Abstract
Metformin remains a widely-used, first-line pharmacotherapy agent for patients with type 2 diabetes mellitus because of its efficacy, mild side effects, and affordability.
However, use of this medication has traditionally been shunned by clinicians in patient populations that are considered at risk of lactic acidosis, such as those with heart failure. The underutilization of metformin can largely be attributed to the historical stigma of its biguanide predecessor, phenformin, and its association with lactic acidosis. Despite various studies finding low rates of lactic acidosis and the United States Federal Drug Administration’s subsequent removal of heart failure from metformin’s contraindication labeling in 2006, this oral hypoglycemic remains underutilized in this patient population. In addition to reports of the safe use of metformin in the heart failure population, a multitude of studies have also additionally suggested a modest reduction in mortality and morbidity. Metformin’s role should be strongly reconsidered in the armamentarium of diabetes management in heart failure patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) has a decidedly complicated relationship with heart failure (HF), acting as both an instigator and a catalyst of disease. At one end of the spectrum, DM is estimated to increase risk of developing HF by 2.5-fold, as observed in the retrospective study in the patients of Kaiser Permanente Northwest [1]. Conversely, at the detriment of patients already diagnosed with HF, DM also worsens clinical outcomes of mortality and hospitalizations due to HF [2,3,4]. In a recent PARADIGM-HF subgroup analysis, Kristensen, et al. found that HF patients with a history of DM and a new DM diagnosis, both had worse outcomes compared to nondiabetics (hazard ratio [HR] 1.64, 95% confidence interval [CI] 1.44–1.88, p < 0.001; HR 1.39, 95% CI 1.18–1.64, p < 0.001, respectively) [5]. Given this deleterious correlation between these two disease states, prevention and treatment of DM is of paramount importance to prevent mortality and morbidity in HF patients.
Metformin, a member of the biguanide class, has traditionally been regarded as first-line pharmacotherapy for DM because of its affordability, favorable safety profile, and proven clinical benefits [6, 7]. However, the narrative has been different for HF patients, owing to concerns about metformin-associated lactic acidosis (LA). This condition, characterized by lactate levels ≥ 5 mmol/L and arterial pH < 7.35 and attributed to metformin overdosing and/or accumulation secondary to renal failure, is potentially life-threatening, requiring close vigilance and management in the hospital setting [8,9,10]. While it is a severe and undesired adverse event, the risk of developing metformin-associated LA are often overstated, which, in the opinion of the authors of this article, can be largely attenuated with judicious use of the medication. In this review article, we seek to investigate the historical basis of the contraindication of metformin in HF, explore mechanisms of pathophysiology for lactic acidosis, and examine the evidence of utilizing this agent in HF patients.
Historical background of biguanides in heart failure
Fear of metformin-associated LA originated from the clinical experience with phenformin several decades ago. Initially, the sole biguanide approved in the USA in the 1950s, phenformin, was eventually estimated to be implicated in 40 to 64 cases per 100,000 patient-years, representing a rate of four to six times higher than that of diabetics not treated with the drug [8, 11]. With mounting evidence of phenformin-associated LA, the medication was ultimately withdrawn from the market in 1976. However, an unintended consequence of this was the stigmatization of metformin, thus dampening the enthusiasm for pursuing Food and Drug Administration (FDA) approval in the USA [8, 10, 11].
Nineteen years after phenformin’s removal, the United States FDA approved metformin for use. Another setback to widespread adoption of the antihyperglycemic agent, however, came on November 6, 1997 when the FDA added “congestive heart failure requiring treatment” to the contraindications of the product labeling. Between May 1995 and June 1996, 47 patients were diagnosed with metformin-associated LA and confirmed to have lactate levels exceeding 5 mmol/L, 18 (38%) of whom carried a diagnosis of HF [12]. It was only until 2006 that the FDA removed HF as a contraindication, instead urging cautious use in patients with acute or unstable HF [11]. Despite these revised recommendations, some clinicians still shy away from metformin because of its predecessor’s troubled history.
