Abstract
Heart failure with preserved ejection fraction (HFpEF) is an increasingly prevalent phenotype affecting over half of today’s heart failure patients. With no proven therapy and no universally accepted diagnostic guideline, many HFpEF patients continue to be misdiagnosed or underdiagnosed at the early stages until the disease has progressed much further along. It is extremely difficult to diagnose the HFpEF patient, because they have a normal ejection fraction and present with non-specific symptoms such as dyspnea or exercise intolerance. To provide greater specificity, the current diagnostic criteria mandate the presence of diastolic dysfunction, where myocardial relaxation is impaired and ventricular filling pressure is elevated as a result of a hypertrophic and stiff heart. Unfortunately, diastolic dysfunction reflects late-stage structural and functional changes and offers a very narrow window, if at all, for successful intervention. In this article, we review the imaging modalities used in the current diagnostic workflow for assessing HFpEF. We also describe the most up-to-date insight into its pathophysiological basis, which attributes systemic inflammation driven by comorbidities as the initiator of disease. With this extramyocardial perspective, we provide our recommendation on new imaging targets that extend beyond the heart to enable early, accurate diagnosis of HFpEF and allow an opportunity for treating this fatal condition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heart failure (HF) is a clinical syndrome characterized by structural and/or functional abnormalities that impair ventricular filling and/or ejection of blood in accordance with the metabolic demands of the body [1]. Our understanding of the pathophysiology of HF continues to evolve, with the current definition recognizing two distinct HF phenotypes: “HF with reduced ejection fraction” (HFrEF) and “HF with preserved ejection fraction” (HFpEF). The latter nomenclature nods to the fact that over the past 30 years, the incidence of HF patients with a near normal, or preserved, left ventricular (LV) ejection fraction (LVEF ≥ 50%) has increased to a staggering ≥ 50% of all HF cases [2]. Continuing to rise in prevalence at 1% a year, HFpEF is projected to be the dominant HF phenotype in a decade [3]. The mortality rate is similar to that of HFrEF, as are signs and symptoms (e.g., dyspnea, exercise intolerance, congestion) [4]. Nonetheless, HFpEF is a distinct disease and has a heterogeneous etiology that remains poorly understood [5]. Treatments that have proven effective in HFrEF fail to provide a survival benefit for HFpEF patients [6]. Our incomplete understanding of the disease presents a barrier not only to treatment but also to accurate and early diagnosis. To date, there is still no universal consensus on a clear diagnostic guideline [7, 8, 5]. In fact, a third category, HFmEF (mid-range ejection fraction 41–49%), was introduced in 2017 in heart failure management guidelines [9]. The physiological significance of HFmEF and whether it should be classified as part of the HFrEF or HFpEF spectrum remain to be determined. Despite all this, what is clear is that patients of the HFpEF phenotype are often misdiagnosed and the severity of disease underestimated [7].
The current diagnosis of HFpEF requires three levels of evidence: (i) clinical symptoms of HF (e.g., dyspnea on exertion, fatigue, exercise intolerance), (ii) LVEF ≥ 50% with normal LV dimensions, and (iii) diastolic dysfunction, defined as impaired myocardial relaxation and passive stiffness (i.e., decreased LV compliance) [10]. It should be noted that the third criterion, diastolic dysfunction, once believed to be the main driver of HFpEF [11], is actually not unique to HFpEF [12] and that extramyocardial factors from comorbidities—hypertension, obesity, diabetes, and kidney disease—have since been uncovered as contributors to the syndrome independent of diastolic dysfunction [4]. According to a 2016 recommendation from the European Society of Cardiology, the current diagnostic criteria need to be reappraised to incorporate new pathophysiological insights in order to diagnose HFpEF patients correctly [13]. At present, HFpEF is commonly missed in the early stages, with the majority of patients diagnosed initially not for HF but for hypertension or type II diabetes [14]. In this review article, we frame our current understanding of the pathophysiology of HFpEF as a backdrop for appreciating what diagnostics are currently used and why, and for evaluating what new diagnostics are needed to diagnose HFpEF patients early and accurately. We focus on non-invasive imaging diagnostics and recommend new applications of advanced imaging techniques to improve the diagnosis of an increasingly prevalent form of HF.
Comorbidities
To shed light on the pathophysiology of HFpEF, it is worth noting that HFpEF patients tend to be older (~ 75 years), female (55–73%), and have multiple comorbidities such as hypertension (~ 75%), obesity/overweight (> 80%), diabetes (~ 40%), and renal disease (25–50%) [15,16,17, 8, 14]. These comorbidities are major risk factors for HFpEF [18, 19]. It is important also to emphasize that they all share a common link—systemic inflammation—which has been hypothesized to introduce an extramyocardial origin in the progression of HFpEF that does not exist in HFrEF [20]. With this hypothesis, inflammation is believed to damage the myocardium by inducing structural and functional abnormalities, including hypertrophy, interstitial fibrosis, impaired myocardial relaxation, and coronary microvascular dysfunction [20, 21]. The insult from systemic inflammation extends beyond the heart, which would explain the symptoms, such as exertional dyspnea and exercise intolerance, with which many HFpEF patients present. Figure 1 illustrates these pathophysiological changes together with HFpEF comorbidities and symptoms.
Common symptoms (orange boxes) and comorbidities (blue boxes) associated with the HFpEF heart, which is characterized by LV hypertrophy and LA volume increase. At the tissue level are various physical and functional changes, including coronary endothelial dysfunction, interstitial fibrosis, and impaired myocardial relaxation. LV hypertrophy cross-section adapted from Patrick J. Lynch, medical illustrator; C. Carl Jaffe, MD, cardiologist. https://creativecommons.org/licenses/by/2.5/
Despite the known associations with HFpEF, patients with the aforementioned comorbid conditions are not screened for heart disease until overt symptoms manifest, which is often too late in disease progression. Diabetic patients, for example, have a 10-fold increase in mortality and a 5-year survival of 15.5% by the time they exhibit HF symptoms [22,23,24]. Obesity is another common comorbidity, and again, patients are not screened for heart disease until HF symptoms appear. The association between obesity and HFpEF is not as well understood, but it is known that in and of itself, obesity can increase aortic stiffness and myocardial load that leads to the hypertrophy seen in HFpEF. A recent clinical trial assessed the outcome of HFpEF patients with abdominal obesity and concluded that the risk of all-cause mortality was significantly higher for obese patients compared to the non-obese cohort [25].
Pathophysiology
The current diagnostic guidelines for HFpEF were developed over a decade ago, when our understanding of the phenotype lagged far behind what we know today. In fact, as recently as the turn of the millennium, the term HFpEF did not exist and the condition was called “diastolic HF” to distinguish it from “systolic HF,” or what we currently refer to as HFrEF [26]. Today, however, we know that depressed systolic function is common in HFpEF. Similarly, HFrEF involves not only systolic dysfunction but, in many patients, diastolic dysfunction also. Given the significant overlap in diastolic and systolic dysfunction between the two HF phenotypes, the more accurate terminology of HFpEF versus HFrEF emerged.
At the macroscopic level, HFpEF is distinguished from HFrEF by virtue of concentric LV remodeling, where there is an increase in LV wall thickness and in LV mass, leading to hypertrophy [27]. This is in stark contrast to the eccentric remodeling and diminished LV wall thickness seen in HFrEF. The increased wall thickness and mass are supported by observations at the tissue level, where fat cardiomyocytes with a higher resting tension have replaced normally long and narrow cardiac muscle cells [15]. What ensues is the defining pathophysiological hallmark of HFpEF: increased LV tissue stiffness. As a result of a stiff ventricle, LV relaxation becomes impaired and LV filling pressure is elevated both overall and at end-diastole [28]. In order to maintain stroke volume and mechanical efficiency, systolic performance increases, thus maintaining the LVEF [29]. Mechanically, the manner in which the LV fills with blood during diastole also changes. In the healthy heart, as the LV untwists during early diastole, a negative pressure is created in the LV, sucking blood from the left atrium (LA). This accounts for 70–80% of the LV filling, while the remaining 20–30% occurs under subsequent LA contraction. With progressive worsening of diastolic dysfunction, LV filling during early diastole is reduced and pressure increases within the LA. The result is an enlarged LA and entry of blood into the LV by positive rather than negative pressure.
