Abstract
Since its discovery in 1988, the endothelin system has been employed in multiple physiological and pathological roles. Endothelin-1 (ET-1) is not only a major regulator of vascular tone and cardiac contractility but also exerts mitogenic effects and is involved in inflammatory responses. ET-1 acts via two endothelin receptors located mainly on smooth muscle and endothelial cells through complex intracellular pathways differing between receptors and cell types. Polymorphisms of the endothelin receptor A have been associated not only with the risk in pulmonary arterial hypertension (PAH), systolic heart failure and systemic hypertension but are also of prognostic significance in dilated cardiomyopathy. Polymorphisms of endothelin receptors might lead to altered endothelin signaling and influence the response to endothelin receptor antagonist therapy in PAH in light of pharmacogenetics. This review will summarize the role of ET-1 within major cardiovascular pathologies and discuss endothelin receptor polymorphisms with special emphasis on potential therapeutic and screening implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endothelin was first described in 1988 by Yanagisawa and was found to be a strong vasoconstrictor but also mediates fundamental cellular processes, such as cell proliferation, fibrosis and inflammation [1–4]. Following the initial description of the endothelin system, there have been multiple experimental and clinical studies employing endothelins in diverse pathologies within the cardiovascular system such as systolic heart failure [5, 6] and pulmonary hypertension [7, 8]. In spite of the extent to which the endothelin system seems involved in cardiovascular pathology, its complexity remains poorly understood. Post-translational modifications of endothelin receptors as well as the existence and relevance of endothelin and endothelin receptor polymorphisms have been studied in several pathologies such as hypertension and dilated cardiomyopathy [9–11]. However, knowledge about relevance of polymorphisms of endothelin receptors in the cardiovascular system especially in sight into antagonist therapy and pharmacogenetics is very limited, and the number of patients studied to date is too small for a formal meta-analysis [11]. Endothelin receptor antagonists are one of the hallmarks of therapy for pulmonary artery hypertension today but are not effective in all patients and furthermore have failed to be of benefit in systolic heart failure for incomplete understood reasons [12–15].
This review will shortly summarize the pathophysiology of the endothelin system with focus on polymorphisms of endothelin receptors within the cardiovascular system.
Physiology of the endothelin system
Endothelin
Endothelins form a family of three peptides, ET-1, ET-2 and ET-3 each located on a distinct chromosome, 6.1 and 20, respectively [16, 17]. ET-1 is the predominant form in vivo and is produced by endothelial cells, whereas ET-2 is mainly expressed in the kidney [18] and ET-3 in the central nervous system and gastrointestinal tract [19, 20]. This review will focus on the role of ET-1.
ET-1 is encoded by EDN1 located on chromosome 6p24.1. Human and mouse EDN1 genes code for a biologically inactive large precursor protein called preproendothelin-1 (PPET1). The biologically active form is generated within the cell in a 2-step proteolytic process. A neutral endopeptidase cleaves prepro-ET-1 to generate the still inactive precursor big-ET1. Big-ET 1 is then converted to the 2,492 Da 21-amino peptide ET-1 by endothelin converting enzymes (ECE) [2, 17, 21–24]. Endothelin-1 is mainly synthesized by endothelial cells but genes encoding for endothelin are also found in several other cell types including vascular smooth muscle cells, bronchial epithelium, cardiomyocytes, glial cells, macrophages and glomerular mesangium [25–27].
Stimuli for ET-1 expression and release from endothelial cells are ET-1, angiotensin II, catecholamines, cardiothrophin-1, thrombin, growth factors, cytokines, free radicals, insulin, hypoxia and sheer stress [19, 28–32].
Endothelin synthesis is inhibited by nitric oxide, natriuretic peptides and prostaglandins. Inhibition of synthesis by nitric oxide and prostacyclines is of special interest since these factors are down regulated in pulmonary hypertension [33, 34]. ET-1 is secreted by endothelial cells mostly at the basal side resulting in much higher concentrations within the vascular wall compared to plasma levels [35]. The quasi irreversible feature of binding to its receptors followed by clearance in the pulmonary circulation via endothelin B receptors further reduces plasma levels, and in normal states, there is no or a minimal pulmonary arteriovenous ET-1 gradient. There is quantitatively similar production and release of ET-1 by the lung [32, 36]. ET-1 therefore acts as a para- and autocrine hormone, and it is released continuously mostly from endothelial cells. ET-1 is a major contributor in regulation of vascular tone and has almost unparalleled vasoconstrictive potential [37, 38]. Besides the regulation of vascular tone, ET-1 is pro-inflammatory via induction of cytokines and adhesion molecules such as vascular cell adhesion molecule (VCAN-1), thrombogenic via induction of platelet endothelial cell adhesion molecule (PECAM-1) and mitogenic on VSMC and fibroblasts [39–42].
ET-1 exerts positive inotropic effects in healthy hearts, whereas its role in contractility in dilated or hypertrophied hearts is debated [43]. However, it is known that ET-1 triggers cardiac hypertrophy via several pathways including cell cycle regulator mitogen-activated protein kinase (MAPK), vascular growth factor and tumor growth factor [44–46].
In the kidney, ET-1 controls glomerular filtration rate but it is debated whether ET-1 predominantly contracts the afferent or efferent arterioles and multiple studies investigated the role of ET-1 on water and sodium reabsorption. Vignon-Zellweger et al. [38] recently reviewed the topic and concluded that ET-1 constricts arterioles via ETA and thereby reduces renal and cortical blood flow but on the other hand enhances medullary blood flow via ETB. Speed et al. also concluded that ET-1 has different effects depending upon the area of production within the kidney. Cortical ET-1 not only causes hypertension via increase in vascular resistance and reducing glomerular filtration but also increases sodium reabsorption. In contrast medullary ET-1 has anti-hypertensive effects via direct inhibition of sodium reabsorption in the collecting duct and the mentioned increased blood flow via ETB [47]. Figure 1 summarizes ET-1 effects.
The various major roles of endothelin-1 in cardiovascular pathologies will be discussed later in this review.
Endothelin receptors
The endothelin receptors A and B (ETA, ETB) were first described in 1990 by Arai et al. [48] and Sakurai et al. [49], respectively. The receptors are members of the heptahelical G-protein-coupled receptor superfamily, range from 45 to 50 kDa in size [19] and are encoded by distinct genes located on chromosomes 4 and 13 [50]. EDNRA is encoding for ETA and maps to chromosome 4q31.22, includes over 63.97 kb of genomic DNA and eight exons with 4 major haplotype blocks [11]. EDNRB encoding for ETB is located on chromosome 13 and has 7 exons and 6 introns; it is 442 amino acid long and has about 59 kDa molecular mass [51]. The receptors are characterized by 60 % amino acid identity and high preservation across mammalian species (85–90 %) [19, 50]. ETA and ETB consist of a long extracellular amino-terminal domain, seven loops of membrane-spanning domains, and an intracellular carboxy-terminus. The C-terminal tail and the third cytoplasmic loop contain several putative phosphorylation sites. The Asp–Arg–Tyr motif in the second cytoplasmic loop is highly conserved among species and is thought to be involved in coupling to G-proteins [32, 52].
Endothelin receptors expressed on vascular smooth muscle cells (VSMC) have synergistic effects and mediate vasoconstriction and cell proliferation when stimulated [53–56]. ETA is primarily localized on VSMC [57] in systemic and pulmonary vessels, whereas ETB is expressed on endothelial cells and on VSMC. Depending on the effector cell type, two ETB functions are known: vasoconstriction on VSMC and vasodilation on endothelial cells. Under physiological conditions, stimulation of ETB has a mild vasodilatory effect outweighing its vasoconstrictor potential. However, in pathological states, this ratio can be altered in [58, 59]. The receptors have the highest expression in lung and heart but have also been identified in numerous tissues including kidney, intestine, adrenal gland, eye and brain [55]. Within the lung, ETA and ETB are mainly located on smooth muscle cells of alveolar walls with predominant ETB [60], whereas in the large pulmonary arteries, ETA is predominant with an increasing portion of ETB in peripheral smaller vessels [61]. The predominant ET-1 has similar affinity to both ETA and ETB, while the affinity of ET-2 and ET-3 varies among receptors [48, 49]. ETA and ETB are G-protein coupled, and activation by ET-1 leads to activation of phospholipase C (PLC). PLC then leads to formation of inositol triphosphate (IP3) and diaglycerol (DAG). Intracellular Ca2+ is increased via stimulation of IP3 receptors from the reticulum as well as opening of dihydropyridine-sensitive voltage-gated transmembrane Ca2+ channels resulting in vasoconstriction [55]. DAG leads to phosphorylation of caldesmon (CAD) via protein kinase C and thereby increases the myofilament sensitivity to Ca2+. DAG can also lead to activation of proto-oncogenes such as the MAPK cascade [39, 62].
