Abstract
Heart failure with preserved ejection fraction (HF-PEF) is a well-recognized complication of long-standing hypertension. However, beyond the control of the traditional cardiovascular risk factors, there are few other recommendations for its management. To examine the potential benefit of renin-angiotensin system (RAS) inhibition in HF-PEF, we performed a systematic review of the published medical literature. MEDLINE, EMBASE, and COCHRANE databases were searched from 1966 to 2011 for longitudinal studies examining HF-PEF patients receiving treatment with RAS inhibitors, either ACE inhibitors (ACE-I) or angiotensin receptor blockers (ARB) in addition to their standard treatment compared to those receiving standard treatment alone. We examined the all-cause mortality, cardiovascular mortality, and hospitalizations for heart failure. A total of 12 studies with 11,259 participants were included in the analysis. Among the randomized clinical trials, with the use of RAS inhibitors over standard treatment, there was no improvement in all-cause mortality (RR: 0.99; 95 % CI: 0.88–1.12; p = 0.88), while there was a trend toward lowered rates of hospitalization (RR: 0.93; 95 % CI: 0.86–1.01; p = 0.08). There were no major differences in the outcomes between the ACE-I or ARB. However, among the observational studies with the use of RAS inhibitors, there was a significant benefit in all-cause mortality (RR: 0.76; 95 % CI: 0.62–0.93; p = 0.009), with no significant impact on the hospitalization rates. RAS inhibition in HF-PEF was not associated with significant reduction in all-cause or cardiovascular mortality, but randomized control trials appear to demonstrate a trend toward reduction in the risk for subsequent hospitalization. Further prospective randomized trials are warranted to confirm the effects of RAS inhibition on mortality and hospitalization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure with preserved ejection fraction (HF-PEF) is the most common and fastest growing form of heart failure in the United States [1]. Despite its importance, there are large gaps in our understanding of the pathophysiology and treatment of HF-PEF. Renin-angiotensin system (RAS) inhibitors, including angiotensin-converting enzyme inhibitors (ACE-I) and angiotensin receptor blockade (ARB), constitute first-line treatment for patients with heart failure with reduced ejection fraction (HF-REF) with proven efficacy in reducing mortality and morbidity. However, their role in HF-PEF remains controversial, with multiple clinical studies examining their role in HF-PEF showing conflicting results [2–6]. The aim of this meta-analysis was to assess efficacy of RAS inhibition on mortality and hospitalizations due to heart failure in HF-PEF patients.
Materials and methods
Search strategy
We systematically searched the electronic databases, MEDLINE, PUBMED, EMBASE, and the Cochrane Library for Central Register of Clinical Trials, using the MESH terms, “heart failure, diastolic”, “angiotensin-converting enzyme inhibitors”, “Angiotensin Receptor Antagonists”, and the names of individual ACE-I or ARB agents. We limited our search to studies in human subjects and English language in peer-reviewed journals from 1966 to June 2011. Additionally, a manual search of all relevant references from the screened articles and reviews of RAS inhibitors was performed for additional clinical studies (Table 2).
Study selection
Eligible studies were (1) prospective (randomized or non-randomized) or retrospective study designs assessing the effectiveness of RAS inhibitors (ACE-I or ARB) for HF-PEF (defined as signs or symptoms of heart failure and EF ≥40 %); (2) studies reporting outcomes of interest, including mortality (all-cause and/or cardiac) and hospitalizations due to heart failure; and (3) studies with at least a 6-month follow-up in each study arm. Exclusion criteria were (1) healthy persons used as controls, (2) patients following heart transplantation, (3) absence of quantitative description of end points, (4) lack of clear and reproducible results, and (5) trials in the abstract form without a published manuscript in a peer-reviewed journal. There were no restrictions based on year of publication. Studies that had duplicated data, including same group of patients or for whom there were updated results available, were excluded.
Data extraction and quality
The data were independently extracted by two authors (V.A. and A.B.), using standardized protocol and reporting form. Disagreements were resolved by arbitration (F.M.), and consensus was reached after discussion. We extracted characteristics of each study (type of design with duration of intervention and methods), baseline demographics, and mortality and hospitalization for heart failure after the initiation of ACE-I/ARB for our analysis. Authors of the studies were individually contacted in case the data were unclear. The study quality was evaluated according to the Jadad composite score [7], which is a five-point quality scale, with low quality studies having a score of ≤2 and high-quality studies a score of ≥3 [8].