Pharmacology of biguanides and implications on lactic acidosis
Inhibition of gluconeogenesis, one of the biguanides’ class effects, is implicated in the development of lactic acidosis. Under normal physiological conditions, pyruvate plays a central role as a substrate for numerous metabolic processes: relevant to this discussion, the Krebs Cycle and gluconeogenesis. Biguanides inhibit the function of three different intracellular targets: pyruvate carboxylase, pyruvate dehydrogenase, and complex 1 in the mitochondrial electron transport chain (see Fig. 1) [8, 10, 13, 14]. Inhibition of pyruvate carboxylase prevents the formation of oxaloacetate, a substrate important in both gluconeogenesis and the Krebs Cycle. By inhibiting pyruvate dehydrogenase, this impacts the production of acetyl co-enzyme A, which further hampers entry into the Krebs Cycle. Regeneration of NAD+ from NADH via the mitochondrial electron transport chain is also affected by biguanides’ inhibition of complex 1. The ultimate result from multiple hindrances in pyruvate’s metabolic pathways is the promotion of an environment favorable to shunting substrates towards lactate formation, mediated by lactate dehydrogenase and elevated levels of NADH. Further adding the proverbial fuel to the fire, gastrointestinal adverse effects of nausea, vomiting, and subsequent lack of appetite at supratherapeutic levels of biguanides induce a state of “starvation,” resulting in lower levels of basal insulin. This in turn leads to protein and fat catabolism, which provides more available substrate for pyruvate formation and promotes ketogenesis in a state of hyperlactatemia.
Pyruvate is a substrate that is central to many processes involved in lactic acidosis. Inhibition of pyruvate carboxylase and pyruvate dehydrogenase impairs the ability to enter into the Krebs Cycle, while the former additionally prevents effective gluconeogenesis. NADH imbalances (not depicted) further decrease entry into the Krebs Cycle. Especially at supratherapeutic levels of biguanides, gastrointestinal adverse effects may lead to a “starvation” state in the body, which results in protein and fat catabolism thus adding more substrates for pyruvate formation and ketones
Given the historical background of phenformin use and complex pathophysiology of LA, it comes as no surprise that metformin has inherited the stigma of its predecessor. However, it is important to highlight the striking difference in biguanide-associated LA rates between phenformin and metformin. Later studies estimated that metformin-associated LA occurred between 3 and 9 cases per 100,000 patient-years, which is interestingly similar in incidence for diabetics who are not treated with metformin [8, 11]. In a large meta-analysis of 96,295 diabetics comparing metformin and non-metformin treatment, it was found that the former cohort had fewer cases of LA than the latter (4.3 cases per 100,000 patient years vs. 5.4 cases per 100,000 patient years) [15]. In other words, there is little cause to believe that metformin is linked to excess cases of LA.
Perhaps then, the difference in rates of biguanide-associated lactic acidosis can be explained by the pharmacological properties of each respective drug (Table 1). Unlike metformin, phenformin undergoes hepatic metabolism by debrisoquin, forming 4-hydroxy-phenformin; however, approximately 8% of British whites have this genetic polymorphism [10, 13, 16]. In a study of eight healthy volunteers in 1983, four “poor metabolizers”—i.e., those with debrisoquin polymorphism—were observed to have elevated peak concentrations and drug exposure compared to the four “extensive metabolizers” (Cmax: 152.2 ± 12.7 vs. 99.8 ± 13.7 ng/mL; 8-h area-under-curve: 779 ± 99 vs. 549 ± 47 ng hr/mL) [16]. Further corroborating the relation between this genetic polymorphism and LA, the investigators also found higher blood lactate levels in the “poor metabolizers” than “extensive metabolizers,” with levels above 0.6 mmol/L in the former and levels below 0.6 mmol/L in the latter over an 8-h observation period. Comparatively, metformin does not undergo hepatic metabolism and is predominantly removed from the body via renal elimination pathways. Another unfavorable characteristic also includes the plasma half-life of phenformin at around 9 to 12 h, compared to 6.2 h for metformin [9, 13, 17]. Phenformin also has stronger binding affinity for complex 1 in the mitochondrial chain and inhibits peripheral glucose oxidation, whereas metformin does not [8, 10, 13]. It may be for these pharmacological advantages that metformin has had lower incidences of LA compared to its predecessor.