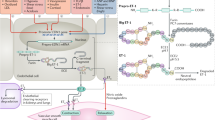
Stimulation of ETB receptors located on endothelial cells leads to activation of phosphoinositide 3-kinase (P13 K) and downstream activation of protein B kinase/Akt [63]. The P13 K/Akt pathway is responsible for activation of endothelial nitric oxide synthase (eNOS). NO on the other hand antagonizes ET synthesis via inhibition of prepro-ET-1 transcription [64]. Other vasodilatory effects following ETB activation are Ca2+/calmodulin dependent, cyclooxygenases, prostacyclins and voltage-dependant Ca2+ channels [65–69]. Figure 2 summarizes endothelin-1 synthesis and receptor signaling.
Endothelin-1 synthesis and signal conduction via its receptors. EDN-1 endothelin-1 gene, ET-1 endothelin-1, ETA endothelin receptor A, ETB endothelin receptor B, G G-protein, DAG diacylglycerol, PKC protein kinase C, CAP calponin, MAPK mitogen-activated protein kinase, IP-3 inositol 1,4,5-trisphosphate, SR sarcoplasmaic reticulum, NO nitric oxide, PGL2 prostaglandin-2, P13 K phosphoinositide-3 kinase, eNOS endothelial nitric oxide synthase, CaM calmodulin, COX-2 cyclooxygenase 2
Selective blockade of ETA enhances the NO-mediated vasodilation by ETB [70]; selective blockade of ETB consequently leads to vasoconstriction [71]. To achieve optimal inhibition of vasoconstriction in isolated human small pulmonary arteries, combined blockade of both ETA and ETB is required [72]. However, under normal circumstances, ETB does not seem to contribute significantly to pulmonary vascular tone [73, 74], and ETB removal of the endothelium does not affect ET-1 induced vasoreactivity significantly [73]. ETB induces significant vasodilation in coronary arteries of healthy pigs but not in diseased explanted human heart suggesting that under pathological circumstances, the vasodilatory role may be lost, and it even has been reported that stimulation of ETB might be detrimental via inducing cell adhesion and contraction [75, 76].
It has recently been observed that endothelin receptors form homo- and heterodimers in vitro. This could partially explain the complexity of ET-1 responses. The types of dimers (ETA/ETA, ETA/ETB, or ETB/ETB) are associated with different binding density [77]. Also there is a different functional response depending on whether homo- or heterodimers are formed: the binding of ET-1 to homodimers induces a transient increase in Ca2+ concentration, while the response mediated by heterodimers has been shown to last for several minutes [78].
Following this short review on the endothelin system, we will discuss its role within three major cardiovascular pathologies and especially the potential importance of endothelin receptor polymorphism: pulmonary hypertension, systolic heart failure and dilated cardiomyopathy as well as systemic arterial hypertension.
Pathophysiology of the endothelin system and receptor polymorphism in pulmonary artery hypertension
Endothelin system in pulmonary artery hypertension and right ventricular adaption
Pulmonary hypertension (PH) is a multifactorial disease leading to an increase in pulmonary vascular resistance secondary to endothelial dysfunction, vasoconstriction, arterial wall thickening and thrombosis [79]. PH is defined as a sustained increase in pulmonary arterial pressure to more than 25 mmHg at rest with a mean pulmonary capillary wedge pressure (PCWP) of less than 15 mmHg. The revised WHO classification of PH forms five groups: group I pulmonary artery hypertension (PAH) that includes idiopathic (IPAH), familial (FPAH) and associated with (APAH) such as congenital heart disease and connective tissue disease, drugs and toxin induced, human immunodeficiency virus (HIV), portal hypertension, hemoglobinopathies and myeloproliferative disorders. Group II is classified secondary to left heart disease; group III is associated with lung diseases and/or hypoxemia; group IV is due to chronic thrombotic and/or embolic disease and group V includes miscellaneous causes [15]. Pulmonary hypertension due to left heart failure is much more common than group I PAH and can result from any pathology leading to increased left heart filling pressures, including systolic dysfunction, diastolic dysfunction and valvular heart disease [15]. This review will focus on group I PAH.
The physiological interplay between ET-1, ETA and ETB is significantly altered in PH with increased pulmonary vascular tone. Under such circumstances, the vasodilatory role of ETB seems to be of significance and can be unmasked with selective ETB stimulation with very low dose ET-1 or specific ETB blockade. With higher ET-1 concentrations, the mild vasodilatory effect of ETB is overcome by vasoconstriction [73, 74].
Stewart et al. [80] described higher serum levels of ET-1 and higher arterial-to-venous ratios in patients with IPAH. Lung tissue from PH patients shows altered endothelin receptor expression, increased ET-1 staining and increased pro-pre-endothelin-1 (PPET-1) in pulmonary arteries with a correlation between the amount of ET-1 staining and hemodynamic parameters such as pulmonary vascular resistance [7, 81]. It is unclear whether this phenomenon results from increased ET-1 production, reduced pulmonary clearance or a combination [82]. Serum levels of ET-1 are markers of disease severity and are of prognostic value in pulmonary artery hypertension [80].
Endothelin converting enzymes are necessary to cleave ET-1 from its less active form. In patients with PH, increased levels of endothelin converting enzyme have been found [83]. Besides the idiopathic form, increased ET-1 levels have also been described for other pathologies. In pulmonary hypertension from chronic hypoxia, increase in ETA was found and treatment with combined ETA/ETB receptor blockade led to amelioration of pulmonary hypertensive changes [84, 85]. Congenital heart disease and chronic thrombembolic pulmonary hypertension are also associated with increased activity of the ET-1 system [82].
PH leads to significant increase in right ventricular (RV) afterload. The upregulated myocardial endothelin system in PH could represent a compensatory mechanism to adapt RV contractility following increased afterload [86]. The RV has to adapt resulting in concentric hypertrophy away from its crescent shape under physiological conditions leading to flattening of the interventricular septum [87]. These adaptive changes of the RV lead to development of left ventricular diastolic dysfunction secondary to the flattened septum. The consequently reduced left ventricular end-diastolic volume contributes to the reduction in stroke volume. Without effective measurements to lower the afterload, the RV will progress to dilation and failure. The reduced perfusion then can lead to RV ischemia even in the absence of coronary artery disease [88]. It is unclear why some patients with PH can maintain normal cardiac outputs over long time periods, while other patients have rapid decompensation and death [87]. The ETA and ETB antagonist bosentan and the selective ETA antagonist ambrisentan are approved for use in group I PAH. In animal studies, endothelin receptor antagonists (ETRAs) are effective in reducing pulmonary artery resistance and vascular remodeling. Bosentan was studied in patients with scleroderma-induced pulmonary hypertension resulting in improvement of 6‐minute walk test, improvement of pulmonary hemodynamics, especially cardiac index and pulmonary vascular resistance and increased time to clinical worsening [89]. Following this primary study, the BREATHE-1 trial for PAH associated with connective tissue disease confirmed the initial findings [90].
It has been reported that endothelin receptor antagonists can have a negative inotropic effect in hypertrophied right- and left ventricle and therefore could partially explain the increased rate of edema upon initiation of ETRA therapy [86].
However, it remains unclear why a certain percentage of patients do not respond to endothelin receptor antagonist therapy.
Endothelin receptor polymorphism in PAH
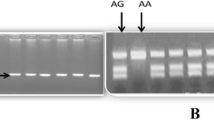
Calabro et al. were the first to study endothelin receptor polymorphisms in idiopathic pulmonary arterial hypertension (IPAH, 34.7 % of patients), PAH due to congenital heart disease (CHD) with systemic to pulmonary shunts (43.9 % of patients) and PAH due to connective tissue disease (CTD, 21.4 % of patients) in a total of 98 Caucasian patients and 100 aged BSA-matched healthy controls. The authors investigated the role of two polymorphisms:
-
EDN1: +134del/insA l
-
EDNRA: His323His
For both polymorphisms, no substantial difference in genotype and allele distribution was identified between the PAH and the control group. When analyzed for subgroups, the main finding of the study is an association between His323His TT-genotype in EDNRA and development of IPAH (the TT subtype is significant more frequently expressed in IPAH with 73.5 % carriers compared to CHD or CTD especially when homozygotic for TT compared to TC). In the overall, IPAH cohort patients with the TT-genotype showed higher pulmonary resistances (2 ± 0.6 vs. 2.3 ± 0.6; p = 0.05) and a higher pulmonary vascular resistance index (18.8 ± 9.6 vs. 14.2 ± 6.9; p = 0.01). A cardiac index < 2 l/min/m2 is a marker for poor prognosis in PAH [91]; the authors therefore hypothesize that patients with IPAH with the TT-genotype have a more aggressive disease. There was significant correlation of idiopathic disease (p = 0.01; OR = 3.8; CI = 1.3–11) and indexed pulmonary vascular resistances (p = 0.01; OR = 1.1; CI = 1–1.2) with the TT-genotype. However, no clinical correlation was found in-between the TT-genotype in IPAH likely secondary to limited sample size [92].