Outcomes assessed
Our primary analysis was aimed at evaluating the difference in the all-cause mortality between the patients on RAS inhibitors compared to patients without RAS inhibition. In addition, we also compared the differences in mortality due to cardiovascular causes and differences in rates of hospitalizations due to heart failure in the two groups of patients.
Data analysis and synthesis
An intention-to-treat traditional meta-analysis was performed in accordance with the recommendations from the Cochrane Collaboration and the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement (for randomized controlled trials) and meta-analysis of observational studies in epidemiology (MOOSE) statement for others. All analyses were performed by metan command of Stata 10.1 (Stata Corp., College Station, TX, USA). A priori, we assumed that substantial clinical heterogeneity would be present in the included trials. We therefore planned to apply both a random-effects model (DerSimonian–Laird approach) [9] and a fixed-effects model (Mantel–Haenszel) [10] to pool the relative risk (RR) estimation for all outcomes. Heterogeneity was assessed with the I2 statistic proposed by Higgins and Thompson [11], with I2 < 25 % considered low and I2 > 75 % considered high. Reported values are two-tailed, and hypothesis testing results were considered statistically significant at p < 0.05. Small study effect, including publication bias, was tested using funnel plot and the regression intercept of Egger et al. [12] and corrected by the nonparametric tri-and-fill method of Duvall and Tweedie [13]. We separately examined if there were any differences in the outcomes between randomized and non-randomized studies, and various groups of RAS inhibitors, including ACE-I, ARB, and ACE-I/ARB combination. To determine whether individual studies had an undue influence on the overall results (because of size or magnitude of effect), we conducted a post hoc influence analysis using the METANINF command in STATA, which systematically excludes each study from the analysis and re-estimates the summary RR; influential studies are identified by a large change in the summary RR after exclusion. Since there was significant heterogeneity between the observational and randomized studies, the cumulative results of the two subgroups are reported separately. Below, we report the results of the random-effects models, while both the fixed- and random-effects models results are listed in the tables.
Results
Study selection
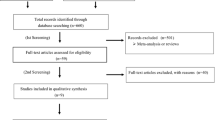
We identified 12 clinical studies (Table 1), with 12 control arms and 13 intervention arms, which examined the effects of RAS inhibitors, on mortality (all cause/cardiovascular) and hospitalizations due to heart failure, based on our inclusion and exclusion criteria (Fig. 1). These include five randomized control trials [2–6] and seven observational studies, with four prospective studies [14–17] and three retrospective studies [18–20].
Baseline characteristics
These studies enrolled a total of 11,259 patients (cases—5,624, controls—5,635), with a total follow-up duration of 39,950 years. While all the studies, that is, 13 comparison groups reported the all-cause mortality, there were 10 groups comparing hospitalization and 6 groups comparing cardiovascular mortality. The mean age of patients was 72.2 years (mean age range, 67–79 years). The mean follow-up duration was 38.6 months (range, 6 months to 5 years). The overall characteristics of the included studies are listed in Table 1.
Quality assessment
Since we included both randomized and observational studies in our analyses, the included studies were of variable quality. There were five studies of good quality (Jadad score ≥3) with low risk of bias and seven studies of low quality (Jadad score <3) with high risk of bias.
All-cause mortality (Table 2, Fig. 2)
When examining only the randomized control trials, there was no benefit of RAS inhibition over standard treatment (RR: 0.99; 95 % CI: 0.88–1.12; p = 0.88). The lack of benefit was consistent across both the ACE-I and ARB subgroups. However, the observational studies showed a significant benefit in all-cause mortality (RR: 0.76; 95 % CI: 0.62–0.93; p = 0.009). As expected with lower-quality studies, there was significant heterogeneity among the observational studies (I2: 73.3 %, p = 0.001).
Cardiovascular mortality (Table 2)
There was no significant reduction in cardiovascular mortality with the use of RAS inhibitors compared to controls in both the randomized control trials (RR: 0.97; 95 % CI: 0.86–1.1; p = 0.63) and the observational studies (RR: 052; 95 % CI: 0.25–1.06; p = 0.07). Similarly, both the ACE-I and ARB subgroups did not show any significant reduction in the cardiovascular mortality.