The structural and functional changes described above take many years to develop, and when a patient is diagnosed with HFpEF based on these changes, he/she is no longer in the early stages of disease progression. Unfortunately, current diagnostic guidelines and the associated technologies are focused on these late-stage cardiac changes, and these guidelines have remained fairly static over the years. Meanwhile, our mechanistic understanding of HFpEF has improved immensely. As discussed in the section “Comorbidities,” the current HFpEF paradigm supports the role of systemic inflammation driven by comorbidities as a key initiator of disease [20]. This paradigm begins with inflammation of the endothelium, which reduces the bioavailability of nitric oxide (NO), a key regulator of vasodilation and smooth muscle relaxation [20]. The action of NO is mediated through the NO-soluble guanyl cyclase (sGC)-cyclic guanosine monophosphate (cGMP) signaling pathway, which is increasingly recognized as a key regulator of cardiac function, exerting inotropic, lusitropic, and chronotropic effects [30]. In heart failure, low NO levels reduce intracellular cGMP and protein kinase G (PKG) activity, promoting cardiomyocyte hypertrophy and delaying myocardial relaxation. This direct impact on cardiac function is further aggravated by NO-mediated effects on the systemic circulation that alters preload and afterload conditions [31]. Another consequence of dysfunction in the systemic circulation is seen in skeletal muscle, where endothelial dysfunction translates to the exercise intolerance frequently seen in HFpEF patients [32, 33]. The coronary microvasculature is also affected by endothelial inflammation, which has been verified in myocardial biopsy samples, and explains the chest pain that results from reduced coronary perfusion and microvascular rarefaction [20]. Beyond these changes, cardiac inflammation will also initiate fibrosis, which has been observed in myocardial specimens from HFpEF patients [34]. In summary, HFpEF is increasingly recognized as a systemic syndrome rather than an isolated cardiac disease [35], a consideration that is pivotal for designing future diagnostic and therapeutic approaches.
Current diagnostics for HFpEF
The current guidelines recommended by the European Society of Cardiology focus on patients at more advanced stages and retain the diagnostic criteria described earlier: clinical symptoms of HF, normal LVEF, and diastolic dysfunction assessed invasively or non-invasively. The first criterion is easily assessed from patient reports of breathlessness, fatigue, and exercise intolerance and from signs such as swelling in the extremities. Many of these symptoms are not specific to HFpEF, however, and are often misdiagnosed and attributed to non-cardiac causes, such as chronic lung disease and anemia [36]. The second criterion is also easily determined, but accuracy depends highly on the imaging approach. Echocardiography, the undisputed workhorse of cardiac examinations, can measure the LVEF and LV end-diastolic volume index, with nominal cut-off values of ≥ 50% and < 97 ml/m2, respectively, as thresholds for normal systolic function and normal ventricular volume [10]. Because these numbers are averages only, sex- and age-dependent cut-off values are recommended instead—for example, the normal LVEF range for men (52–72%) differs from that for women (54–74%); see the American Society of Echocardiography 2015 quantitative guidelines for sex- and age-related changes in LV size and function [37]. Traditional two-dimensional (2D) echocardiography provides the easiest access to LVEF and LV volume estimation, but there can be substantial variability due to dependence on clear endocardial definition, which is absent in one third of cases [38]. To address this hurdle, ultrasound contrast agents can be administered to enhance the endocardial border for more accurate estimates of LV function and size [39]. Three-dimensional (3D) echocardiography has the potential for higher accuracy and reproducibility approaching that of cardiac MRI; the challenge with 3D echocardiography, however, is even greater susceptibility to operator expertise compared to 2D imaging, and both 3D ultrasound and MRI require breath-holding, which is a challenge for patients dyspneic at rest [40]. Accuracy and reproducibility are crucial, because diagnosing HFpEF is premised on correctly establishing a normal LVEF.
The most discriminatory piece of information comes from the third criterion of diastolic dysfunction, which of all three criteria is the most difficult to establish. It should be noted that diastolic dysfunction is a pathophysiological condition and can present in the absence of HF [41, 13, 11]. Only when diastolic dysfunction is present with the other two criteria is a diagnosis of HFpEF confirmed. To diagnose diastolic dysfunction definitively, there must be evidence of elevated LV filling pressures. Invasive catheterization of the LV remains the gold standard technique and involves measuring the LV end-diastolic pressure (> 16 mmHg), the time constant of LV relaxation (> 48 ms), and the pulmonary capillary wedge pressure (> 12 mmHg) [10]. However, since invasive techniques are not viable in most patients, non-invasive imaging based on echocardiography is used instead.
On a 2D echocardiogram, the HFpEF heart rarely appears normal in the late stages: the LV wall is usually thickened and the left atrial volume increased. These structural indices are suggestive of diastolic failure but are not surrogate measures of filling pressures, however. Functional indices based on Doppler echocardiography of mitral flow and tissue Doppler are used to determine if LV filling pressures are elevated, which is recommended as the first step to diagnosing diastolic dysfunction [42, 43]. In brief, tissue Doppler is used to measure the LV basal, longitudinal cardiac shortening, and/or lengthening velocity; measurements are taken at the position of the mitral annulus, and several velocities are taken, including early diastolic mitral annular velocity (e′) and late (atrial) diastolic mitral annular velocity (a′). Flow Doppler is used to measure the peak blood flow velocity through the mitral valve at early filling (E) and at late filling (A) due to atrial contraction. The ratio E/e′ is then calculated, and this index has been shown to be highly specific for increased LV filling pressures [44]. The ratio E/e′ < 8 in the normal heart but increases in diastolic dysfunction due to a lower e′, or less blood entering the LV during early filling, from impaired relaxation. The likelihood of increased filling pressures is much higher if the lateral E/e′ > 12 (or septal E/e′ > 15) and there is no mitral annual calcification, mitral regurgitation or valve prosthesis, tachycardia, atrioventricular block, atrial fibrillation, constrictive physiology, ventricular dyssynchrony, or focal wall motion abnormalities [45]. An alternative means to measure filling pressure (e.g., invasive hemodynamic testing) is required when these aforementioned conditions exist [45]. In addition to E/e′, it is currently recommended for improved specificity that the annular e′ velocity (septal e′ < 7 cm/s, lateral e′ < 10 cm/s), peak tricuspid regurgitation velocity (> 2.8 m/s), and left atrial volume index (> 34 mL/m2) also be considered. Taking all four parameters together decreases the likelihood of false positive findings; with this approach, diastolic dysfunction is diagnosed only when over half of these parameters meet respective abnormal cut-off values [43]. Once diastolic dysfunction is determined, disease grading is performed by calculating the mitral valve flow velocity ratio (E/A), which is typically ≥ 1.5 in the normal heart. In healthy individuals, the E-wave prevails due to efficient LV filling in early and mid-diastole, whereas in older individuals or in mild diastolic dysfunction, early LV filling decreases and the A-wave dominates (E/A < 0.8). With worsening diastolic dysfunction, the E/A ratio increases above 0.8 back into the normal range as left atrial pressure increases; the Valsalva maneuver is helpful in this scenario for distinguishing normal diastolic function from dysfunction [45]. Full details on the diagnostic tree are found in the 2016 guidelines from the American Society of Echocardiography and the European Association of Cardiovascular Imaging [43]. Finally, plasma levels of brain natriuretic peptide (BNP) and N-terminal pro brain natriuretic peptide (NT-proBNP) should be tested to make a diagnosis for HFpEF, since the production of biomarkers is minimal in healthy individuals but becomes elevated (BNP > 200 pg/mL or NT-proBNP > 220 pg/mL) in both HFpEF and HFrEF [46, 47]. However, caution must be exercised with the use of these biomarkers, since BNP levels tend to be lower in HFpEF and even normal in some HFpEF patients [48, 49]. Figure 2 illustrates various echocardiographic techniques [37, 45, 39, 50].