Previously it has been reported that the +134ins/delA polymorphism correlates with levels of endothelin-1 ex vivo; however, this was not confirmed in this study with PAH [10]. There was no difference in the +134del/insA polymorphism among different groups of patients [92]. This study of Calabro included 200 age-matched controls but the diversity of the patient’s conditions might limit the investigations power as concluded by Cattano et al. [93]; however, the study suggests a possible role for the EDNRA polymorphism in the pathogenesis of PAH but lacks demonstration of an experimental functional role.
The physiological effects of the TT-phenotype are unknown but could include altered gene expression and modified post-transcriptional processing. The polymorphism also has been studied in DCM, and it was hypothesized that His323His could affect splicing leading to production of an altered receptor. Furthermore, the His323His variant might be in linkage disequilibrium with another functional variant [9, 92].
There could be a potential link between specific genotypes in the EDNRA gene and susceptibly for PAH with worse hemodynamic profiles [92]. A failing RV is a major prognostic factor in PH. At this point, it is unclear why the compensatory capacity of the RV is so different among patients with PH.
ETRA therapy in light of pharmacogenetics with focus on barriers for clinical practice
Possibly the described polymorphisms of ETA are linked to responsiveness to endothelin receptor antagonist treatment and could thereby in part explain the different degree of RV recovery or compensation following initiation of therapy. Tailored therapy in light of pharmacogenetics therefore could potentially improve treatment results.
Furthermore, ETRAs have been reported to be negative inotropic in hypertrophied RV and patients with relatively worse RV hypertrophy could have polymorphism promoting stronger hypertrophic effects and therefore would be more sensitive to negative inotropic effects of ETRA therapy.
However, effects of endothelin receptor polymorphisms on cell signaling have not yet been studied. The complex regulation and function of the endothelin system in physiology and pathology remains poorly understood, and therefore, substantial research is required to clarify the relevance of these polymorphisms in human disease especially with focus on potential therapeutic implications. In spite of its promising concept, pharmacogenetics is not yet used widely in clinical settings. Johnson and Cavallari have extensively reviewed current implications of pharmacogenetics with special emphasis on cardiovascular disease [94].
Cardiovascular pharmacogenetics used to focus on implications for already existing therapies but now starts to involve in new drug development as the concept of personalized medicine is emerging [94]. Thus, pharmacogenetics may be used to predict response to therapy as well as drug toxicity. However, to date general acceptance of pharmacogenetics in clinical practice remains low. There are knowledge barriers such as unawareness, uncertainty how to interpret pharmacogenetic test results and necessary actions based on these results. Furthermore, logistical barriers such as turning around time when genetic information is delivered after a treatment decision was made as well as financial barriers of genetic testing. Finally, evidence barriers represent a challenge for pharmacogenetics given lack of randomized controlled trials proving improved outcome following a pharmacogenetic approach. The authors then discuss the following solution approaches: It has been suggested to obtain genetic information at one point in life and then keep available for future use to eliminate turn round time. This is also more cost-effective than genotyping for single nucleotide polymorphisms (SNP) or one gene at a time. In terms of evidence barriers, it has been argued that randomized controlled trials may not be an absolute requirement but rather the little likelihood of causing harm with pharmacogenetics compared to potential benefit [94].
In summary, pharmacogenetics has made great progress in the last decade and the implementation of genetics to tailor therapy in clinical practice appears very promising once above discussed barriers have been overcome. Implementation of endothelin receptor polymorphisms as concept of personalized medicine could be one future example.
Pathophysiology of the endothelin system and receptor polymorphism in dilated cardiomyopathy and heart failure
Endothelin system in systolic heart failure
Dilated cardiomyopathy (DCM) is characterized by biventricular chamber enlargement and systolic dysfunction that can result in progressive systolic heart failure. DCM is frequently caused by acquired factors such as ischemia, infectious agents, autoimmune disorders, alcohol excess, chemotherapeutic drugs or nutritional deficiencies [95].
The presence of left ventricular dilation and systolic dysfunction when other causes of dilated cardiomyopathy are ruled out are the hallmarks of idiopathic dilated cardiomyopathy (IDC) [96]. Inherited genetic variations form the subgroup of familial dilative cardiomyopathy (FDC). The genetic base includes a variety of genes coding for proteins compromising the nuclear envelope, the cardiac sarcomere, ion channels, transcription factors and the dystrophin-associated cytoskeletal complex [97]. DCM then potentially leads to heart failure where the endothelin system has been studied in the last two decades.
In systolic heart failure, not only plasma ET-1 levels are increased but also the myocardial endothelin axis with increased receptor expression and endothelin synthesis [5, 98]. Plasma levels of ET-1 and its precursor ET-1 are independent predictors of outcome in systolic heart failure [99, 100].
ETA and ETB receptors are expressed on cardiomyocytes and coronary vasculature. Cardiomyocytes express predominately ETA, while cardiac fibroblasts express both receptor subtypes equally [101]. ET-1 induces myocardial hypertrophy and remodeling via multiple routes including MAPK, vascular endothelial growth factor and TGF-beta pathways [44–46]. Bosentan has been shown to reduce cardiac fibrosis and hypertrophy in systolic heart failure [102, 103]. On the other hand cardioprotective effects of ET-1 have been described. Zhao et al. reported that ET-1 might prevent excessive apoptosis after cardiac overload following aortic banding [38, 104].
The effect of ET-1 and ETRAs on contractility is debated and seems to differ in healthy, hypertrophied and failing hearts [38].
-
In healthy hearts, ET-1 has a small positive inotropic effect mediated by an increase in intracellular calcium and increased sensitivity of myofilaments to calcium via the PLC/IP3 pathway as discussed earlier [105, 106]. This small positive inotropic effect is not affected by bosentan in healthy hearts [102].
-
In hypertrophied hearts, Piuhola et al. [102] describe a significant role for ET-1 in maintaining cardiac function. In this setting, ET-1 and ETA are significantly upregulated and blocking the endothelin system has significant negative inotropic effects. ETRAs have negative inotropic effects in hypertrophied RV [86].
-
In systolic heart failure, McCarthey et al. [43] also described a negative inotropic effect of ET-1 that was antagonized by use of a specific ETA blocker. ET-1 has also been described as negative inotropic via ETB receptors and consecutive activation of Na+/Ca+ exchanger that is known to be overexpressed in systolic heart failure [107, 108].
The described negative inotropic effect of endothelin receptor antagonists in hypertrophied LV and RV could explain the increased rate of edema upon initiation of ETRA therapy in 25 % of patients with group I PAH [86, 102]. Despite a significant reduction in pulmonary artery pressure, there was no improvement in cardiac index described after ETRA use possibly explained by negative inotropy in this setting [109].
At this point, it is unclear why edema develops in a certain percentage of patients on ETRA therapy and potential effects on the RV need to be studied in human and experimental settings [86].
Endothelin receptor polymorphism in DCM and heart failure
Based on the evident role of the endothelin system in cardiovascular pathology, genetic variations have been studied [110, 111]. The first study on an association between endothelin receptor polymorphisms and DCM was part of the CARDIGENE group studies to identify genetic risk factors for idiopathic dilated cardiomyopathy. The following polymorphisms were investigated in French Caucasian patients [112]:
-
EDN-1: +138A insertion on exon 1, +61G/T polymorphism on exon 5
-
EDNRA: −231A/G and +1363 C/T on exon 8
-
EDNRB: +30 A/G on exon 4
A total of 433 patients with DCM LVEF < 40 % and 400 age- and gender-matched controls were included in the study. The ET-1 and ETB polymorphism did not reveal significant results but analysis of the exon 8 +1,363 C/T polymorphism in the endothelin A gene (EDNRA) showed significantly different genotype frequencies in patients and controls (p = 0.02) as well as distribution of allele frequencies (p = 0.045). This association with the T allele and the disease was secondary to an increased frequency of T/T homozygoty among DCM patients. Therefore, the polymorphism seems to have a recessive mode of action in idiopathic dilative cardiomyopathy. The odds ratio for the disease in patients with the T/T genotype in EDNRA was 1.9 (95 % CI 1.2–3.01; p < 0.006). While there was significantly increased risk in the development of DCM, there was no association between the polymorphism (CC, CT and TT) and disease severity as assessed by LVEF, left ventricular dilation or cardiac transplantation [112].