Hospitalization (Table 2, Fig. 3)
With the use of RAS inhibitors, the randomized control trials showed a trend toward lower rates of hospitalization (RR: 0.93; 95 % CI: 0.86–1.01; p = 0.08), while the observational studies did not show any significant reduction in hospitalization rates (RR: 0.97; 95 % CI: 0.77–1.21; p = 0.76). On examining the individual subgroups among the randomized control trials, while the ARB subgroup showed a trend toward lower rates of hospitalization (RR: 0.94; 95 % CI: 0.86–1.0; p = 0.14), there was no significant change in hospitalization rates with the use of ACE-I (RR: 0.85; 95 % CI: 0.63–1.13; p = 0.26). Also, there was no significant heterogeneity between the studies among the randomized studies group and the individual subgroups of ACE-Is and ARBs.
Discussion
In an effort to evaluate the effects of RAS inhibition on HF-PEF, we performed a systematic review of the published medical literature on the effects of RAS inhibitors on patients with HF-PEF. While we found that regardless of the study design, there was little evidence of reduced all-cause or cardiovascular mortality, there was a trend toward lower rates of hospitalization among patients on RAS inhibitors, especially among the high-quality randomized clinical trials. However, we observed significant heterogeneity between trials in the efficacy of RAS inhibitors to improve outcomes. Much of this heterogeneity could be explained by differences in the methodological quality of the trials. Lower rates of hospitalization are an important component of heart failure outcomes, as it has been shown that readmission portends a significantly worse short- and long-term prognosis [21].
Stiffening of the peripheral vasculature and the ventricles with aging and hypertension has been implicated to play a central role in the pathogenesis of HF-PEF [1, 22, 23]. As is the case with HF-REF, activation of the RAS has been suggested as a contributor to HF-PEF development [24]. Hence, tighter control of blood pressure by various agents, including RAS inhibitors, potentially forms an important part of the armamentarium of management of HF-PEF. In addition, ACE-Is have been shown to additionally reduce arterial stiffness, independent of their blood pressure lowering effect [25]. Thus, treatment strategies with RAS inhibitors should have a favorable effect on inhibiting the underlying pathophysiological process in HF-PEF. While most studies included in our meta-analysis reported improved blood pressure control with the use of RAS inhibitors, this reduction of blood pressure did not translate into improved clinical hard end points. There are several potential reasons for a lack of an effect.
First, it is conceivable that many of the patients included in the clinical trials may not actually have chronic heart failure. The diagnosis of HF-PEF is challenging and has been constantly evolving, with some authors even questioning the existence of a clinical picture of HF-PEF [26]. Initial clinical trials looking at HF-PEF enrolled patients with a myriad of underlying clinical conditions; limited systolic dysfunction due to limited myocardial infarction and cardiac remodeling secondary to arterial hypertension, obesity, and diabetes without evidence of coronary artery disease [3]. Recent guidelines have focused on introducing more stringent criterion for HF-PEF, with increasing emphasis on evidence of diastolic dysfunction [27, 28]. Hence, newer studies, especially the I-PRESERVE trial [4] as compared to the relatively older CHARM-Preserved study [3] enrolled patients with greater incidence of heart failure due to hypertension (63 vs. 23 %), as compared to an ischemic etiology (24 vs. 63 %). However, even the I-PRESERVE inclusion criteria did not include abnormal LV filling kinetics on mitral flow velocity Doppler. In addition, there were variations between the cutoff ejection fractions for the various studies, ranging from 40 to 50 %. It is possible that the maximum benefit with the use of RAS inhibitors may have been obtained in patients with prior myocardial damage due to an ischemic etiology, resulting in principally underlying systolic dysfunction with relatively preserved ejection.
Second, while there is significant neurohormonal upregulation in HF-REF, the same degree of upregulation is not present in HF-PEF. In majority of trials, many patients were already taking other RAS-modifying agents, including mineralocorticoid receptor antagonists, like spironolactone, and beta-blockers. In the I-PRESERVE trial [4], 40 % of patients were using ACE-I, 29 % were using spironolactone, and 73 % of patients were on beta-blockers. Similarly, in the CHARM-Preserved study [3], about 19 % of patients were using ACE-I, 12 % were on spironolactone, and 56 % were on a beta-blocker. In the PEP-CHF study [2], 55 % of patients were on beta-blockers, and 10 % were on low-dose spironolactone. Based on these numbers, it appears that a large proportion of patients were on multiple inhibitors of the renin–angiotensin–aldosterone system (RAAS), which may have left little room for incremental benefit from the addition of another RAS inhibitor.