Echocardiography of LV diastolic function. a Doppler transmitral flow demonstrating early (E) and late/atrial (A) waves on pulsed-wave Doppler at the mitral leaflet tips in the apical 4-chamber view (left). Pulsed-wave tissue Doppler velocities at the lateral mitral annulus (e′ = early diastolic, a′ = late/atrial diastolic, and s′ = systolic tissue velocities) (right). (Reprinted from Mitter et al. [45]). b Differences in end-diastolic/systolic volumes observed without contrast (top) and with contrast and low-mechanical index imaging (bottom). A marked increase in volume size is noted post-contrast. (Reprinted from Porter et al. [39]). c Myocardial perfusion via contrast. (Reprinted from Porter and Xie [50]). d Measurement of global longitudinal strain via speckle tracking. (Reprinted from Lang et al. [37])
Cardiac magnetic resonance imaging (MRI) is seldom used in routine clinical practice and is not part of the current diagnostic flowcharts for HFpEF due to high costs and limited availability. For patients who have poor quality echocardiographic findings, however, cardiac MRI is the only other alternative able to measure cardiac structure and function. Its inherent superior soft-tissue contrast and high spatial resolution make cardiac MRI the gold-standard modality for measuring LA volume, LV volume, and LV mass [51]. In fact, cardiac MRI is preferred over echocardiography for its reproducibility and for monitoring small changes in LV mass or LV volume during disease progression. Dynamic CINE acquisitions of the whole heart allow us to measure LVEF much more reproducibly than from echocardiography [52] in addition to a wide range of LV filling parameters identical to those from echocardiography [10]. Myocardial perfusion and viability can also be assessed with the aid of an intravascular injection of gadolinium-based MR contrast agent [53, 54]. Perhaps the most important capability of cardiac MRI, one for which there is no echocardiographic analog, is myocardial tissue characterization—only cardiac MRI can identify and delineate ischemic tissue, inflammation, and infiltrative diseases [55]. Quantitative MRI methods such as parametric cardiac T1, T2, and T2* mapping reflect intrinsic tissue magnetic properties and are increasingly accessible on clinical MR scanners [56]. If implemented robustly, quantitative mapping can provide information on fibrosis (T1) [57, 58], edema and inflammation (T2) [59, 60], and iron overload and hemorrhage (T2*) [61, 62]. The first multicenter randomized, controlled clinical trial to evaluate the role of cardiac MRI in non-ischemic heart failure or HFpEF (IMAGE-HF project 1-B) is underway to investigate if routine cardiac MRI can identify more specific heart failure etiologies compared to echocardiography alone [63]. Depending on the results of the trial, the inclusion of cardiac MRI in future strategies for HFpEF diagnosis may be recommended. Figure 3 demonstrates the capabilities of cardiac MRI [64,65,66,67,68].
Cardiac MRI. a Acquisition protocol includes assessment at rest and during vasodilator stress. (Reproduced from Coelho-Filho et al. [64]). b Short-axis perfusion MRI showing mid-anteroseptal and anterior wall motion abnormality, rest (left) and stress (middle) perfusion abnormality (black arrows), and myocardial delayed enhancement (right, white arrows) consistent with infarct. (Adapted from Jenson et al. [65]). c Bright-blood and dark-blood late gadolinium enhancement reveals contrast between the subendocardial scar and adjacent blood pool. (Adapted from Francis et al. [66]). d Subvalvular obstruction (red arrows) secondary to subaortic membrane seen on 3-chamber (left) and coronal (right) views. (Reprinted from Cavalcante et al. [67]). e Color-coded strain map of tagged MRI shows heterogeneous strain values in the left ventricle throughout one cardiac cycle (left upper: first frame at end-diastole; right upper: end-systole; lower row: diastole). The time-strain curves of six myocardial segments show variations in the peak time of each strain curve; left ventricular dyssynchrony is identified (right lower). (Figure reproduced from Nagao and Yamasaki [68])
The structural and functional metrics described above for both echocardiography and cardiac MRI reflect the status of the heart at rest. However, a stress echocardiogram or a stress MRI may be performed to assess cardiac performance under stress. The stress could be triggered either by exercise on a treadmill or by dobutamine, which is injected intravenously to raise heart rates in patients who cannot exercise. Using stress echocardiography, myocardial viability is then assessed via measurement of contractile reserve and biphasic response. Using cardiac MRI, myocardial viability is assessed via spatial distribution of intravenously injected contrast agents. The same injection also provides information on the perfusion status of heart muscle. Table 1 summarizes the role of various imaging modalities in the diagnosis of HFpEF. Table 2 highlights imaging modalities that are useful for assessing various physical or functional parameters relevant to HFpEF.
Challenges of diagnosing HFpEF and future directions
One of the major limitations of existing diagnostic workup is lack of information on heart function when the patient is under stress (e.g., from exercise). Some patients are asymptomatic at rest and do not exhibit cardiac structural or functional abnormalities, particularly those at the early stages of disease progression [69, 5]. These patients in the early stages may report dyspnea and/or fatigue on exertion but otherwise have negative findings on routine physical and radiological examination. Hemodynamic assessment during exercise then becomes the only approach to determine the existence of HFpEF [4]. For example, since elevated LV filling pressure is critical to the current diagnosis of HFpEF, if echocardiography and invasive hemodynamic measurements all yield inconclusive results, then one should resort to exercise stress testing and/or manoeuvers such as leg raises and fluid challenge [11]. There is evidence that many patients who have normal echocardiography findings at rest have significantly altered hemodynamics during exercise; increases in the E/e′ ratio and impaired ventricular-arterial coupling have all been reported [70, 71]. Chronotropic incompetence and abnormalities outside the heart, such as impaired vascular reserve, can also help uncover the presence of HFpEF during exercise [70].
It is also important to recall that diastolic dysfunction is not present in all HFpEF patients and that a diagnosis of diastolic dysfunction alone does not constitute a diagnosis of HFpEF [11]. In other words, heart failure with diastolic dysfunction is only a subset of HFpEF. This distinction between a pathophysiological state (diastolic dysfunction) and a clinical syndrome (HFpEF) highlights the importance of thinking broadly about the complex etiologies of HFpEF when advancing new technologies and approaches for earlier, more sensitive, and more specific diagnosis. Furthermore, we must remember that many of the current indices recommended for diagnosis (e.g., elevated E/e′ ratio, LV hypertrophy, left atrial enlargement) are indirect measures of LV stiffness, the hallmark of HFpEF and the parameter that provides the most direct evidence of disease if it can be measured accurately [72]. In the following, we discuss new targets and imaging-based tools for improving diagnostic accuracy.
Myocardial stiffness
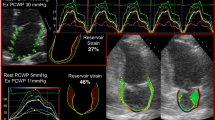
Although the E/e′ ratio is regarded as the echocardiographic gold standard for measuring LV diastolic function, recent studies have shown inaccuracies in its measurement when regional wall motion is abnormal. This is problematic, as several studies have shown that abnormalities in regional contractility are common in HFpEF patients [32]. To address this dilemma, newer echocardiographic methods have been applied to quantify regional cardiac stiffness directly through an assessment of myocardial deformation [73]. This technique, known as speckle-tracking echocardiography [74], uses frame-by-frame tracking of small myocardial regions-of-interest, each with a unique speckle pattern, and calculates cardiac strain (i.e., tissue deformation) from temporal changes in the segment length. The calculated strain, together with its temporal derivative, the strain rate, can be measured on a regional basis or can be averaged to yield a global strain score, longitudinally, radially, and circumferentially [75, 76]. A strong linear association between the global strain score and functional capacity has been reported in HFpEF patients [77]. Recent insights from the RELAX trial of sildenafil in HFpEF patients showed that the LV global longitudinal strain as determined by speckle-tracking was significantly impaired in HFpEF and was associated with collagen synthesis and diastolic dysfunction [78]. However, impaired LV global longitudinal strain was not associated with quality of life or exercise tolerance, indicating that other factors, intra- or extra-myocardial, are at play. Another difficulty is the low reproducibility of strain value, particularly circumferential and radian strain, due to variability in software algorithms for analysis [79]. Clearly, more work lies ahead to determine the prognostic value of strain measurements.