Following the identification of EDNRA polymorphism as a risk factor for the development of DCM, Herrmann et al. investigated differences in DCM (LVEF < 56 %) related cardiac phenotypes and/or clinical outcomes. Authors genotyped 125 patients with idiopathic DCM for the following polymorphisms:
-
EDN-1: T1370G, K198 N (G/T), +138ex1 ins/del
-
EDNRA: H232H (C/T), G+70C
-
EDNRB: L277L (G/A)
The EDNRA polymorphism His323His was found to be associated with a significantly shorter cumulative survival despite no baseline differences in the severity of heart failure [9]. The odds ratio to die within 2 years was 5.5 (95 % CI 1.4–2.1, p = 0.013) for T allele carriers. The His323His polymorphism was identified as predictor of survival independent of the known predictors NYHA class, left ventricular function and end-diastolic diameter [9].
Colombo et al. then for the first time studied the effect of two-combined polymorphisms in EDN-1 and EDNRA in 122 Italian patients with systolic heart failure LVEF < 40 % of ischemic or idiopathic etiology:
-
EDN1: Lys198Asn (G/T)
-
EDNRA: His323His (T/C)
In the present study, the ET-1 Lys198Asn polymorphism in patients homozygotous for Asn/Asn represents an OR of 4.3 (95 % CI 1.7–11.1, p = 0.001) for systolic heart failure. The percentage of Asn/Asn in the systolic heart failure group was 13.1 % versus 3.6 % in the control group (p = 0.005), and Asn/Asn was identified as an independent risk factor for the development of systolic heart failure (p = 0.03) [113]. The His232His polymorphism was found to be distributed equally among the groups what seems to be contradictory to the description of His323His as an independent risk factor for survival in DCM. This might be related to the chosen control group of patients hospitalized for elective valve replacements but LVEF > 50 %. However, Lys198Asn and His323His together were found to be a major risk factor for the development of systolic heart failure. After stratification for His323His, the Lys198Asn was significantly associated with the presence of heart failure in T/T carriers (43.7, 35.9, 20.3 vs. 60.0, 37.0, 3.0 %, p = 0.0009 for Lys/Lys, Lys/Asn, Asn/Asn, patients vs. controls) with an OR of 8.6 (95 % CI 1.5–48.1, p = 0.005). There were no differences in allele distribution among ischemic and idiopathic etiology of systolic heart failure [113]. The Asn/Asn SNP was also associated with significant lower LVEF 48.0 ± 0.8 vs. 39.8 ± 2.3 %, p = 0.0006) but no significant difference in left ventricular end-diastolic diameter (LVDD) and left ventricular end-systolic diameter (LVSD). When both EDN-1 Asn/Asn and EDNRA T/T SNPs were simultaneously present, LVEF and LVDD/LVSD were significantly reduced compared to Lys and C-allele carriers. Asn/Asn and TT double genotype was found to be an independent predictor of LVEF [113].
Genetic variations of the endothelin system seem to play an important role in development and deterioration of systolic heart failure although the exact mechanisms are not understood mainly in lack of functional studies. The identified SNP Lys198Asn leads to G/T transversion and a change in amino acid sequence that is, however, not located in the regulatory region of EDN-1 but in a region coding for preproendothelin-1. Colombo et al. also conclude similar to Hermann et al. that the SNP might not be functional itself but could be in linkage disequilibrium with a functional polymorphism located within the regulatory side [113]. Lys198Asn has previously been associated with higher ET-1- and blood pressure levels but controversially did not directly affect big-ET-1 production [114–116]. Therefore, the real functional significance of the SNP remains unclear [113]. His323His is located on the sixth exon of the EDNRA gene that codes for the sixth membrane-spanning domain and the third extracellular loop of the protein. The polymorphism substitutes a thymine (T) for a cytosine (C) which does not alter the amino acid sequence in contrast to the Lys198Asn. However, His323His could influence gene transcription or affect splicing leading to production of an altered receptor but as per Herman et al. [9] the existence of another functional variant is more likely. At this point, there are only clinical association studies available for both the +1,363 C/T on exon 8 and the His323His SNP in EDNRA.
It is unknown how a potentially altered receptor expression would influence ET-1 signaling in the cardiovascular system especially in pathologic states. His323His was not identified as a risk factor for the development of systolic heart failure but as an independent prognostic marker for survival in DCM [9]. Particularly interesting is the greater combined effect of Lys198Asn and His323His polymorphism with significantly higher risk of development of HF and lower LVEF representing two altered steps in endothelin effect: ET-1 production and receptor activation [113]. Genetic variations of the endothelin system therefore are not only associated with the risk of development of systolic heart failure but are of prognostic value raising the question of potential therapeutic interventions.
Charron et al. [112] discuss that a possible loss of function of ETA caused by polymorphisms could have negative effects in systolic heart failure when the endothelin system might be needed to maintain cardiac function. On the other hand, ETRA therapy can ameliorate DCM phenotypes and improve cardiac function and survival in experimental settings supporting a gain of function theory [117, 118].
The paradox disappointment of ETRAs in systolic heart failure and the potential role of endothelin receptor polymorphisms
The failure of ETRAs in clinical use for heart failure seems paradox to date especially given initial extensive evidence for potential benefit. In 1997, Muldner et al. used bosentan in a post-infarction rat model of heart failure and showed markedly increased survival. They described decrease in preload and afterload as well as an increase in cardiac output, decreased left ventricular (LV) hypertrophy, LV dilatation and cardiac fibrosis. The speculation was made that chronic treatment with ETRAs might be beneficial in systolic heart failure and could improve long-term survival [103]. Following this, one small human study used bosentan as adjunct to established heart failure therapy and was able to show short-term benefits on hemodynamic parameters [119]. Unfortunately, these very promising results could not be translated in clinical long-term use. Two large trials on the use of bosentan in symptomatic systolic heart failure were prematurely stopped because of an increase in adverse events and no improvement of symptoms [12, 13]. Following this, the non-selective intravenous antagonist tezosentan and ETA selective darusentan both demonstrated favorable hemodynamic effects with increased cardiac index and reduced pulmonary and systemic vascular resistance in a dose dependent manner in heart failure [120, 121]. However, both drugs did not provide any beneficial effect in chronic heart failure, and specific ETA-receptor antagonists seem to have the same lack of efficiency as non-selective drugs [122, 123].
While ETRAs disappointed for use in systolic heart failure, there was rising evidence for beneficial effects in pulmonary hypertension and a possible benefit from ETRAs was suspected for patients with pulmonary hypertension from left ventricular dysfunction [82]. In 2008, disappointing results were published; bosentan did not provide any measurable hemodynamic benefit and was associated with more frequent adverse events, requiring drug discontinuation in this subgroup of patients [123].
Kaluski et al. used the term “the hemodynamic paradox” for the ETRA group. These drugs have impressive initial effect on hemodynamics but no measurable long-term effect in heart failure. In addition, there is no sustained improvement in morphological or functional cardiac parameters [123]. In the majority of studies, both hemodynamic and toxic ETRA effects were directly dose related and after investigating wide dose ranges investigators chose lower doses to avoid toxicity. Directly following ETRA use, there is immediate endothelin overproduction resulting in significant increase in serum levels that usually resolves within hours [124]. Described adverse effects of ETRA use such as anemia, lower extremity edema and hypotension do not seem to be related to direct toxic effects of increased endothelin or potential interaction with neurohormonal and inflammatory pathways. Kaluski et al. speculated that the drugs or their metabolites might have direct drug toxicity such as headaches associated with high dose tezosentan therapy [123].
In 2011, Handoko et al. wrote an editorial named “The rise and fall of endothelin receptor antagonists in congestive heart failure” addressing the paradox of the failure of ETRAs to provide any clinical benefit despite promising results in multiple animal studies [14].
The authors especially commented on the results of Jiang et al. who have conducted similar experiments on the use of bosentan in an animal model of ischemic heart failure as published in 1997 by Muldner et al. Controversially, they were not able to show any benefit from bosentan in cardiac as well pulmonary remodeling and no improvement of RV hemodynamic parameters was shown in ischemic heart failure [125]. Handoko et al. [14] postulate that these results probably will end the discussion on the role of ETRAs in systolic heart failure. However, there is a recent report on 17 patients with NYHA III–IV symptoms treated with bosentan for pulmonary hypertension from left ventricular failure. Nine patients responded to therapy with improved hemodynamics and 4 became eligible for heart transplantation and 3 for left ventricular assist device implantation. In 6 patients, bosentan was discontinued because of hypotension, increased liver enzymes or acute decompensation of heart failure. The authors concluded that Bosentan decreased pulmonary pressures and PVR in the majority of patients with PH secondary to systolic HF, thereby allowing them to be considered candidates for heart transplantation [126].