Evidence of metformin use in HF: safety perspective
Although there is limited literature focusing exclusively on HF populations, metformin use appears to be safe. While the 47 reports of LA between May 1995 and June 1996 became inevitably entwined with HF, later studies suggest there is weak evidence that metformin causes LA in HF patients. A large observational database study comparing different combinations of sulfonylureas, metformin, and insulin was conducted in Danish HF patients from 1997 to 2006, where metformin was used in 2952 (27%) patients [18]. While the primary focus of the study was on all-cause mortality among different treatment regimens, no hospitalizations or deaths due to metformin-associated LA were reported. However, it is noteworthy that concurrent renal disease was low at 2%. No other safety outcomes were reported. In another study in Spain between January 1, 2001 and December 31, 2009, Romero, et al. reviewed the national database for patients with HF and new-onset DM, identifying 592 patients taking metformin and 592 patients who were not prescribed metformin [19]. Over a median follow-up period of 56.9 months, the authors reported no events of metformin-associated LA occurring in the overall cohort. In contrast with the Danish retrospective analysis, 29.6% patients taking metformin had concomitant renal disease.
In two case series of diabetics who experienced LA, metformin usage appears to be a coincidence, rather than the culprit of the acute episode. In fact, there was poor correlation between mortality from LA in metformin users and both serum concentrations of metformin and lactate, suggesting other risk factors resulted in severity of hyperlactatemia and mortality. In 49 French patients, Lalau and Race compared the metformin serum concentrations in the survivors (n = 27) and the deceased (n = 22), which were 20.6 and 6.3 mcg/mL, respectively [20]. If solely focusing on the 15 patients with HF, the median metformin serum levels were 25.1 and 4.4 mcg/mL in the survivors (n = 10) and the deceased (n = 5) (see Fig. 2). In a literature search summarizing 47 LA cases, Stades and colleagues also revealed similar findings, with the 32 survivors reflecting elevated metformin concentrations compared to the 15 deceased patients (37.4 vs. 4.9 mcg/mL, respectively) [21]. Further analysis showed that neither plasma metformin or lactate levels correlated with mortality (p = 0.16 and 0.19, respectively). Upon closer inspection of 23 patients with chronic cardiovascular conditions (the authors did not separately identify HF patients), median metformin levels were 23.2 and 4.1 mcg/mL in the survivors (n = 4) and the deceased (n = 3), respectively. Instead, the authors concluded that a combination of other factors likely resulted in hyperlactatemia.
A comparison of median metformin and lactate levels in HF patients who developed LA in the Lalau, et al. and Stades, et al. case series is depicted here. The average metformin serum levels were typically higher in patients who recovered from LA, compared to those who did not survive. On the other hand, lower lactate levels were observed in survivors than in those who perished from LA. Asterisks denote that data was not provided/available for certain patients
While these studies were observational in nature, the data revealed no reports of metformin-associated LA in these large cohorts of patients, suggesting that metformin can be used safely in HF patients. Even in clinical scenarios where metformin was suspected to be the offending agent, severity of LA and subsequent mortality did not appear to be related to the drug. For these reasons, metformin is likely safe for HF patients.
Evidence of metformin use in HF: Efficacy perspective
A review of the current literature suggests metformin may have a beneficial effect on clinical outcomes in patients with concurrent HF and DM.