Cardiac MRI is also capable of providing information on regional myocardial strain by using a method to “tag” myocardial tissue with a “grid” and track movement during systole [80]. In applying the method to diastole, however, a major limitation is fading of grid lines over the cardiac cycle interval. The consequence is that while the early diastolic strain rate can be measured in 80% of segments analyzed, atrial-induced strain could be measured in only 32% of patients [81]. More recently, a method known as feature tracking using CINE balanced steady-state free precession acquisitions was introduced as a robust and rapid method for measuring myocardial strain and diastolic strain rate [82]. This technique requires considerably less data processing time than tissue tagging and has even been applied to both obese and diabetic patients to assess LA strain for early detection of diastolic dysfunction [83] and to echocardiography-confirmed HFpEF patients without co-morbidities to assess LV diastolic strain rate [84]. To advance this promising cardiac MRI technique into widespread clinical implementation, more rapid image acquisition solutions are needed.
Ventricular-arterial coupling
Hypertension is a well-known antecedent to the development of HF. In the HFpEF population, approximately 75% of patients are hypertensive, and in these patients, there is evidence of stiffening in the LV and arteries [4, 85]. One school of thought has proposed arterial-ventricular stiffening as a main contributor to increases in blood pressure, which then impairs diastolic LV relaxation [86]. The result of this stiffening and non-compliance in both the ventricular and arterial compartments negatively alters ventricular-arterial coupling, which is defined by the ratio of arterial elastance (afterload Ea) and end-systolic ventricular elastance (end-systole elastance Ees) [7]. In HFpEF patients, the coupling ratio (Ea/Ees) is decreased relative to control hypertensive subjects without HF [87]. This decrease stems from a proportionally higher change in ventricular stiffening, or Ees. Alterations in ventricular-arterial coupling have an especially pronounced impact on cardiac function during exercise and variations of volume load, and its measurement may provide valuable information for managing patients. In a recent study of patients who had negative results on stress echocardiography, higher rates of mortality and hospitalization were predicted by altered ventricular-arterial coupling [88].
Although measuring ventricular-arterial coupling remains challenging, both echocardiography and cardiac MRI have demonstrated the potential for non-invasive assessment. The arterial elastance, Ea, is calculated as the ratio of end-systolic pressure to stroke volume. The end-systolic ventricular elastance, Ees, is calculated as the change in pressure for a given change in chamber volume. From these two numbers, the ventricular-arterial coupling (Ea/Ees) is computed. Echocardiography provides continuous temporal monitoring and measures ventricular-arterial coupling using a single-beat method developed by Chen et al. [89]. This method involves complex mathematical formulae that require computer algorithms for easy calculation of the ratio Ea/Ees in the clinical setting. More recent application of 3D echocardiography has demonstrated higher reproducibility and sensitivity over 2D methods [90]. In contrast, cardiac MRI does not have sufficient temporal resolution to capture the beat-to-beat changes in the LV pressure-volume relationship required to measure Ees. Using conventional CINE acquisitions, Ees is approximated as the ratio of end-systolic pressure to end-systolic volume [91]. Improved estimation of Ees using MRI is possible using real-time acquisition approaches to allow essentially continuous measurement of LV volume changes [92]; however, these custom sequences are not widely accessible. Irrespective of the relative advantage of echocardiography over cardiac MRI in this setting, determination of ventricular-arterial coupling is simply not in the current diagnostic workflow due to complexity of measurement. Prognostic clinical trials are needed to determine its predictive value on the outcomes of cardiovascular disease.
Myocardial fibrosis
The diffuse myocardial fibrosis observed in the hypertrophic HFpEF heart is a result of chronic inflammation. Activated fibroblasts (myofibroblasts), in their role of inflammatory supporter cells, deposit collagen and release cytokines that drive a vicious circle triggering further inflammation and fibrosis [93]. Although diffuse fibrosis has not traditionally been considered as an early marker of HFpEF [94], there is recent evidence that suggests otherwise: patients at risk for HFpEF (elevated BNP levels) exhibited the same degree of fibrosis as those with a confirmed HFpEF diagnosis [95]. The temporal relationship between myocardial fibrosis and the progression of HFpEF is not well characterized due to the scarcity of methods able to assess fibrosis non-invasively. Consequently, the evaluation of myocardial fibrosis should be considered in future investigations and explorations of early biomarkers.
To date, most attempts to detect diffuse cardiac fibrosis have been achieved using cardiac MRI because of its exquisite spatial delineation [96, 97]. However, the gadolinium-contrast-enhanced MRI method employed is sensitive to the extracellular volume fraction and, therefore, is not specific to fibrosis. Ambiguity is a problem, as other pathologies, including cell death and edema, can give rise to a higher extracellular volume fraction. In order to detect fibrosis with certainty, molecular probes with an affinity for collagen are required. Currently, there is very little effort in the development of targeted probes for imaging fibrosis, although there are a few reports in animals in various anatomical regions of the body [98,99,100]. An important future emphasis is to translate these molecular probes into human patients.
Endothelial dysfunction
A potentially very powerful diagnostic approach is to look also for extramyocardial evidence of disease, in contrast to focusing exclusively on the heart. As described earlier, the current paradigm on the etiology of HFpEF attributes coronary microvascular dysfunction and impaired vascular reserve, amongst other, to endothelial dysfunction, which is a deleterious outcome of inflammation. Given that the most common comorbidities of HFpEF all involve inflammation, it is reasonable, even logical, to place the occurrence of endothelial dysfunction at the same time as, if not earlier than, the earliest development of abnormalities in the heart. If our ultimate goal is to achieve early detection of HFpEF, it may very well be that waiting until overt indications of diagnostic dysfunction appear is already too late. With this philosophy, we would need to detect and diagnose endothelial dysfunction directly. It is important to note that the complications of endothelial dysfunction—such as impaired vasodilation, microvessel rarefaction, and a reduced systemic vasodilatory response [101]—are all consistent with HFpEF symptoms (dyspnea on exertion, exercise intolerance) that have been observed in the absence of diastolic dysfunction [32]. While it is true that endothelial dysfunction and inflammation may not necessarily lead to HFpEF, they do put patients at risk for heart disease and represent the earliest known biomarker than can be assessed on diagnostic imaging.
The gold standard for measuring endothelial function is angiography under injection of acetylcholine, a vasodilator [102]. This method, however, is invasive and limited to large blood vessels such as the coronary artery. Another large-vessel but non-invasive method is ultrasound-measured flow-mediated dilation of the brachial artery. Laser Doppler allows non-invasive assessment of microvessel flow, but it can only be applied to superficial tissue [103]. PET imaging involving radioactive tracers allows deep-tissue assessment of myocardial perfusion [104] and sympathetic nerve activity [105, 106]. Importantly, HFpEF patients with compromised coronary flow reserve as identified on PET stress/rest perfusion have been shown in separate studies to have markedly greater risk of HFpEF events [107, 108], but this capability must be balanced against the risk of exposing patients to radioactive tracers. A completely non-invasive alternative is MRI, which offers superior spatial resolution to PET imaging. To probe endothelial dysfunction, blood oxygenation level-dependent (BOLD) MRI methods developed from neuroimaging has been applied to the heart, but this method is inappropriate due to inherent sensitivity to many factors unrelated to microvascular tone [109]. The only MRI technique that has been reported in the literature to date for the direct assessment of endothelial function and dysfunction is specifically sensitized to microvascular volume [110]. This approach was developed for high- and low-flow organs and has been applied to ischemic muscle tissue to elucidate compromised microvessel dilation [111]. The translation of this method to humans is underway and should be considered in future investigations on the prognostic and early diagnostic value of assessing endothelial dysfunction both intra- and extramyocardially.