Nonetheless unfortunately ETRAs have failed to be beneficial in large clinical trials leading to even more adverse outcomes in heart failure patients treated with ETRA possibly secondary to too high dosages but the exact reason for the failure of ETRAs in systolic heart failure is unknown to the best of our knowledge [122, 127].
However, EDNRA polymorphisms could potentially identify patients who could profit from ETRA therapy to antagonize an over active endothelin system in support of the gain of function theory. Nevertheless, this is only a speculative thought pending functional studies on the actual effect of EDRNA polymorphisms in receptor function and cell signaling in response to ET-1.
Although in a different setting, the endothelin-1 Lsy198Asn polymorphism has recently also been associated with transplant free survival in children with hypoplastic left heart syndrome [128]. Therefore, polymorphisms of the endothelin system could be useful as screening tools to identify high-risk patient populations and potentially identify those who might benefit from endothelin receptor antagonist therapy.
Table 1 summarizes identified endothelin receptor polymorphism and their clinical implications.
Pathophysiology of the endothelin system and receptor polymorphism in essential hypertension
Endothelin system in essential hypertension
Essential hypertension is defined as increased blood pressure of unknown etiology leading to a higher risk of cardiac, renal and cerebral events [129]. Interestingly, ET-1plasma levels were found to be predictors for the development of hypertension in a Japanese population [130]. African American hypertensive patients are known to have a salt-sensitive hypertension with particularly activated endothelin system [131].
ET-1 is a very strong vasoconstrictor and EDNRA was found to be overexpressed in arteries of hypertensive patients, and increased ET-1 production in hypertension might lead to an enhanced EDNRA-dependant vasoconstriction and consecutively ET-1-induced hypertrophic remodeling of small arteries [2, 132–134]. Thus, dual endothelin receptor blockade has been shown to decrease peripheral vascular resistance and blood pressure in healthy humans [135] but selective receptor blockade leads to different effects depending on the model of hypertension in animal studies [136, 137]. In vascular tissue of deoxycortisone acetate (DOCA)- salt hypertensive rats and Dahl salt-sensitive rats, ET-1 protein and mRNA were found to be increased contributing to the development of hypertension [138, 139]. ETA-receptor antagonist therapy and non-selective ETA/ETB-therapy have been shown to prevent hypertension induced tissue injuries in salt-sensitive animal models [140, 141].
In a different mouse model of proendothelin-1 overexpression, hypertrophic remodeling of the vascular wall was characterized by increased inflammation caused by enhanced expression of vascular cell adhesion molecules, presence of macrophages and activation of pro-inflammatory transcription factors promoting endothelial dysfunction [142]. Interestingly, these mice have increased aortic expression of ETB as compensatory mechanism and no significant hypertension. Upon exposure to a high-salt diet, these mice have a greater increase in blood pressure than wild-type mice and worsened endothelial dysfunction. These effects are lessened with ETA antagonists and worsened with ETB antagonists. This study clearly shows the different effect of ETA and ETB on vascular tone and enhances the important role of ET-1 in vascular pathology [143].
Endothelin receptor polymorphism in hypertension
About 20–40 % of the total variation in blood pressure is attributed to genetic influences especially at night [11, 144]. Rahman et al. have studied common genetic variations of EDNRA in ambulatory blood pressure. One common single nucleotide polymorphism (SNP) was selected from each of the 4 EDNRA haplotype blocks:
-
rs1801708 (referred to in previous studies as G-231A) from 5`untranslated region
-
rs1568136; from intron 2
-
rs5333 SNP (referred to in previous studies as H323H) from exon 6
-
rs5335 SNP (previously referred to as C p 211GorC p 70) from exon 8 [11].
There was a significant association between the rs5335 (C+70G) SNP and mean and diastolic blood pressure (DBP) during night and marginally less significant association with daytime systolic blood pressure (SBP), DBP and mean pressure. These associations appeared to be codominant with each C-allele counting for a percentage of lower blood pressures especially at night. Each of the phenotypes had a 1–2 % contribution to the variability of overall blood pressure what appears to be a rather small number. The CC genotype was associated with 34.7 % of hypertension within the carriers, the CG genotype with 37.9 % and the GC genotype with 42.4 %. The odds ratio for hypertension was 1.19 (95 % confidence interval 1.00–1.41; p = 0.05) per G-allele carried. This study of Rahman et al. proved that natural variations of the endothelin signal transduction pathway have significant effects on blood pressure; carriers of the SNP have a 20 % higher risk of hypertension [11].
Nicaud et al. studied the C+1363T and rs5335 (C+70 G) among other SNPs in 773 males who where controls in the ECTIM myocardial infarction study. The +1363T SNP C/T substitution was associated with modestly higher SBP (p = 0.043) and carrier of the G-allele of rs5335 had a trend toward higher SBP (p = 0.008) due to strong association between the two SNP on EDNRA exon 8. No association of other SNPs with hypertension including ETB was found in this study [110]. Benjafield et al. [145] also studied the C+1363T SNP and found an association with hypertension in 155 hypertensive patients and 245 controls.
The endothelins and consequently endothelin receptors are major regulators of vascular tone and as previously addressed ETA is overexpressed in patients with hypertension [133]. Rahman et al. [11] conclude that variation in the 3’ untranslated region of EDNRA is a significant determinant of blood pressure possibly via an effect on arterial compliance.
ETRAs: potential role in treatment of hypertension
Bosentan was studied by Krum et al. [146] in hypertensive patients and could achieve blood pressure control similar to treatment with the ACE inhibitor enalapril without activation of the rennin-angiotensin system or the sympatric nervous system. Darusentan, a selective ETA antagonist, has also been proven effective in treatment of essential hypertension without major side effects [147]. Despite the promising results for blood pressure control, ETRAs were not perused for routine treatment for essential hypertension because of potential severe side effects like liver toxicity, pulmonary vascular dilation and development of lower extremity edema also in light of lack of superiority to established regimens like ACE inhibitors [137].
In resistant hypertension, darusentan was used in a small, randomized, placebo-controlled study and resulted in significant blood pressure reduction after 10 weeks of treatment without significant adverse effects [148]. Following this, a larger trial with 379 patients confirmed the findings with substantial reduction in blood pressure with addition of darusentan to triple therapy compared to placebo [149]. Later a phase 3 clinical trial failed to show improvement of office blood pressure over placebo and led to cessation of darusentan by the manufacturer [150]. The use of ETRAs on essential and resistant hypertension seems to have failed for lack of superiority to established agents like ACE inhibitors and increased overall risk of adverse events.
However, ETRAs might be beneficial in selective groups of patients. For example, hypertension has a very high incidence in patients after solid organ transplantation [151]. Plasma endothelin levels increase after heart and kidney transplantation and are associated with allograft rejection [152, 153]. Myocardial expression of ET-1 is also increased post-transplant and seems associated with ischemic injury and subsequent development of coronary vasculopathy [153]. Calcineurin inhibitors used for immunosuppression also increase endothelin-1 plasma levels contributing to hypertension and promoting renal dysfunction after transplantation [154]. ETRAs have been used in experimental settings after cardiac transplantation with improved survival and reduced vasculopathy independent of immunosuppression [155]. Furthermore, preclinical after renal transplantation ETRAs have been shown to inhibit cyclosporin-induced hypertension and limited end-organ damage [156]. Recently, ambrisentan was proven save for use in combination with tacrolimus and mycophenolate [151].
It is now compelling to speculate if patients carrying endothelin receptor polymorphisms associated with hypertension are especially prone to development of post-transplant hypertension? If so would these patients particularly benefit from ETRA therapy not only in light of hypertension but also protection against nephropathy and vasculopathy?
Conclusion
The endothelin system is of vast importance in cardiovascular physiology and plays major roles in its pathologies. The interplay between endothelins and their receptors varies between cell types and changes in states of pathology. Polymorphisms of EDNRA have been associated with the development of group I pulmonary hypertension, dilated cardiomyopathy and essential hypertension. The polymorphisms are not only risk factors for development of these cardiovascular pathologies but are of prognostic value in systolic heart failure. Awareness for genetic variations therefore is crucial in understanding and treating pathology offering potentially valuable screening tools. The studies conducted to date have several limitations; one major is that they were mainly conducted in Caucasian European populations, and the possibility of interracial differences in relevance of endothelin receptor polymorphisms has not yet been addressed. Endothelin receptor antagonists are of great value in treatment of group I pulmonary hypertension but unfortunately have not proven to be beneficial in systolic heart failure despite promising pre-clinical results for incompletely understood reasons. Nevertheless, despite the effectiveness of ETRAs in PAH, it remains unclear why some patients do not respond to treatment. Endothelin receptor polymorphisms might alter response to antagonist therapy, and patients might benefit from tailored therapy in light of pharmacogenetics. ETRAs might be of benefit in patients with systolic heart failure carrying polymorphisms with potentially hyperactive endothelin system, and moreover, endothelin receptor polymorphism might be useful as screening tools for adverse outcome in these patients? The use of ETRAs for post-transplant hypertension might be especially effective in carriers of endothelin receptor polymorphism prone to hypertension?