Third, the lack of efficacy of RAS inhibitors may be linked to the patients’ baseline systolic and diastolic blood pressures. In HF-REF, aggressive control of blood pressure is recommended with blood pressure targets of ≤130/80 mmHg, or better, ≤120/80 mmHg [29]. However, multiple studies in HF-REF have suggested mortality has either an inverse linear relationship or a J-shaped relationship with systolic blood pressure [30–33]. A recent study comparing the hemodynamic responses to vasodilator therapy in patients with HF-PEF and HF-REF found that as compared to HF-REF, HF-PEF experienced greater reductions in blood pressure, less enhancement of cardiac output, and greater likelihood of stroke volume reduction with vasodilators [34]. This may be particularly true in heart failure patients with ejection fraction ≥50 %, where a J-shaped relationship between systolic blood pressure and mortality has been reported [33]. In addition, in the PEP-CHF study, hypertensive patients with systolic blood pressure ≥140 mmHg showed a greater benefit with the use of perindopril. Conceivably, therefore, HF-PEF patients with higher systolic blood pressures may exhibit greater benefit, and consequently better outcomes, with the use of RAS inhibitors.
Fortunately, two major randomized studies will be further exploring the role of RAAS inhibitors in HF-PEF and help clarify the role of RAAS inhibitors in HF-PEF. The Treatment of Preserved Cardiac function heart failure with an Aldosterone Antagonist (TOPCAT) will focus on the effects of spironolactone in HF-PEF patients, with the primary end point examining a composite of cardiovascular mortality, aborted cardiac arrest, or hospitalization for the management of heart failure, and secondary end points including quality of life, non-fatal cardiovascular events, and new-onset atrial fibrillation [35]. The Aldo-DHF study will test whether spironolactone will help improving maximal exercise capacity (peak VO2, spiroergometry) and diastolic function [36]. In addition, future studies should focus on including more homogeneous patient populations after excluding patients with underlying ischemia, which may be contributing to systolic dysfunction with limited reduction in ejection fraction. The inclusion parameters should not only include ejection fraction cutoffs, but they should also incorporate the various echocardiographic parameters which form the basis of diagnosis of HF-PEF. Also, it may be worth exploring if the benefits in clinical end points, including mortality and hospitalization, correlate with changes in echocardiographic parameters with the use of RAS inhibitors.
Limitations
This systematic review has several limitations. Although a few of the studies included in the analysis were double-blinded randomized controlled trials, many of the included studies were of relatively poor quality. It is known that studies with inadequate concealment of treatment allocation may overestimate the actual treatment effect [37]. Also, the results are subject to limitations inherent to any meta-analysis based on pooling of data from different trials with different inclusion criteria, different designs, variable follow-up duration with differing attrition rates, and different patient populations. As in other meta-analyses, given the lack of data in each trial, we did not adjust our analyses for compliance to assigned therapy. In addition, the doses of the RAS inhibitors in the different studies were different and may not have achieved adequate or equipotent RAS blockade.
Conclusion
In spite of the small reduction in hospitalization rates, especially with the use of ACE-Is and a trend toward decreased mortality, the inconsistency of the results between the studies along with the negative results of the larger trials allow no firm conclusions on therapy in patients with HF-PEF. For now, treatment of these patients should empirically focus on strict blood pressure control and paying attention to the other comorbidities often found in this patient population. Whether or not RAS inhibitors should be used as first-line drugs remains to be determined.