Mortality
An early case-control study from the United Kingdom General Practice Research Database evaluated patients older than 35 years of age with newly diagnosed HF and DM between 1988 and 2007 [22]. The investigators found that metformin monotherapy was associated with reduction in all-cause mortality compared to other treatment modalities in diabetics with HF (adjusted odds ratio [OR] 0.65, 95% CI 0.48–0.87). Even when metformin was combined with other pharmacotherapy agents, metformin-containing regimens had lower rates of mortality than antihyperglycemic regimens lacking metformin (adjusted OR 0.72, 95% CI 0.59–0.90). A US observational study between 1998 and 2001 echoed similar observations in discharged elderly Medicare beneficiaries with concurrent HF and DM, where metformin therapy reduced the risk of all-cause mortality by 14% compared to regimens utilizing sulfonylureas or insulin (HR 0.86, 95% CI 0.78–0.97) [23]. In a study of 2874 American veterans with concurrent HF and DM, metformin use was associated with a 24% relative reduction in 2-year mortality (16.1 vs. 19.8%; adjusted HR 0.76, 95% CI 0.63–0.92, p < 0.01) [24].
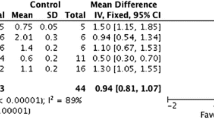
The most recent meta-analysis by Crowley, et al. suggests that metformin in HF patients can improve clinical outcomes in all-cause mortality as well as cardiovascular mortality [25]. Metformin use was associated with a 22% reduction in all-cause mortality based on the combined data from 35,410 patients across 11 observational studies (HR 0.78, 95% CI 0.71–0.87, Q = 26.6 [p = 0.003], I 2 = 62.3%). When specifically evaluating cardiovascular mortality (n = 6486), metformin treatment exhibited a non-significant reduction compared to other antidiabetic medications (HR 0.77; 95% CI 0.53–1.12; Q = 7.8 [p = 0.02]; I 2 = 74.3%). However, the heterogeneity was substantial, which influences the interpretation of the results.
Renal dysfunction also plays an important role in clinicians’ decision to utilize metformin in their diabetic patients with concurrent HF. In the subgroup analysis from the Veterans Administration study, Aguilar and his colleagues found metformin was associated with a significant reduction in all-cause mortality for patients with an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 (adjusted HR 0.70, 95% CI 0.52–0.94), whereas patients with eGFR ≥ 60 mL/min/1.73 m2 did not demonstrate benefit (adjusted HR 1.00, 95% CI 0.75–1.35) [24, 26]. Given the discrepancy in these two cohorts, metformin appears to, at a minimum, not increase harm in patients with impaired renal function and carries potential benefit (p = 0.09 for interaction). In a 2013 meta-analysis, Eurich, et al. determined in a cohort of patients with HF, DM, and chronic renal insufficiency that less metformin users died compared to their counterparts not taking metformin (pooled adjusted risk estimate: 0.81; 95% CI 0.64–1.02; p = 0.08) [26]. While the difference in mortality rates was not statistically significant, these findings challenge the notion of metformin causing harm in the setting of renal dysfunction. While the characteristics of HF and chronic renal insufficiency were evaluated separately in an international DM registry analysis, lower rates of all-cause mortality were also seen in both the HF and eGFR 30–60 mL/min/1.73 m2 subgroups (HR 0.69, 95% CI 0.54–0.90, p = 0.006; HR 0.64, 95% CI 0.48–0.86, p = 0.003, respectively) [27]. The results from the Swedish National Diabetes Register also support these findings, as patients with eGFR between 45 and 60 mL/min/1.73 m2 experienced less deaths with metformin-based regimens than those treated with other modalities (HR 0.87, 95% CI 0.77–0.99) [28].