At this point, basic science studies need to clarify functional effects of endothelin receptor polymorphism to understand how endothelin signaling is altered and clinical studies should evaluate ETRA therapy with respect to pharmacogenetics.
References
Mullol J et al (1996) Endothelin-1 induces GM-CSF, IL-6 and IL-8 but not G-CSF release from a human bronchial epithelial cell line (BEAS-2B). Neuropeptides 30(6):551–556
Yanagisawa M et al (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332(6163):411–415
Janakidevi K et al (1992) Endothelin-1 stimulates DNA synthesis and proliferation of pulmonary artery smooth muscle cells. Am J Physiol 263(6 Pt 1):C1295–C1301
Guarda E et al (1993) Effects of endothelins on collagen turnover in cardiac fibroblasts. Cardiovasc Res 27(12):2130–2134
Sakai S et al (1996) Endogenous endothelin-1 participates in the maintenance of cardiac function in rats with congestive heart failure. Marked increase in endothelin-1 production in the failing heart. Circulation 93(6):1214–1222
Pacher R et al (1996) Prognostic impact of big endothelin-1 plasma concentrations compared with invasive hemodynamic evaluation in severe heart failure. J Am Coll Cardiol 27(3):633–641
Giaid A et al (1993) Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med 328(24):1732–1739
Stelzner TJ et al (1992) Increased lung endothelin-1 production in rats with idiopathic pulmonary hypertension. Am J Physiol 262(5 Pt 1):L614–L620
Herrmann S et al (2001) A polymorphism in the endothelin-A receptor gene predicts survival in patients with idiopathic dilated cardiomyopathy. Eur Heart J 22(20):1948–1953
Popowski K et al (2003) Functional significance of a hereditary adenine insertion variant in the 5′-UTR of the endothelin-1 gene. Pharmacogenetics 13(8):445–451
Rahman T et al (2008) Common genetic variation in the type A endothelin-1 receptor is associated with ambulatory blood pressure: a family study. J Hum Hypertens 22(4):282–288
Mylona P, Cleland JG (1999) Update of REACH-1 and MERIT-HF clinical trials in heart failure. Cardio.net Editorial Team. Eur J Heart Fail 1(2):197–200
Kalra PR, Moon JC, Coats AJ (2002) Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol 85(2–3):195–197
Handoko ML, de Man FS, Vonk-Noordegraaf A (2011) The rise and fall of endothelin receptor antagonists in congestive heart failure. Eur Respir J 37(3):484–485
McLaughlin VV et al (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 53(17):1573–1619
Michael JR, Markewitz BA (1996) Endothelins and the lung. Am J Respir Crit Care Med 154(3 Pt 1):555–581
Inoue A et al (1989) The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A 86(8):2863–2867
Masaki T (1998) The discovery of endothelins. Cardiovasc Res 39(3):530–533
Levin ER (1995) Endothelins. N Engl J Med 333(6):356–363
Shinmi O et al (1989) Endothelin-3 is a novel neuropeptide: isolation and sequence determination of endothelin-1 and endothelin-3 in porcine brain. Biochem Biophys Res Commun 164(1):587–593
Inoue A et al (1989) The human preproendothelin-1 gene. Complete nucleotide sequence and regulation of expression. J Biol Chem 264(25):14954–14959
Laporte S et al (1993) Presence of furin mRNA in cultured bovine endothelial cells and possible involvement of furin in the processing of the endothelin precursor. J Cardiovasc Pharmacol 22(Suppl 8):S7–S10
Takahashi M et al (1993) Purification and characterization of endothelin-converting enzyme from rat lung. J Biol Chem 268(28):21394–21398
Xu D et al (1994) ECE-1: a membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell 78(3):473–485
Sakurai T et al (1991) cDNA cloning, sequence analysis and tissue distribution of rat preproendothelin-1 mRNA. Biochem Biophys Res Commun 175(1):44–47
Ehrenreich H et al (1990) Endothelins, peptides with potent vasoactive properties, are produced by human macrophages. J Exp Med 172(6):1741–1748
Yu JC, Davenport AP (1995) Secretion of endothelin-1 and endothelin-3 by human cultured vascular smooth muscle cells. Br J Pharmacol 114(2):551–557
Emori T et al (1991) Cellular mechanism of endothelin-1 release by angiotensin and vasopressin. Hypertension 18(2):165–170
Jougasaki M et al (2002) Cardiotrophin-1 stimulates endothelin-1 via gp130 in vascular endothelial cells. Peptides 23(8):1441–1447
Love MP et al (1996) Vasodilator effects of endothelin-converting enzyme inhibition and endothelin ETA receptor blockade in chronic heart failure patients treated with ACE inhibitors. Circulation 94(9):2131–2137
Masaki T et al (1991) Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation 84(4):1457–1468
Motte S, McEntee K, Naeije R (2006) Endothelin receptor antagonists. Pharmacol Ther 110(3):386–414
Boulanger C, Luscher TF (1990) Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest 85(2):587–590
Prins BA et al (1994) Prostaglandin E2 and prostacyclin inhibit the production and secretion of endothelin from cultured endothelial cells. J Biol Chem 269(16):11938–11944
Wagner OF et al (1992) Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem 267(23):16066–16068
Dupuis J, Goresky CA, Fournier A (1996) Pulmonary clearance of circulating endothelin-1 in dogs in vivo: exclusive role of ETB receptors. J Appl Physiol 81(4):1510–1515
Thorin E, Clozel M (2010) The cardiovascular physiology and pharmacology of endothelin-1. Adv Pharmacol 60:1–26
Vignon-Zellweger N et al (2012) Endothelin and endothelin receptors in the renal and cardiovascular systems. Life Sci 91(13–14):490–500
Clerk A et al (2002) Up-regulation of c-jun mRNA in cardiac myocytes requires the extracellular signal-regulated kinase cascade, but c-Jun N-terminal kinases are required for efficient up-regulation of c-Jun protein. Biochem J 368(Pt 1):101–110
MacNulty EE, Plevin R, Wakelam MJ (1990) Stimulation of the hydrolysis of phosphatidylinositol 4,5-bisphosphate and phosphatidylcholine by endothelin, a complete mitogen for Rat-1 fibroblasts. Biochem J 272(3):761–766
Anggrahini DW et al (2009) Vascular endothelial cell-derived endothelin-1 mediates vascular inflammation and neointima formation following blood flow cessation. Cardiovasc Res 82(1):143–151
Schiffrin EL (2001) Role of endothelin-1 in hypertension and vascular disease. Am J Hypertens 14(6 Pt 2):83S–89S
MacCarthy PA et al (2000) Contrasting inotropic effects of endogenous endothelin in the normal and failing human heart: studies with an intracoronary ET(A) receptor antagonist. Circulation 101(2):142–147
Marshall AK et al (2010) ERK1/2 signaling dominates over RhoA signaling in regulating early changes in RNA expression induced by endothelin-1 in neonatal rat cardiomyocytes. PLoS ONE 5(4):e10027
Shimojo N et al (2007) Contributory role of VEGF overexpression in endothelin-1-induced cardiomyocyte hypertrophy. Am J Physiol Heart Circ Physiol 293(1):H474–H481
Shimojo N et al (2006) Eicosapentaenoic acid prevents endothelin-1-induced cardiomyocyte hypertrophy in vitro through the suppression of TGF-beta 1 and phosphorylated JNK. Am J Physiol Heart Circ Physiol 291(2):H835–H845
Speed JS, Pollock DM (2013) Endothelin, kidney disease, and hypertension. Hypertension 61(6):1142–1145
Arai H et al (1990) Cloning and expression of a cDNA encoding an endothelin receptor. Nature 348(6303):730–732
Sakurai T et al (1990) Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature 348(6303):732–735
Sakurai T, Yanagisawa M, Masaki T (1992) Molecular characterization of endothelin receptors. Trends Pharmacol Sci 13(3):103–108
Hynynen MM, Khalil RA (2006) The vascular endothelin system in hypertension–recent patents and discoveries. Recent Pat Cardiovasc Drug Discov 1(1):95–108
Bourne HR (1997) How receptors talk to trimeric G proteins. Curr Opin Cell Biol 9(2):134–142
LaDouceur DM et al (1993) ETA and ETB receptors coexist on rabbit pulmonary artery vascular smooth muscle mediating contraction. Biochem Biophys Res Commun 196(1):209–215
MacLean MR, McCulloch KM, Baird M (1994) Endothelin ETA- and ETB-receptor-mediated vasoconstriction in rat pulmonary arteries and arterioles. J Cardiovasc Pharmacol 23(5):838–845
Simonson MS, Dunn MJ (1990) Cellular signaling by peptides of the endothelin gene family. FASEB J 4(12):2989–3000