References
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (2006) Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 355(3):251–259. doi:10.1056/NEJMoa052256
Cleland JG, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J (2006) The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J 27(19):2338–2345. doi:10.1093/eurheartj/ehl250
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Ostergren J (2003) Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet 362(9386):777–781. doi:10.1016/S0140-6736(03)14285-7
Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A (2008) Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med 359(23):2456–2467. doi:10.1056/NEJMoa0805450
Yip GW, Wang M, Wang T, Chan S, Fung JW, Yeung L, Yip T, Lau ST, Lau CP, Tang MO, Yu CM, Sanderson JE (2008) The Hong Kong diastolic heart failure study: a randomised controlled trial of diuretics, irbesartan and ramipril on quality of life, exercise capacity, left ventricular global and regional function in heart failure with a normal ejection fraction. Heart 94(5):573–580. doi:10.1136/hrt.2007.117978
Zi M, Carmichael N, Lye M (2003) The effect of quinapril on functional status of elderly patients with diastolic heart failure. Cardiovasc Drugs Ther 17(2):133–139
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
Kjaergard LL, Villumsen J, Gluud C (2001) Reported methodologic quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med 135(11):982–989
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Demets DL (1987) Methods for combining randomized clinical trials: strengths and limitations. Stat Med 6(3):341–350
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21(11):1539–1558. doi:10.1002/sim.1186
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56(2):455–463
Philbin EF, Rocco TA Jr (1997) Use of angiotensin-converting enzyme inhibitors in heart failure with preserved left ventricular systolic function. Am Heart J 134(2 Pt 1):188–195
Philbin EF, Rocco TA Jr, Lindenmuth NW, Ulrich K, Jenkins PL (2000) Systolic versus diastolic heart failure in community practice: clinical features, outcomes, and the use of angiotensin-converting enzyme inhibitors. Am J Med 109(8):605–613
Grigorian Shamagian L, Roman AV, Ramos PM, Veloso PR, Bandin Dieguez MA, Gonzalez-Juanatey JR (2006) Angiotensin-converting enzyme inhibitors prescription is associated with longer survival among patients hospitalized for congestive heart failure who have preserved systolic function: a long-term follow-up study. J Card Fail 12(2):128–133. doi:10.1016/j.cardfail.2005.09.001
Tribouilloy C, Rusinaru D, Leborgne L, Peltier M, Massy Z, Slama M (2008) Prognostic impact of angiotensin-converting enzyme inhibitor therapy in diastolic heart failure. Am J Cardiol 101(5):639–644. doi:10.1016/j.amjcard.2007.10.026
Dauterman KW, Go AS, Rowell R, Gebretsadik T, Gettner S, Massie BM (2001) Congestive heart failure with preserved systolic function in a statewide sample of community hospitals. J Card Fail 7(3):221–228. doi:10.1054/jcaf.2001.26896
Ahmed A, Roseman JM, Duxbury AS, Allman RM, DeLong JF (2002) Correlates and outcomes of preserved left ventricular systolic function among older adults hospitalized with heart failure. Am Heart J 144(2):365–372
Sueta CA, Russo A, Schenck A, Brown DW, Simpson RJ (2003) Effect of angiotensin-converting inhibitor or angiotensin receptor blocker on one-year survival in patients > or =65 years hospitalized with a left ventricular ejection fraction > or =50 %. Am J Cardiol 91(3):363–365
Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, Yusuf S, Swedberg K, Young JB, Michelson EL, Pfeffer MA (2007) Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation 116(13):1482–1487. doi:10.1161/CIRCULATIONAHA.107.696906
Melenovsky V, Borlaug BA, Rosen B, Hay I, Ferruci L, Morell CH, Lakatta EG, Najjar SS, Kass DA (2007) Cardiovascular features of heart failure with preserved ejection fraction versus nonfailing hypertensive left ventricular hypertrophy in the urban Baltimore community: the role of atrial remodeling/dysfunction. J Am Coll Cardiol 49(2):198–207. doi:10.1016/j.jacc.2006.08.050
Tartiere-Kesri L, Tartiere JM, Logeart D, Beauvais F, Cohen Solal A (2012) Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol 59(5):455–461. doi:10.1016/j.jacc.2011.10.873
Yamamoto K, Masuyama T, Sakata Y, Mano T, Nishikawa N, Kondo H, Akehi N, Kuzuya T, Miwa T, Hori M (2000) Roles of renin-angiotensin and endothelin systems in development of diastolic heart failure in hypertensive hearts. Cardiovasc Res 47(2):274–283