Hospitalization
Overall, the data from available clinical studies corroborate with the notion that metformin may have beneficial effects on morbidity. The investigators in a Spanish database study found that metformin users were admitted for HF at a rate of 14.7 ± 3.3%, compared to non-metformin users at a rate of 16.5 ± 3.6% (p = 0.003) [19]. Masoudi, et al. also observed similar trends: metformin patients had a lower likelihood of HF readmission than their counterparts who did not take metformin (adjusted HR 0.92, 95% CI 0.86–0.99), while having a nonsignificant reduction in all-cause readmissions (adjusted HR 0.94, 95% CI 0.89–1.01) [23]. Meanwhile, Aguilar and his co-investigators observed no difference in HF readmission (adjusted HR 0.93, 95% CI 0.74–1.18, p = 0.56) or all-cause readmission (HR 0.94, 95% CI 0.83–1.07, p = 0.35) between metformin-treated patients and patients receiving alternate agents [24]. After conglomerating the data across four studies from 27,050 patients, Crowley and his colleagues found metformin was associated with reducing HF hospitalizations by 13% (HR 0.87, 95% CI 0.78–0.97, Q = 11.7 [p = 0.009], I 2 = 74.3%) [25]. Another recent database analysis also found metformin, compared to sulfonylureas, having lower rates of the primary outcome (defined as a composite endpoint of cardiovascular death or hospital admission due to HF) that were primarily driven by hospitalization events [29]. In the subset consisting of 8128 patients with a baseline history of HF, 397 primary outcome events occurred in patients taking sulfonylureas, compared to 307 in their metformin counterparts (adjusted HR 1.39, 95% CI 1.20–1.62).
From the available literature involving this subset of the diabetic population, the evidence collectively suggests that at a minimum, metformin is not harmful to diabetics with concurrent HF and may even be beneficial in reducing mortality and morbidity. However, caution must be exercised in its interpretation. While these retrospective, observational studies and meta-analyses prove to be a valuable resource in the absence of rigorously tested data, their study designs and subsequent results are subject to confounders and heterogeneity that obfuscates our ability to delineate the true effects of metformin in a patient having both HF and DM. Another unaddressed gap in the literature is the appropriateness of metformin in the setting of renal insufficiency. Some data suggests that eGFR above 30 or 45 mL/min/1.73 m2 would be beneficial. Further muddying the waters are the advances in HF management across the timespan covered in these articles, with some patient data originating in the late 1980s. Robustly designed, prospective, randomized clinical trials in the modern setting of optimal HF treatment are needed to truly elucidate metformin’s efficacy in this patient population.
Appropriate use of metformin in HF
Based on the evidence provided earlier in this article, having a medical history of HF does not necessarily preclude patients from incorporating metformin into their medication regimens. However, clinicians should remain wary of severe renal insufficiency, i.e., eGFR < 45 mL/min/1.73 m2. While the human body possesses innate lactate clearance via the liver (~ 60%) and kidneys (~ 30%), potential situations increasing the risk of metformin-associated LA should alarm the clinician to discontinue the medication, i.e., lactate overproduction or impaired lactate elimination [8]. Situations that warrant closer monitoring include, but are not limited to those where hypoxia may be a concern (i.e., sepsis and inadequate organ perfusion during acute decompensated heart failure), hepatic impairment (i.e., impaired lactate clearance), and renal impairment (i.e., impaired metformin clearance, leading to lactate overproduction). At the behest of the authors of this article, safe prescribing practices for metformin should follow the recommendations from the FDA (Table 2) and the available clinical evidences are summarized above [9, 30].
Conclusion
In the absence of randomized clinical trials, current evidence from retrospective studies suggests that metformin is a viable treatment option for patients with concurrent HF and DM, and perhaps even renal insufficiency. The concern for drug-induced LA largely stems from phenformin’s storied past and unfairly dismisses metformin from the armamentarium of oral hypoglycemics that are compatible with HF patients. While risk factors for metformin-associated LA should not be overlooked, a multitude of studies report low rates of this life-threatening complication. Furthermore, numerous efficacy analyses suggest that HF patients derive a modest benefit in mortality and morbidity from metformin treatment. With the inherent low risks of LA and possible mortality and morbidity reduction, we believe that this embargo on metformin treatment in HF patients should be lifted, and metformin should be used judiciously in eligible candidates.