Sumner MJ et al (1992) Endothelin ETA and ETB receptors mediate vascular smooth muscle contraction. Br J Pharmacol 107(3):858–860
Hosoda K et al (1991) Cloning and expression of human endothelin-1 receptor cDNA. FEBS Lett 287(1–2):23–26
Davenport AP et al (1993) Human endothelin receptors characterized using reverse transcriptase-polymerase chain reaction, in situ hybridization, and subtype-selective ligands BQ123 and BQ3020: evidence for expression of ETB receptors in human vascular smooth muscle. J Cardiovasc Pharmacol 22(Suppl 8):S22–S25
Ogawa Y et al (1991) Molecular cloning of a non-isopeptide-selective human endothelin receptor. Biochem Biophys Res Commun 178(1):248–255
Goldie RG et al (1996) The distribution and density of receptor subtypes for endothelin-1 in peripheral lung of the rat, guinea-pig and pig. Br J Pharmacol 117(4):729–735
Davie N et al (2002) ET(A) and ET(B) receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am J Respir Crit Care Med 165(3):398–405
Mazzuca MQ, Khalil RA (2012) Vascular endothelin receptor type B: structure, function and dysregulation in vascular disease. Biochem Pharmacol 84(2):147–162
Liu S et al (2003) Endothelin-1 activates endothelial cell nitric-oxide synthase via heterotrimeric G-protein betagamma subunit signaling to protein jinase B/Akt. J Biol Chem 278(50):49929–49935
Alonso D, Radomski MW (2003) The nitric oxide-endothelin-1 connection. Heart Fail Rev 8(1):107–115
Sato K et al (1995) Effects of separate and combined ETA and ETB blockade on ET-1-induced constriction in perfused rat lungs. Am J Physiol 269(5 Pt 1):L668–L672
Muramatsu M et al (1999) Chronic hypoxia augments endothelin-B receptor-mediated vasodilation in isolated perfused rat lungs. Am J Physiol 276(2 Pt 1):L358–L364
Clozel M et al (1992) The endothelin ETB receptor mediates both vasodilation and vasoconstriction in vivo. Biochem Biophys Res Commun 186(2):867–873
de Nucci G et al (1988) Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A 85(24):9797–9800
Haynes WG, Webb DJ (1993) Endothelium-dependent modulation of responses to endothelin-I in human veins. Clin Sci (Lond) 84(4):427–433
Verhaar MC et al (1998) Endothelin-A receptor antagonist-mediated vasodilatation is attenuated by inhibition of nitric oxide synthesis and by endothelin-B receptor blockade. Circulation 97(8):752–756
Pernow J, Modin A (1993) Endothelial regulation of coronary vascular tone in vitro: contribution of endothelin receptor subtypes and nitric oxide. Eur J Pharmacol 243(3):281–286
Sauvageau S et al (2006) Evaluation of endothelin-1-induced pulmonary vasoconstriction following myocardial infarction. Exp Biol Med (Maywood) 231(6):840–846
Sauvageau S et al (2007) Endothelin-1-induced pulmonary vasoreactivity is regulated by ET(A) and ET(B) receptor interactions. J Vasc Res 44(5):375–381
Dupuis J et al (2000) Importance of local production of endothelin-1 and of the ET(B)Receptor in the regulation of pulmonary vascular tone. Pulm Pharmacol Ther 13(3):135–140
Schneider MP, Boesen EI, Pollock DM (2007) Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol 47:731–759
Sen U et al (2009) Fibrinogen-induced endothelin-1 production from endothelial cells. Am J Physiol Cell Physiol 296(4):C840–C847
Evans NJ, Walker JW (2008) Endothelin receptor dimers evaluated by FRET, ligand binding, and calcium mobilization. Biophys J 95(1):483–492
Evans NJ, Walker JW (2008) Sustained Ca2+ signaling and delayed internalization associated with endothelin receptor heterodimers linked through a PDZ finger. Can J Physiol Pharmacol 86(8):526–535
Pietra GG et al (2004) Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 43(12 Suppl):25S–32S
Stewart DJ et al (1991) Increased plasma endothelin-1 in pulmonary hypertension: marker or mediator of disease? Ann Intern Med 114(6):464–469
Takahashi H et al (2001) Discrepant distribution of big endothelin (ET)-1 and ET receptors in the pulmonary artery. Eur Respir J 18(1):5–14
Rubin LJ (2012) Endothelin receptor antagonists for the treatment of pulmonary artery hypertension. Life Sci 91(13–14):517–521
Giaid A (1998) Nitric oxide and endothelin-1 in pulmonary hypertension. Chest 114(3 Suppl):208S–212S
Eddahibi S et al (1995) Protection from pulmonary hypertension with an orally active endothelin receptor antagonist in hypoxic rats. Am J Physiol 268(2 Pt 2):H828–H835
Chen SJ et al (1997) The orally active nonpeptide endothelin A-receptor antagonist A-127722 prevents and reverses hypoxia-induced pulmonary hypertension and pulmonary vascular remodeling in Sprague-Dawley rats. J Cardiovasc Pharmacol 29(6):713–725
Nagendran J et al (2013) Endothelin axis is upregulated in human and rat right ventricular hypertrophy. Circ Res 112(2):347–354
Chin KM, Kim NH, Rubin LJ (2005) The right ventricle in pulmonary hypertension. Coron Artery Dis 16(1):13–18
Farb A, Burke AP, Virmani R (1992) Anatomy and pathology of the right ventricle (including acquired tricuspid and pulmonic valve disease). Cardiol Clin 10(1):1–21
Channick R et al (2001) Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary hypertension: a placebo-controlled study. J Heart Lung Transplant 20(2):262–263
Rubin LJ et al (2002) Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 346(12):896–903
Galie N et al (2009) Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 30(20):2493–2537
Calabro P et al (2012) Analysis of endothelin-1 and endothelin-1 receptor A gene polymorphisms in patients with pulmonary arterial hypertension. Intern Emerg Med 7(5):425–430
Cattano D, Doursout MF (2012) Pulmonary hypertension: have we learned enough yet? Intern Emerg Med 7(5):395–397
Johnson JA, Cavallari LH (2013) Pharmacogenetics and cardiovascular disease–implications for personalized medicine. Pharmacol Rev 65(3):987–1009
Maron BJ et al (2006) Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113(14):1807–1816
Burkett EL, Hershberger RE (2005) Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 45(7):969–981
Hershberger RE et al (2010) Coding sequence rare variants identified in MYBPC3, MYH6, TPM1, TNNC1, and TNNI3 from 312 patients with familial or idiopathic dilated cardiomyopathy. Circ Cardiovasc Genet 3(2):155–161
Ponicke K et al (1998) Endothelin receptors in the failing and nonfailing human heart. Circulation 97(8):744–751
McMurray JJ et al (1992) Plasma endothelin in chronic heart failure. Circulation 85(4):1374–1379
Omland T et al (1994) Plasma endothelin determination as a prognostic indicator of 1-year mortality after acute myocardial infarction. Circulation 89(4):1573–1579
Fareh J et al (1996) Endothelin-1 and angiotensin II receptors in cells from rat hypertrophied heart. Receptor regulation and intracellular Ca2+ modulation. Circ Res 78(2):302–311
Piuhola J et al (2003) Endothelin-1 contributes to the Frank-Starling response in hypertrophic rat hearts. Hypertension 41(1):93–98
Mulder P et al (1997) Role of endogenous endothelin in chronic heart failure: effect of long-term treatment with an endothelin antagonist on survival, hemodynamics, and cardiac remodeling. Circulation 96(6):1976–1982
Zhao XS et al (2006) Endogenous endothelin-1 is required for cardiomyocyte survival in vivo. Circulation 114(8):830–837
Talukder MA et al (2001) Inotropic response of rabbit ventricular myocytes to endothelin-1: difference from isolated papillary muscles. Am J Physiol Heart Circ Physiol 281(2):H596–H605
Endoh M (2006) Signal transduction and Ca2+ signaling in intact myocardium. J Pharmacol Sci 100(5):525–537
Nishimaru K, Miura Y, Endoh M (2007) Mechanisms of endothelin-1-induced decrease in contractility in adult mouse ventricular myocytes. Br J Pharmacol 152(4):456–463
Namekata I et al (2008) Intracellular mechanisms and receptor types for endothelin-1-induced positive and negative inotropy in mouse ventricular myocardium. Naunyn Schmiedebergs Arch Pharmacol 376(6):385–395
Galie N et al (2005) Ambrisentan therapy for pulmonary arterial hypertension. J Am Coll Cardiol 46(3):529–535
Nicaud V et al (1999) Polymorphisms of the endothelin-A and -B receptor genes in relation to blood pressure and myocardial infarction: the Etude Cas-Temoins sur l’Infarctus du Myocarde (ECTIM) Study. Am J Hypertens 12(3):304–310