Inflammation
In our discussion of future perspectives for earlier and more accurate HFpEF diagnosis, we have highlighted entities (stress testing, myocardial stiffness, ventricular-arterial coupling, fibrosis, and endothelial dysfunction) that have the potential to be included in a new imaging diagnostic workflow. Of these, endothelial dysfunction is one of the earliest changes that manifest, possibly first extramyocardially before the heart is even affected. Another pathophysiology that likely forebodes cardiac changes is inflammation. Using radiotracer probes on nuclear medicine imaging, immune cell migration to the myocardium may be visualized [112] and acute and post-inflammatory reaction may be distinguished [113]. MRI is less useful for this purpose, because inflammation is identified indirectly through increased extravasation of intravenously injected contrast agents. Since the current HFpEF paradigm places inflammation as the initiator of disease, it would be worthwhile to consider the value of imaging systemic inflammation in the prognosis of HFpEF.
Conclusions
HFpEF is a heterogeneous disease whose pathophysiological basis is still being uncovered. There is no uniformly accepted set of validated diagnostic guidelines and no proven therapy. What is certain, however, is that HFpEF patients are often misdiagnosed at the early stages but then are far along in disease progression when a HFpEF diagnosis is eventually confirmed. This review article summarized our current understanding of the etiology of the syndrome and proposed new approaches to diagnosis that take a systemic perspective to enable earlier, more accurate diagnosis of a prevalent and equally fatal phenotype of HF. We also reviewed the mainstay of cardiac diagnostics, namely, echocardiography, cardiac MRI, and cardiac PET. Each modality provides complementary information, but a different modality may assume a more dominant role in diagnosis depending on the stage of disease. In the early stages of HFpEF development, where tissue-level changes—such as microstructural alterations, reactive fibrosis, and vascular changes—manifest in the absence of overt functional and structural alterations, cardiac MRI may be arguably the best modality for early detection of disease. As disease progresses and the increasingly stiffer myocardium begins to impair mechanics, both echocardiography and cardiac MRI can inform on the severity of disease. One distinct advantage that echocardiography has over the other modalities, however, is its unique real-time acquisition ability, which provides specific information on diastolic function and dysfunction. Ultimately, the relative diagnostic value of each modality at different stages of HFpEF progression can only be determined with improved understanding of the etiology and development of HFpEF.
References
Garg A, Virmani D, Agrawal S, Agarwal C, Sharma A, Stefanini G, Kostis JB (2017) Clinical application of biomarkers in heart failure with a preserved ejection fraction: a review. Cardiology 136(3):192–203. https://doi.org/10.1159/000450573
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. New England Journal of Medicine 355:251–259
Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC (2012) Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 126(1):65–75
Borlaug BA, Paulus WJ (2011) Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J 32(6):670–679. https://doi.org/10.1093/eurheartj/ehq426
Tschöpe C, Lam CSP (2012) Diastolic heart failure: what we still don’t know. Looking for new concepts, diagnostic approaches, and the role of comorbidities. Herz 37(8):875–879. https://doi.org/10.1007/s00059-012-3719-5
Ho JE, Enserro D, Brouwers FP, Kizer JR, Shah SJ, Psaty BM, Bartz TM, Santhanakrishnan R, Lee DS, Chan C, Liu K, Blaha MJ, Hillege HL, van der Harst P, van Gilst WH, Kop WJ, Gansevoort RT, Vasan RS, Gardin JM, Levy D, Gottdiener JS, de Boer RA, Larson MG (2016) Predicting heart failure with preserved and reduced ejection fraction: The International Collaboration on Heart Failure Subtypes. Circ Heart Fail 9(6). https://doi.org/10.1161/circheartfailure.115.003116
Becher PM, Lindner D, Fluschnik N, Blankenberg S, Westermann D (2013) Diagnosing heart failure with preserved ejection fraction. Expert Opinion on Medical Diagnostics 7(5):463–474. https://doi.org/10.1517/17530059.2013.825246
Campbell RT, McMurray JJV (2014) Comorbidities and differential diagnosis in heart failure with preserved ejection fraction. Heart Fail Clin 10(3):481–501. https://doi.org/10.1016/j.hfc.2014.04.009
Ezekowitz JA, O’Meara E, McDonald MA, Abrams H, Chan M, Ducharme A, Giannetti N, Grzeslo A, Hamilton PG, Heckman GA, Howlett JG, Koshman SL, Lepage S, McKelvie RS, Moe GW, Rajda M, Swiggum E, Virani SA, Zieroth S, Al-Hesayen A, Cohen-Solal A, D’Astous M, De S, Estrella-Holder E, Fremes S, Green L, Haddad H, Harkness K, Hernandez AF, Kouz S, MH L, Fa M, Rose HJ, Roussin A, Sussex B (2017) 2017 Comprehensive update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Canadian Journal of Cardiology 33(11):1342–1433
Paulus WJ, Tschöpe C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbély A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 28(20):2539–2550. https://doi.org/10.1093/eurheartj/ehm037
Oktay AA, Shah SJ (2015) Diagnosis and management of heart failure with preserved ejection fraction: 10 key lessons. Curr Cardiol Rev 11(1):42–52
Hadano Y, Murata K, Yamamoto T, Kunichika H, Matsumoto T, Akagawa E, Sato T, Tanaka T, Nose Y, Tanaka N, Matsuzaki M (2006) Usefulness of mitral annular velocity in predicting exercise intolerance in patients with impaired left ventricular systolic function. American Journal of Cardiology 97:1025–1028
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González-Juanatey JR, Harjola V-P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37(27):2129–2200. https://doi.org/10.1093/eurheartj/ehw128
Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, Paulus WJ (2016) Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation 134(1):73–90. https://doi.org/10.1161/circulationaha.116.021884
Borlaug BA, Redfield MM (2011) Diastolic and systolic heart failure are distinct phenotypes within the heart failure spectrum. Circulation 123(18):2006–2013; discussion 2014. https://doi.org/10.1161/circulationaha.110.954388
Altara R, Giordano M, Nordén ES, Cataliotti A, Kurdi M, Bajestani SN, Booz GW (2017) Targeting obesity and diabetes to treat heart failure with preserved ejection fraction. Front Endocrinol (Lausanne) 8:160. https://doi.org/10.3389/fendo.2017.00160
Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM (2018) Sex differences in cardiovascular pathophysiology: why women are overrepresented in heart failure with preserved ejection fraction. Circulation 138(2):198–205. https://doi.org/10.1161/circulationaha.118.034271
Afsar B, Rossignol P, van Heerebeek L, Paulus WJ, Damman K, Heymans S, van Empel V, Sag A, Maisel A, Kanbay M (2017) Heart failure with preserved ejection fraction: a nephrologist-directed primer. Heart Fail Rev. https://doi.org/10.1007/s10741-017-9619-2
Haass M, Kitzman DW, Anand IS, Miller A, Zile MR, Massie BM, Carson PE (2011) Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail 4(3):324–331. https://doi.org/10.1161/CIRCHEARTFAILURE.110.959890
Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction. comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62(4):263–271. https://doi.org/10.1016/j.jacc.2013.02.092