References
Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB (2004) The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care 27(8):1879–1884. https://doi.org/10.2337/diacare.27.8.1879
Nasir S, Aguilar D (2012) Congestive heart failure and diabetes mellitus: balancing glycemic control with heart failure improvement. Am J Cardiol 110(9 Suppl):50B–57B. https://doi.org/10.1016/j.amjcard.2012.08.031
Targher G, Dauriz M, Laroche C, Temporelli PL, Hassanein M, Seferovic PM, Drozdz J, Ferrari R, Anker S, Coats A, Filippatos G, Crespo-Leiro MG, Mebazaa A, Piepoli MF, Maggioni AP, Tavazzi L, investigators E-HHL-TR (2017) In-hospital and 1-year mortality associated with diabetes in patients with acute heart failure: results from the ESC-HFA heart failure long-term registry. Eur J Heart Fail 19 (1):54–65. doi:https://doi.org/10.1002/ejhf.679
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr., Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL, American College of Cardiology Foundation/American Heart Association Task Force on Practice G (2013) 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 128 (16):e240–e327. doi:https://doi.org/10.1161/CIR.0b013e31829e8776
Kristensen SL, Preiss D, Jhund PS, Squire I, Cardoso JS, Merkely B, Martinez F, Starling RC, Desai AS, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, McMurray JJ, Packer M, Investigators P-H, Committees (2016) Risk related to pre-diabetes mellitus and diabetes mellitus in heart failure with reduced ejection fraction: insights from prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial. Circ Heart Fail 9 (1). doi:https://doi.org/10.1161/CIRCHEARTFAILURE.115.002560
Chamberlain JJ, Herman WH, Leal S, Rhinehart AS, Shubrook JH, Skolnik N, Kalyani RR (2017) Pharmacologic therapy for type 2 diabetes: synopsis of the 2017 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med 166(8):572–578. https://doi.org/10.7326/M16-2937
Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo-Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE (2017) Consensus statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the comprehensive type 2 diabetes management algorithm—2017 executive summary. Endocr Pract 23(2):207–238. https://doi.org/10.4158/EP161682.CS
DeFronzo R, Fleming GA, Chen K, Bicsak TA (2016) Metformin-associated lactic acidosis: current perspectives on causes and risk. Metabolism 65(2):20–29. https://doi.org/10.1016/j.metabol.2015.10.014
Glucophage® [prescribing information] (2017) Princeton, NJ: Bristol-Myers Squibb Company
Bailey CJ, Turner RC (1996) Metformin. N Engl J Med 334(9):574–579. https://doi.org/10.1056/NEJM199602293340906
Swift TMM (2009) Metformin use in patients with diabetes and heart failure: cause for concern? Diabetes Spectrum 22(1):18–20. https://doi.org/10.2337/diaspect.22.1.18
Center for Drug Evaluation and Research (1996) Supplemental new drug application approval for Glucophage®. Federal Drug Administration https://wwwaccessdatafdagov/drugsatfda_docs/nda/97/020357a_s006pdf Accessed 3 May 2017
Kwong SC, Brubacher J (1998) Phenformin and lactic acidosis: a case report and review. J Emerg Med 16(6):881–886
Dembo AJ, Marliss EB, Halperin ML (1975) Insulin therapy in phenformin-associated lactic acidosis; a case report, biochemical considerations and review of the literature. Diabetes 24(1):28–35
Salpeter SR, Greyber E, Pasternak GA, Salpeter EE (2010) Risk of fatal and nonfatal lactic acidosis with metformin use in type 2 diabetes mellitus. Cochrane Database Syst Rev 4:CD002967. https://doi.org/10.1002/14651858.CD002967.pub4
Oates NS, Shah RR, Idle JR, Smith RL (1983) Influence of oxidation polymorphism on phenformin kinetics and dynamics. Clin Pharmacol Ther 34(6):827–834