Tiret L et al (1999) The Lys198Asn polymorphism in the endothelin-1 gene is associated with blood pressure in overweight people. Hypertension 33(5):1169–1174
Charron P et al (1999) Identification of a genetic risk factor for idiopathic dilated cardiomyopathy. Involvement of a polymorphism in the endothelin receptor type A gene. CARDIGENE group. Eur Heart J 20(21):1587–1591
Colombo MG et al (2006) ET-1 Lys198Asn and ET(A) receptor H323H polymorphisms in heart failure. A case-control study. Cardiology 105(4):246–252
Tanaka C et al (2004) Evaluation of the Lys198Asn and -134delA genetic polymorphisms of the endothelin-1 gene. Hypertens Res 27(5):367–371
Treiber FA et al (2003) Endothelin-1 gene Lys198Asn polymorphism and blood pressure reactivity. Hypertension 42(4):494–499
Barden AE et al (2001) Association between the endothelin-1 gene Lys198Asn polymorphism blood pressure and plasma endothelin-1 levels in normal and pre-eclamptic pregnancy. J Hypertens 19(10):1775–1782
Fraccarollo D et al (1997) Chronic endothelin receptor blockade attenuates progressive ventricular dilation and improves cardiac function in rats with myocardial infarction: possible involvement of myocardial endothelin system in ventricular remodeling. Circulation 96(11):3963–3973
Yamauchi-Kohno R et al (1999) Role of endothelin in deterioration of heart failure due to cardiomyopathy in hamsters: increase in endothelin-1 production in the heart and beneficial effect of endothelin-A receptor antagonist on survival and cardiac function. Circulation 99(16):2171–2176
Sutsch G et al (1998) Short-term oral endothelin-receptor antagonist therapy in conventionally treated patients with symptomatic severe chronic heart failure. Circulation 98(21):2262–2268
Torre-Amione G et al (2001) Hemodynamic effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients with class III to IV congestive heart failure. Circulation 103(7):973–980
Cotter G et al (2001) Tezosentan (an intravenous endothelin receptor A/B antagonist) reduces peripheral resistance and increases cardiac power therefore preventing a steep decrease in blood pressure in patients with congestive heart failure. Eur J Heart Fail 3(4):457–461
Anand I et al (2004) Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the EndothelinA Receptor Antagonist Trial in Heart Failure (EARTH): randomised, double-blind, placebo-controlled trial. Lancet 364(9431):347–354
Kaluski E et al (2008) Clinical and hemodynamic effects of bosentan dose optimization in symptomatic heart failure patients with severe systolic dysfunction, associated with secondary pulmonary hypertension–a multi-center randomized study. Cardiology 109(4):273–280
Torre-Amione G et al (2003) Hemodynamic and clinical effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 42(1):140–147
Jiang BH et al (2011) Bosentan does not improve pulmonary hypertension and lung remodeling in heart failure. Eur Respir J 37(3):578–586
Padeletti M et al (2013) Effect of bosentan on pulmonary hypertension secondary to systolic heart failure. Pharmacology 92(5–6):281–285
O’Connor CM et al (2003) Tezosentan in patients with acute heart failure and acute coronary syndromes: results of the Randomized Intravenous TeZosentan Study (RITZ-4). J Am Coll Cardiol 41(9):1452–1457
Kirshbom PM et al (2008) The endothelin-1 G5665T polymorphism impacts transplant-free survival for single ventricle patients. J Thorac Cardiovasc Surg 136(1):117–122
Messerli FH, Williams B, Ritz E (2007) Essential hypertension. Lancet 370(9587):591–603
Kumagae S et al (2010) High level of plasma endothelin-1 predicts development of hypertension in normotensive subjects. Am J Hypertens 23(10):1103–1107
Ergul S et al (1996) Racial differences in plasma endothelin-1 concentrations in individuals with essential hypertension. Hypertension 28(4):652–655
Campia U, Cardillo C, Panza JA (2004) Ethnic differences in the vasoconstrictor activity of endogenous endothelin-1 in hypertensive patients. Circulation 109(25):3191–3195
Hasegawa K et al (1994) Endothelin-1-selective receptor in the arterial intima of patients with hypertension. Hypertension 23(3):288–293
Schiffrin EL et al (1996) Enhanced expression of the endothelin-1 gene in blood vessels of DOCA-salt hypertensive rats: correlation with vascular structure. J Vasc Res 33(3):235–248
Haynes WG et al (1996) Systemic endothelin receptor blockade decreases peripheral vascular resistance and blood pressure in humans. Circulation 93(10):1860–1870
Ohkita M et al (2012) Pathophysiological roles of endothelin receptors in cardiovascular diseases. J Pharmacol Sci 119(4):302–313
Rautureau Y, Schiffrin EL (2012) Endothelin in hypertension: an update. Curr Opin Nephrol Hypertens 21(2):128–136
Lariviere R, Day R, Schiffrin EL (1993) Increased expression of endothelin-1 gene in blood vessels of deoxycorticosterone acetate-salt hypertensive rats. Hypertension 21(6 Pt 2):916–920
Barton M et al (1998) ET(A) receptor blockade prevents increased tissue endothelin-1, vascular hypertrophy, and endothelial dysfunction in salt-sensitive hypertension. Hypertension 31(1 Pt 2):499–504
Li JS, Lariviere R, Schiffrin EL (1994) Effect of a nonselective endothelin antagonist on vascular remodeling in deoxycorticosterone acetate-salt hypertensive rats. Evidence for a role of endothelin in vascular hypertrophy. Hypertension 24(2):183–188
Matsumura Y et al (1999) Different contributions of endothelin-A and endothelin-B receptors in the pathogenesis of deoxycorticosterone acetate-salt-induced hypertension in rats. Hypertension 33(2):759–765
Amiri F et al (2008) Vascular inflammation in absence of blood pressure elevation in transgenic murine model overexpressing endothelin-1 in endothelial cells. J Hypertens 26(6):1102–1109
Amiri F et al (2010) Deleterious combined effects of salt-loading and endothelial cell restricted endothelin-1 overexpression on blood pressure and vascular function in mice. J Hypertens 28(6):1243–1251
Ward RH (1983) Genetic and sociocultural components of high blood pressure. Am J Phys Anthropol 62(1):91–105
Benjafield AV, Katyk K, Morris BJ (2003) Association of EDNRA, but not WNK4 or FKBP1B, polymorphisms with essential hypertension. Clin Genet 64(5):433–438
Krum H et al (1998) The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan Hypertension Investigators. N Engl J Med 338(12):784–790
Nakov R et al (2002) Darusentan: an effective endothelinA receptor antagonist for treatment of hypertension. Am J Hypertens 15(7 Pt 1):583–589
Black HR et al (2007) Efficacy and safety of darusentan in patients with resistant hypertension: results from a randomized, double-blind, placebo-controlled dose-ranging study. J Clin Hypertens (Greenwich) 9(10):760–769
Weber MA et al (2009) A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet 374(9699):1423–1431
Bakris GL et al (2010) Divergent results using clinic and ambulatory blood pressures: report of a darusentan-resistant hypertension trial. Hypertension 56(5):824–830
Raina A, Horn ET, Benza RL (2012) The pathophysiology of endothelin in complications after solid organ transplantation: a potential novel therapeutic role for endothelin receptor antagonists. Transplantation 94(9):885–893
Dorent R et al (1994) Endothelin levels after orthotopic heart transplantation. Transplant Proc 26(1):250
Ferri C et al (2002) Patterns of myocardial endothelin-1 expression and outcome after cardiac transplantation. Circulation 105(15):1768–1771
Cauduro RL et al (2005) Endothelin-1 plasma levels and hypertension in cyclosporine-treated renal transplant patients. Clin Transplant 19(4):470–474
Simonson MS et al (1999) Inhibition of endothelin-converting enzyme attenuates transplant vasculopathy and rejection in rat cardiac allografts. Transplantation 67(12):1542–1547
Takeda Y et al (1995) Effects of an endothelin receptor antagonist in rats with cyclosporine-induced hypertension. Hypertension 26(6 Pt 1):932–936
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Holzhauser, L., Zolty, R. Endothelin receptor polymorphisms in the cardiovascular system: potential implications for therapy and screening. Heart Fail Rev 19, 743–758 (2014). https://doi.org/10.1007/s10741-014-9426-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-014-9426-y