O’Meara E, de Denus S, Rouleau J-L, Desai A (2013) Circulating biomarkers in patients with heart failure and preserved ejection fraction. Curr Heart Fail Rep 10(4):350–358. https://doi.org/10.1007/s11897-013-0160-x
Hippisley-Cox J, Coupland C (2016) Diabetes treatments and risk of heart failure, cardiovascular disease, and all cause mortality: cohort study in primary care. BMJ 354:i3477. https://doi.org/10.1136/bmj.i3477
Sandesara PB, O’Neal WT, Kelli HM, Samman-Tahhan A, Hammadah M, Quyyumi AA, Sperling LS (2018) The prognostic significance of diabetes and microvascular complications in patients with heart failure with preserved ejection fraction. Diabetes Care 41(1):150–155. https://doi.org/10.2337/dc17-0755
Boonman-de Winter LJM, Cramer MJ, Hoes AW, Rutten FH (2016) Uncovering heart failure with preserved ejection fraction in patients with type 2 diabetes in primary care: time for a change. Neth Heart J 24(4):237–243. https://doi.org/10.1007/s12471-016-0809-7
Tsujimoto T, Kajio H (2017) Abdominal obesity is associated with an increased risk of all-cause mortality in patients with HFpEF. J Am Coll Cardiol 70(22):2739–2749. https://doi.org/10.1016/j.jacc.2017.09.1111
How to diagnose diastolic heart failure European study group on diastolic heart failure (1998). Eur Heart J 19(7):990–1003
Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM (2012) Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community-based study. Circ Heart Fail 5(6):710–719. https://doi.org/10.1161/circheartfailure.112.968594
Hay I, Rich J, Ferber P, Burkhoff D, Maurer MS (2005) Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Physiol Heart Circ Physiol 288(3):H1203–H1208. https://doi.org/10.1152/ajpheart.00681.2004
Gladden JD, Linke WA, Redfield MM (2014) Heart failure with preserved ejection fraction. Pflugers Arch 466(6):1037–1053. https://doi.org/10.1007/s00424-014-1480-8
Lim SL, Lam CS, Segers VF, Brutsaert DL, De Keulenaer GW (2015) Cardiac endothelium-myocyte interaction: clinical opportunities for new heart failure therapies regardless of ejection fraction. Eur Heart J 36(31):2050–2060. https://doi.org/10.1093/eurheartj/ehv132
van Heerebeek L, Hamdani N, Falcao-Pires I, Leite-Moreira AF, Begieneman MP, Bronzwaer JG, van der Velden J, Stienen GJ, Laarman GJ, Somsen A, Verheugt FW, Niessen HW, Paulus WJ (2012) Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 126(7):830–839. https://doi.org/10.1161/CIRCULATIONAHA.111.076075
Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, Redfield MM (2010) Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56(11):845–854. https://doi.org/10.1016/j.jacc.2010.03.077
Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, Ohba K, Matsubara J, Maeda H, Horibata Y, Sakamoto K, Sugamura K, Yamamuro M, Sumida H, Kaikita K, Iwashita S, Matsui K, Kimura K, Umemura S, Ogawa H (2012) Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol 60(18):1778–1786. https://doi.org/10.1016/j.jacc.2012.07.036
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss H-P, Tschöpe C (2011) Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4(1):44–52. https://doi.org/10.1161/circheartfailure.109.931451
Lam CS, Lyass A, Kraigher-Krainer E, Massaro JM, Lee DS, Ho JE, Levy D, Redfield MM, Pieske BM, Benjamin EJ, Vasan RS (2011) Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation 124(1):24–30
Bell DSH (2003) Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 26(8):2433–2441
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European heart journal cardiovascular imaging 16(3):233–270
Hare JL, Brown JK, Marwick TH (2008) Performance of convetional echocardiographic parameters and myocardial measurements in the sequential evaluation of left ventricular function. American Journal of Cardiology 101:706–711
Porter TR, Mulvagh SL, Abdelmoneim SS, Becher H, Belcik JT, Bierig M, Choy J, Gaibazzi N, Gillam LD, Janardhanan R, Kutty S, Leong-Poi H, Lindner JR, Main ML, Mathias WJ, Park MM, Senior R, Villanueva F (2018) Clinical applications of ultrasonic enhancing agents in echocardiography: 2018 American Society of Echocardiography Guidelines Update. Journal of the American Society of Echocardiography 31(3):241–274
Soliman OI, Kirschbaum SW, van Dalen BM, van der Zwaan HB, Mahdavian Delavary B, Vletter WB, van Geuns RJ, Ten Cate FJ, Geleijnse ML (2008) Accuracy and reproducibility of quantitation of left ventricular function by real-time three-dimensional echocardiography versus cardiac magnetic resonance. American Journal of Cardiology 102(6):778–783
Butrous H, Pai RG (2013) Heart failure with normal ejection fraction: current diagnostic and management strategies. Expert Rev Cardiovasc Ther 11(9):1179–1193. https://doi.org/10.1586/14779072.2013.827468
Gillebert TC, De Pauw M, Timmermans F (2013) Echo-doppler assessment of diastole: flow, function and haemodynamics. Heart 99:55–64
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD (2016) Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. European Heart Journal Cardiovascular Imaging 17(12):1321–1360
Kasner M, Westermann D, Steendijk P, Gaub R, Wilkenshoff U, Weitmann K, Hoffmann W, Poller W, Schultheiss HP, Pauschinger M, Tschöpe C (2007) Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation 116(6):637–647
Mitter SS, Shah SJ, Thomas JD (2017) A test in context: E/A and E/e' to assess diastolic dysfunction and LV filling pressure. J Am Coll Cardiol 69(11):1451–1464. https://doi.org/10.1016/j.jacc.2016.12.037
Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW (2009) 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol 53(15):e1–e90
Hunt PJ, Richards AM, Nicholls MG, Yandle TG, Doughty RN, Espiner EA (1997) Immunoreactive amino-terminal pro-brain natriuretic peptide (NT-PROBNP): a new marker of cardiac. Clinical endocrinology 47(3):287–296
Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, Shah SJ (2012) Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. American Journal of Cardiology 110(6):870–876
Mehra MR, Uber PA, Park MH, Scott RL, Ventura HO, Harris BC, Frohlich ED (2004) Obesity and suppressed B-type natriuretic peptide levels in heart failure. American Journal of Cardiology 43(9):1590–1595
Porter TR, Xie F (2010) Myocardial perfusion imaging with contrast ultrasound. JACC Cardiovasc Imaging 3(2):176–187. https://doi.org/10.1016/j.jcmg.2009.09.024
Rademakers FE (2003) Magnetic resonance imaging in cardiology. Lancet 361(9355):359–360
Mertens LL, Friedberg MK (2010) Imaging the right ventricle -- current state of the art. Nat Rev Cardiol 7(10):551–563
Hendel RC, Friedrich MG, Schulz-Menger J, Zemmrich C, Bengel F, Berman DS, Camici PG, Flamm SD, Le Guludec D, Kim R, Lombardi M, Mahmarian J, Sechtem U, Nagel E (2016) CMR first-pass perfusion for suspected inducible myocardial ischemia. JACC Cardiovascular Imaging 9(11):1338–1348
Shehata ML, Basha TA, Hayeri MR, Hartung D, Teytelboym OM, Vogel-Claussen J (2014) MR myocardial perfusion imaging: insights on techniques, analysis, interpretation, and findings. Radiographics 34(6):1636–1657
Kanagala P, Cheng ASH, Singh A, McAdam J, Marsh A-M, Arnold JR, Squire IB, Ng LL, McCann GP (2018) Diagnostic and prognostic utility of cardiovascular magnetic resonance imaging in heart failure with preserved ejection fraction - implications for clinical trials. Journal of Cardiovascular Magnetic Resonance 20(1):4. https://doi.org/10.1186/s12968-017-0424-9