Alkalay D, Khemani L, Wagner WE, Bartlett MF (1975) Pharmacokinetics of phenformin in man. J Clin Pharmacol 15(5–6):446–448
Andersson C, Olesen JB, Hansen PR, Weeke P, Norgaard ML, Jorgensen CH, Lange T, Abildstrom SZ, Schramm TK, Vaag A, Kober L, Torp-Pedersen C, Gislason GH (2010) Metformin treatment is associated with a low risk of mortality in diabetic patients with heart failure: a retrospective nationwide cohort study. Diabetologia 53(12):2546–2553. https://doi.org/10.1007/s00125-010-1906-6
Romero SP, Andrey JL, Garcia-Egido A, Escobar MA, Perez V, Corzo R, Garcia-Domiguez GJ, Gomez F (2013) Metformin therapy and prognosis of patients with heart failure and new-onset diabetes mellitus. A propensity-matched study in the community. Int J Cardiol 166(2):404–412. https://doi.org/10.1016/j.ijcard.2011.10.141
Lalau JD, Race JM (1999) Lactic acidosis in metformin-treated patients. Prognostic value of arterial lactate levels and plasma metformin concentrations. Drug Saf 20(4):377–384
Stades AM, Heikens JT, Erkelens DW, Holleman F, Hoekstra JB (2004) Metformin and lactic acidosis: cause or coincidence? A review of case reports. J Intern Med 255(2):179–187
MacDonald MR, Eurich DT, Majumdar SR, Lewsey JD, Bhagra S, Jhund PS, Petrie MC, McMurray JJ, Petrie JR, McAlister FA (2010) Treatment of type 2 diabetes and outcomes in patients with heart failure: a nested case-control study from the U.K. general practice research database. Diabetes Care 33(6):1213–1218. https://doi.org/10.2337/dc09-2227
Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM (2005) Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation 111(5):583–590. https://doi.org/10.1161/01.CIR.0000154542.13412.B1
Aguilar D, Chan W, Bozkurt B, Ramasubbu K, Deswal A (2011) Metformin use and mortality in ambulatory patients with diabetes and heart failure. Circ Heart Fail 4(1):53–58. https://doi.org/10.1161/CIRCHEARTFAILURE.110.952556
Crowley MJ, Diamantidis CJ, McDuffie JR, Cameron B, Stanifer J, Mock CK, Kosinski A, Wang X, Tang S, Williams JW, Jr. (2016) Metformin use in patients with contraindications or precautions. VA ESP project #09-010
Eurich DT, Weir DL, Majumdar SR, Tsuyuki RT, Johnson JA, Tjosvold L, Vanderloo SE, McAlister FA (2013) Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail 6(3):395–402. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000162
Roussel R, Travert F, Pasquet B, Wilson PW, Smith SC, Jr., Goto S, Ravaud P, Marre M, Porath A, Bhatt DL, Steg PG, Reduction of Atherothrombosis for Continued Health Registry I (2010) Metformin use and mortality among patients with diabetes and atherothrombosis. Arch Intern Med 170 (21):1892–1899. doi:https://doi.org/10.1001/archinternmed.2010.409
Ekstrom N, Schioler L, Svensson AM, Eeg-Olofsson K, Miao Jonasson J, Zethelius B, Cederholm J, Eliasson B, Gudbjornsdottir S (2012) Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open 2(4). https://doi.org/10.1136/bmjopen-2012-001076
Roumie CL, Min JY, D'Agostino McGowan L, Presley C, Grijalva CG, Hackstadt AJ, Hung AM, Greevy RA, Elasy T, Griffin MR (2017) Comparative safety of sulfonylurea and metformin monotherapy on the risk of heart failure: a cohort study. J Am Heart Assoc 6(4). https://doi.org/10.1161/JAHA.116.005379
Administration FD (2016) FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function: safety announcement https://www.fda.gov/downloads/drugs/drugsafety/ucm494140.pdf. Accessed 3 May 2017
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Kuan, W., Beavers, C.J. & Guglin, M.E. Still sour about lactic acidosis years later: role of metformin in heart failure. Heart Fail Rev 23, 347–353 (2018). https://doi.org/10.1007/s10741-017-9649-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-017-9649-9