Tropeano AI, Boutouyrie P, Pannier B, Joannides R, Balkestein E, Katsahian S, Laloux B, Thuillez C, Struijker-Boudier H, Laurent S (2006) Brachial pressure-independent reduction in carotid stiffness after long-term angiotensin-converting enzyme inhibition in diabetic hypertensives. Hypertension 48(1):80–86. doi:10.1161/01.HYP.0000224283.76347.8c
Packer M (2011) Can brain natriuretic peptide be used to guide the management of patients with heart failure and a preserved ejection fraction? The wrong way to identify new treatments for a nonexistent disease. Circ Heart Fail 4(5):538–540. doi:10.1161/CIRCHEARTFAILURE.111.963710
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite-Moreira AF, Borbely A, Edes I, Handoko ML, Heymans S, Pezzali N, Pieske B, Dickstein K, Fraser AG, Brutsaert DL (2007) How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography associations of the european society of cardiology. Eur Heart J 28(20):2539–2550. doi:10.1093/eurheartj/ehm037
Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, Waggoner AD, Flachskampf FA, Pellikka PA, Evangelista A (2009) Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr 22(2):107–133. doi:10.1016/j.echo.2008.11.023
Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL Jr, Kaplan NM, O’Connor CM, O’Gara PT, Oparil S (2007) Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the councils on clinical cardiology and epidemiology and prevention. Circulation 115(21):2761–2788. doi:10.1161/CIRCULATIONAHA.107.183885
Lee TT, Chen J, Cohen DJ, Tsao L (2006) The association between blood pressure and mortality in patients with heart failure. Am Heart J 151(1):76–83. doi:10.1016/j.ahj.2005.03.009
Ather S, Chan W, Chillar A, Aguilar D, Pritchett AM, Ramasubbu K, Wehrens XH, Deswal A, Bozkurt B (2011) Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J 161(3):567–573. doi:10.1016/j.ahj.2010.12.009
Lee DS, Ghosh N, Floras JS, Newton GE, Austin PC, Wang X, Liu PP, Stukel TA, Tu JV (2009) Association of blood pressure at hospital discharge with mortality in patients diagnosed with heart failure. Circ Heart Fail 2(6):616–623. doi:10.1161/CIRCHEARTFAILURE.109.869743
Nunez J, Nunez E, Fonarow GC, Sanchis J, Bodi V, Bertomeu-Gonzalez V, Minana G, Merlos P, Bertomeu-Martinez V, Redon J, Chorro FJ, Llacer A (2010) Differential prognostic effect of systolic blood pressure on mortality according to left-ventricular function in patients with acute heart failure. Eur J Heart Fail 12(1):38–44. doi:10.1093/eurjhf/hfp176
Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA (2012) Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol 59(5):442–451. doi:10.1016/j.jacc.2011.09.062
Desai AS, Lewis EF, Li R, Solomon SD, Assmann SF, Boineau R, Clausell N, Diaz R, Fleg JL, Gordeev I, McKinlay S, O’Meara E, Shaburishvili T, Pitt B, Pfeffer MA (2011) Rationale and design of the treatment of preserved cardiac function heart failure with an aldosterone antagonist trial: a randomized, controlled study of spironolactone in patients with symptomatic heart failure and preserved ejection fraction. Am Heart J 162(6):966–972 e910. doi:10.1016/j.ahj.2011.09.007
Edelmann F, Schmidt AG, Gelbrich G, Binder L, Herrmann-Lingen C, Halle M, Hasenfuss G, Wachter R, Pieske B (2010) Rationale and design of the ‘aldosterone receptor blockade in diastolic heart failure’ trial: a double-blind, randomized, placebo-controlled, parallel group study to determine the effects of spironolactone on exercise capacity and diastolic function in patients with symptomatic diastolic heart failure (Aldo-DHF). Eur J Heart Fail 12(8):874–882. doi:10.1093/eurjhf/hfq087
Schulz KF, Chalmers I, Hayes RJ, Altman DG (1995) Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 273(5):408–412
Acknowledgments
Franz Messerli has served as an ad hoc consultant for Novartis, Boehringer Ingelheim, Daiichi Sankyo, Sanofi, and Takeda and has received research funding and grants from Novartis, Boehringer Ingelheim, and Forest.
Conflict of interest
Vikram Agarwal: no relationships to disclose. Alexandros Briasoulis: no relationships to disclose
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Agarwal, V., Briasoulis, A. & Messerli, F.H. Effects of renin-angiotensin system blockade on mortality and hospitalization in heart failure with preserved ejection fraction. Heart Fail Rev 18, 429–437 (2013). https://doi.org/10.1007/s10741-012-9329-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-012-9329-8