Salerno M, Kramer CM (2013) Advances in parametric mapping with CMR imaging. JACC Cardiovascular Imaging 6(7):806–822
Reiter G, Reiter C, Krauter C, Fuschsjager M, Reiter U (2018) Cardiac magnetic resonance T1 mapping. Part 1: aspects of acquisition and evaluation. European journal of radiology 109:223–234
Zhuang B, Sirajuddin A, Wang S, Arai A, Zhao S, Lu M (2018) Prognostic value of T1 mapping and extracellular volume fraction in cardiovascular disease: a systematic review and meta-analysis. Heart Fail Rev 23(5):723–731
Bohnen S, Radunski UK, Lund GK, Ojeda F, Looft Y, Senel M, Radziwolek L, Avanesov M, Tahir E, Stehning C, Schnackenburg B, Adam G, Blankenberg S, Muellerleile K (2017) Tissue characterization by T1 and T2 mapping cardiovascular magnetic resonance imaging to monitor myocardial inflammation in healing myocarditis. Eur Heart J Cardiovasc Imaging 18(7):744–751
Hartung D, Hueper K, Chen R, Gutberlet M, Wacker F, Meier M, Rong S, Jang MS, Bräsen JH, Gueler F (2018) T2 mapping for noninvasive assessment of interstitial edema in acute cardiac allograft rejection in a mouse model of heterotopic heart transplantation. Investigative Radiology 53(5):271–277
Krittayaphong R, Zhang S, Saiviroonporn P, Viprakasit V, Tanapibunpon P, Rerkudom B, Yindeengam A, Wood JC (2019) Assessment of cardiac iron overload in thalassemia with MRI on 3.0-T: high-field T1, T2, and T2* quantitative parametric mapping in comparison to T2* on 1.5-T. JACC Cardiovascular Imaging 12(4):752–754
Ghugre NR, Ramanan V, Pop M, Yang Y, Barry J, Qiang B, Connelly KA, Dick AJ, Wright GA (2011) Quantitative tracking of edema, hemorrhage, and microvascular obstruction in subacute myocardial infarction in a porcine model by MRI. Magn Reson Med 66(4):1129–1141
Paterson I, Wells GA, Ezekowitz JA, White JA, Friedrich MG, Mielniczuk LM, O’Meara E, Chow BS, DeKemp RA, Klein R, Dennie C, Dick A, Coyle D, Dwivedi G, Rajda M, Wright GA, Laine M, Hanninen H, Larose E, Connelly KA, Leong-Poi H, Howarth AG, Davies RA, Duchesne L, Yla-Herttuala S, Saraste A, Farand P, Garrard L, Tardif JC, Arnold M, Knuuti J, Beanlands R, Chan KL (2013) Routine versus selective cardiac magnetic resonance in non-ischemic heart failure - OUTSMART-HF: study protocol for a randomized controlled trial (IMAGE-HF (heart failure) project 1-B). Trials 14:332
Coelho-Filho OR, Rickers C, Kwong RY, Jerosch-Herold M (2013) MR myocardial perfusion imaging. Radiology 266(3):701–715. https://doi.org/10.1148/radiol.12110918
Jenson S, Craig S, York G, Steel K (2013) Review of cardiac MR perfusion imaging. Journal of the American Osteopathic College of Radiology 2(2):1–7
Francis R, Kellman P, Kotecha T, Baggiano A, Norrington K, Martinez-Naharro A, Nordin S, Knight DS, Rakhit RD, Lockie T, Hawkins PN, Moon JC, Hausenloy DJ, Xue H, Hansen MS, Fontana M (2017) Prospective comparison of novel dark blood late gadolinium enhancement with conventional bright blood imaging for the detection of scar. J Cardiovasc Magn Reson 19(1):91. https://doi.org/10.1186/s12968-017-0407-x
Cavalcante JL, Lalude OO, Schoenhagen P, Lerakis S (2016) Cardiovascular magnetic resonance imaging for structural and valvular heart disease interventions. JACC Cardiovasc Interv 9(5):399–425. https://doi.org/10.1016/j.jcin.2015.11.031
Nagao M, Yamasaki Y (2018) Cardiac strain analysis using cine magnetic resonance imaging and computed tomography. Cardiovascular Imaging Asia 2:76–84
Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM (2010) Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circulation Heart failure 3(5):588–595
Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA (2006) Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation 114(20):2138–2147
Donal E, Thebault C, Lund LH, Kervio G, Reynaud A, Simon T, Drouet E, Nonotte E, Linde C, Daubert JC (2012) Heart failure with a preserved ejection fraction additive value of an exercise stress echocardiography. European Heart Journal Cardiovascular Imaging 13(8):656–665
Oren O, Goldberg S (2017) Heart failure with preserved ejection fraction: diagnosis and management. Am J Med 130(5):510–516. https://doi.org/10.1016/j.amjmed.2016.12.031
Ohtani T, Mohammed SF, Yamamoto K, Dunlay SM, Weston SA, Sakata Y, Rodeheffer RJ, Roger VL, Redfield MM (2012) Diastolic stiffness as assessed by diastolic wall strain is associated with adverse remodelling and poor outcomes in heart failure with preserved ejection fraction. Eur Heart J 33(14):1742–1749
Kaluzynski K, Chen X, Emelianov SY, Skovoroda AR, O’Donnell M (2001) Strain rate imaging using two-dimensional speckle tracking. IEEE transactions on ultrasonics, ferroelectrics, and frequency control 48(4):1111–1123
Korinek J, Wang J, Sengupta PP, Miyazaki C, Kjaergaard J, McMahon E, Abraham TP, Belohlavek M (2005) Two-dimensional strain--a Doppler-independent ultrasound method for quantitation of regional deformation: validation in vitro and in vivo. Journal of the American Society of Echocardiography 18(12):1247–1253
Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, Støylen A, Ihlen H, Lima JA, Smiseth OA, Slørdahl SA (2006) Noninvasive myocardial strain measurement by speckle tracking echocardiography. validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol 47(4):789–798
Petersen JW, Nazir TF, Lee L, Garvan CS, Karimi A (2013) Speckle tracking echocardiography-determined measures of global and regional left ventricular function correlate with functional capacity in patients with and without preserved ejection fraction. Cardiovascular ultrasound 11:20
DeVore AD, McNulty S, Alenezi F, Ersboll M, Vader JM, Oh JK, Lin G, Redfield MM, Lewis G, Semigran MJ, Anstrom KJ, Hernandez AF, Velazquez EJ (2017) Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail 19(7):893–900
Risum N, Ali S, Olsen NT, Jons C, Khouri MG, Lauridsen TK, Samad Z, Velazquez EJ, Sogaard P, Kisslo J (2012) Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. Journal of the American Society of Echocardiography 25(11):1195–1203
McVeigh ER, Zerhouni EA (1991) Noninvasive measurement of transmural gradients in myocardial strain with MR imaging. Radiology 180(3):677–683
Edvardsen T, Rosen BD, Pan L, Jerosch-Herold M, Lai S, Hundley WG, Sinha S, Kronmal RA, Bluemke DA, Lima JA (2006) Regional diastolic dysfunction in individuals with left ventricular hypertrophy measured by tagged magnetic resonance imaging--the Multi-Ethnic Study of Atherosclerosis (MESA). Am Heart J 151(1):109–114
Hor KN, Baumann R, Pedrizzetti G, Tonti G, Gottliebson WM, Taylor M, Benson DW, Mazur W (2011) Magnetic resonance derived myocardial strain assessment using feature tracking. Journal of Visualized Experiments: JoVE 48:2356
Evin M, Broadhouse KM, Callaghan FM, McGrath RT, Glastras S, Kozor R, Hocking SL, Lamy J, Redheuil A, Kachenoura N, Fulcher GR, Figtree GA, Grieve SM (2016) Impact of obesity and epicardial fat on early left atrial dysfunction assessed by cardiac MRI strain analysis. Cardiovascular Diabetology 15(1):164
Mahmod M, Pal N, Rayner J, Holloway C, Raman B, Dass S, Levelt E, Ariga R, Ferreira V, Banerjee R, Schneider JE, Rodgers C, Francis JM, Karamitsos TD, Frenneaux M, Ashrafian H, Neubauer S, Rider O (2018) The interplay between metabolic alterations, diastolic strain rate and exercise capacity in mild heart failure with preserved ejection fraction: a cardiovascular magnetic resonance study. Journal of Cardiovascular Magnetic Resonance 20(1):88
Borlaug BA, Kass DA (2011) Ventricular-vascular interaction in heart failure. Cardiology clinics 29(3):447–459
