490 doi:10.1006/jhev.1995.1071.
Willermet, C. M., and Hill, J. B. (1997). Fuzzy Set Theory and Its Implications for Speciation Models. In Clark, G. A., and Willermet, C. M. (eds.), Conceptual Issues in Modern Human Origins Research. Aldine de gruyter, New York, pp. 77–88.
Wobst, H. M. (1974). Boundary Conditions for Paleolithic Social Systems: A Simulation Approach. American Antiquity 39: 147–178.
Wolf, D. E., Takebayashi, N., and Rieseberg, L. H. (2001). Predicting the Risk of Extinction Through Hybridization. Conservation Biology 15: 1039–1053.
Wolpoff, M. H., Mannheim, B., Mann, A., et al. (2004). Why Not the Neandertals? World Archaeology 36: 527–546.
Zilhão, J. (2007). The Emergence of Ornaments and Art: An Archaeological Perspective on the Origins of Behavioural “Modernity.”. Journal of Archaeological Research 15: 1–54 doi:10.1007/s\10814-006-9008-1.
Zilhão, J., and d’Errico, F. (2003). The Chronology of the Aurignacian and of the Transitional Technocomplexes: Dating, Stratigraphies, Cultural Implications. Instituto Português de Arqueologia, Lisbon.
Zilhão, J., Angelucci, D. E., Badal-García, E., et al. (2010). Symbolic use of Marine Shells and Mineral Pigments by Iberian Neandertals. Proceedings of the National Academy of Sciences 107: 1023–1028 doi:10.1073/pnas.0914088107.
Acknowledgements
This research was supported in part by the National Science Foundation (grant BCS-0526073), and a Fulbright Senior Research Fellowship and Graduate Student Fellowship from the Committee for the International Exchange of Scholars. We want to thank Kim Hill, Bill Kimbel, and Geoff Clark for their comments on versions of this manuscript. We are, of course, fully responsible for all ideas and their expression herein.
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant Information
This research was supported in part by the National Science Foundation (grant BCS-0526073), a Fulbright Senior Research Fellowship (Committee for the International Exchange of Scholars), and a Fulbright Graduate Student Fellowship (Committee for the International Exchange of Scholars.)
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1.33 mb)
Rights and permissions
About this article
Cite this article
Barton, C.M., Riel-Salvatore, J., Anderies, J.M. et al. Modeling Human Ecodynamics and Biocultural Interactions in the Late Pleistocene of Western Eurasia. Hum Ecol 39, 705–725 (2011). https://doi.org/10.1007/s10745-011-9433-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10745-011-9433-8