Borlaug BA, Jaber WA, Ommen SR, Lam CS, Redfield MM, Nishimura RA (2011) Diastolic relaxation and compliance reserve during dynamic exercise in heart failure with preserved ejection fraction. Heart 97(12):964–969
Gillebert TC, Leite-Moreira AF, De Hert SG (2000) Load dependent diastolic dysfunction in heart failure. Heart Fail Rev 5:345–355
Bombardini T, Costantino MF, Sicari R, Ciampi Q, Pratali L, Picano E (2013) End-systolic elastance and ventricular-arterial coupling reserve predict cardiac events in patients with negative stress echocardiography. Biomed Res Int 2013:235194
Chen CH, Fetics B, Nevo E, Rochitte CE, Chiou KR, Ding PA, Kawaguchi M, Kass DA (2001) Noninvasive single-beat determination of left ventricular end-systolic elastance in humans. J Am Coll Cardiol 38(7):2028–2034
Gayat E, Mor-Avi V, Weinert L, Yodwut C, Lang RM (2011) Noninvasive quantification of left ventricular elastance and ventricular-arterial coupling using three-dimensional echocardiography and arterial tonometry. Am J Physiol Heart Circ Physiol 301(5):H916–H923
Wong RC, Dumont CA, Austin BA, Kwon DH, Flamm SD, Thomas JD, Starling RC, Desai MY (2010) Relation of ventricular-vascular coupling to exercise capacity in ischemic cardiomyopathy: a cardiac multi-modality imaging study. The International Journal of Cardiovascular Imaging 26(2):151–159
Witschey WR, Contijoch F, McGarvey JR, Ferrari VA, Hansen MS, Lee ME, Takebayashi S, Aoki C, Chirinos JA, Yushkevich PA, Gorman JH, Pilla JJ, Gorman RC (2014) Real-time magnetic resonance imaging technique for determining left ventricle pressure-volume loops. The annals of thoracic surgery 97(5):1597–1603
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschöpe C (2011) Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4(1):44–52
Desai S, Haines P, Zamani P, Konda P, Shiva-Kumar P, Peddireddy S, Shekhar RC, Jain S, Kers S, Ferrari V, Chirinos J (2018) Diffuse myocardial fibrosis is greatly elevated in males with heart failure with reduced ejection fraction, but not heart failure with preserved ejection fraction. J Am Coll Cardiol 63(12 Supplement):A967. https://doi.org/10.1016/s0735-1097(14)60967-0
Schelbert EB, Fridman Y, Wong TC, Abu Daya H, Piehler KM, Kadakkal A, Miller CA, Ugander M, Maanja M, Kellman P, Shah DJ, Abebe KZ, Simon MA, Quarta G, Senni M, Butler J, Diez J, Redfield MM, Gheorghiade M (2017) Temporal relation between myocardial fibrosis and heart failure with preserved ejection fraction: association with baseline disease severity and subsequent outcome. JAMA cardiology 2(9):995–1006
Su MY, Lin LY, Tseng YH, Chang CC, Wu CK, Lin JL, Tseng WY (2014) CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. JACC Cardiovascular imaging 7(10):991–997
Duca F, Kammerlander AA, Zotter-Tufaro C, Aschauer S, Schwaiger ML, Marzluf BA, Bonderman D, Mascherbauer J (2016) Interstitial fibrosis, functional status, and outcomes in heart failure with preserved ejection fraction: insights from a prospective cardiac magnetic resonance imaging study. Circulation, cardiovascular imaging 9(12):e005277
Helm PA, Caravan P, French BA, Jacques V, Shen L, Xu Y, Beyers RJ, Roy RJ, Kramer CM, Epstein FH (2008) Postinfarction myocardial scarring in mice: molecular MR imaging with use of a collagen-targeting contrast agent. Radiology 247(3):788–796
Tassali N, Bianchi A, Lux F, Raffard G, Sanchez S, Tillement O, Crémillieux Y (2016) MR imaging, targeting and characterization of pulmonary fibrosis using intra-tracheal administration of gadolinium-based nanoparticles. Contrast media and molecular imaging 11(5):396–404
Caravan P, Das B, Dumas S, Epstein FH, Helm PA, Jacques V, Koerner S, Kolodziej A, Shen L, Sun WC, Zhang Z (2007) Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angewandte chemie 46(43):8171–8173
Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ (2005) Endothelial function and dysfunction. Part I: Methodological issues for assessment in the different vascular beds. a statement by the Working Group on Endothelin and Endothelial Factors of the European Society of Hypertension. J Hypertens 23(1):7–17
Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, Ganz P (1986) Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med 315(17):1046–1051. https://doi.org/10.1056/NEJM198610233151702
Felmeden DC, Lip GY (2005) Endothelial function and its assessment. Expert Opin Investig Drugs 14(11):1319–1336. https://doi.org/10.1517/13543784.14.11.1319
Berman DS, Kiat H, Van Train KF, Friedman J, Garcia EV, Maddahi J (1990) Comparison of SPECT using technetium-99m agents and thallium-201 and PET for the assessment of myocardial perfusion and viability. American Journal of Cardiology 66(13):72E–79E
Thackeray JT, Radziuk J, Harper ME, Suuronen EJ, Ascah KJ, Beanlands RS, Dasilva JN (2011) Thackeray JT, Radziuk J, Harper ME, Suuronen EJ, Ascah KJ, Beanlands RS, Dasilva JN. Cardiovascular Diabetology 10:75
Schwaiger M, Kalff V, Rosenspire K, Haka MS, Molina E, Hutchins GD, Deeb M, Wolfe EJ, Wieland DM (1990) Noninvasive evaluation of sympathetic nervous system in human heart by positron emission tomography. Circulation 82(2):457–464
Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF (2018) Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 39(10):840–849
Srivaratharajah K, Coutinho T, deKemp R, Liu P, Haddad H, Stadnick E, Davies RA, Chih S, Dwivedi G, Guo A, Wells GA, Bernick J, Beanlands R, Mielniczuk LM (2016) Reduced myocardial flow in heart failure patients with preserved ejection fraction. Circ Heart Fail 9(7):e002562
Gillies RJ, Bhujwalla ZM, Evelhoch J, Garwood M, Neeman M, Robinson SP, Sotak CH, Van Der Sanden B (2000) Applications of magnetic resonance in model systems: tumor biology and physiology. Neoplasia 2(1–2):139–151
Ganesh T, Estrada M, Yeger H, Duffin J, Margaret Cheng H-L (2017) A non-invasive magnetic resonance imaging approach for assessment of real-time microcirculation dynamics. Sci Rep 7(1):7468. https://doi.org/10.1038/s41598-017-06983-6
Ganesh T, Zakher E, Estrada M, Cheng HL (2018) Assessment of microvascular dysfunction in acute limb ischemia-reperfusion injury. Journal of magnetic resonance imaging 49(4):1174–1185
Rischpler C, Dirschinger RJ, Nekolla SG, Kossmann H, Nicolosi S, Hanus F, van Marwick S, Kunze KP, Meinicke A, Götze K, Kastrati A, Langwieser N, Ibrahim T, Nahrendorf M, Schwaiger M, Laugwitz K-L (2016) Prospective evaluation of 18F-fluorodeoxyglucose uptake in postischemic myocardium by simultaneous positron emission tomography/magnetic resonance imaging as a prognostic marker of functional outcome. Circulation: Cardiovascular Imaging 9(4):1–13
Werner RA, Wakabayashi H, Bauer J, Schütz C, Zechmeister C, Hayakawa N, Javadi MS, Lapa C, Jahns R, Ergün S, Jahns V, Higuchi T (2018) Longitudinal 18F-FDG PET imaging in a rat model of autoimmune myocarditis. Eur Heart J Cardiovasc Imaging 20(4):467–474
Funding sources
This work was supported in part by grants from the Natural Sciences and Engineering Research Council of Canada (#355795) and the Ted Rogers Centre for Heart Research. S. L. is funded by a Heart & Stroke Richard Lewar Centre of Excellence Studentship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Loai, S., Cheng, HL.M. Heart failure with preserved ejection fraction: the missing pieces in diagnostic imaging. Heart Fail Rev 25, 305–319 (2020). https://doi.org/10.1007/s10741-019-09836-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-019-09836-8