Abstract
The present study aimed to investigate the protective effects of rAAV9-CyclinA2 combined with fibrin glue (FG) in vivo in rats after myocardial infarction (MI). Ninety male Sprague–Dawley rats were randomized into 6 groups (15 in each group): sham, MI, rAAV9-green fluorescent protein (GFP) + MI, rAAV9-CyclinA2 + MI, FG + MI, and rAAV9-CyclinA2 + FG + MI. Packed virus (5 × 1011vg/ml) in 150 µl of normal saline or FG was injected into the infarcted myocardium at five locations in rAAV9-GFP + MI, rAAV9-CyclinA2 + MI, and rAAV9-CyclinA2 + FG + MI groups. The sham, MI, and FG + MI groups were injected with an equal volume of normal saline or FG at the same sites. Five weeks after injection, echocardiography was performed to evaluate the left ventricular function. The expressions of CyclinA2, proliferating cell nuclear antigen (PCNA), and phospho-histone-H3 (H3P), vascular density, and infarct area were assessed by Western blot, immunohistochemistry, immunofluorescence, and Masson staining. As a result, the combination of rAAV9-CyclinA2 and FG increased ejection fraction and fractional shortening compared with FG or rAAV9-CyclinA2 alone. The expression level of CyclinA2 was significantly higher in the rAAV9-CyclinA2 + FG + MI group compared with the rAAV9-CyclinA2 + MI and FG + MI groups (70.1 ± 1.86% vs. 14.74 ± 2.02%, P < 0.01; or vs. 50.13 ± 3.80%; P < 0.01). A higher expression level of PCNA and H3P was found in the rAAV9-CyclinA2 + FG + MI group compared with other groups. Comparing with other experiment groups, collagen deposition and the infarct size significantly decreased in rAAV9-CyclinA2 + Fibrin + MI group. The vascular density was much higher in the rAAV9-CyclinA2 + FG + MI group compared with the rAAV9-CyclinA2 + MI group. We concluded that fibrin glue combined with rAAV9-CyclinA2 was found to be effective in cardiac remodeling and improving myocardial protection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Myocardial infarction (MI) is a serious threat to human health worldwide, often leading to a high rate of mortality and morbidity (Yusuf et al. 2014). The incidence of MI in China has increased in recent years (Li et al. 2015). After MI, various inflammatory reactions lead to the formation of scar tissue, left ventricular wall remodeling, ventricular wall thinning, and continuous deterioration of heart function, eventually causing heart failure (Van Aelst et al. 2015). At present, the treatments of MI include drug therapy, percutaneous coronary intervention, and coronary artery bypass graft, which can neither reverse the deterioration of cardiac function nor promote the regeneration of myocardial cells.
However, studies have shown that in nontreated MI, a very small number of cardiomyocytes can restart the cell cycle (Li et al. 2013). CyclinA2, an important cell cycle regulator, plays a key role in promoting entry of cells into mitosis (G1/S phase, G2/M phase) (Li et al. 2013). Previous studies have confirmed that the transfection of CyclinA2 into cardiomyocytes could promote the regeneration of cardiomyocytes after MI (Cheng et al. 2007; Woo et al. 2006; Shapiro et al. 2014). Also, tail vein injection was used in this study to transfect rAAV9-CyclinA2 into cardiomyocytes after infarction, and the feasibility and safety of rAAV9-CyclinA2 were demonstrated (Ma et al. 2015). Studies have shown that AAV9 can achieve efficient and uniform myocardial transfection (White et al. 2011; Prasad et al. 2011; Pleger et al. 2011).
While using CyclinA2 alone can promote cardiomyocytes to reenter the cell cycle, it is difficult to achieve a better outcome because the poor microenvironment can affect the expression of CyclinA2, and a large number of adenoviruses entering the heart are lost.
Fibrin glue (FG), a new scaffold material for tissue engineering, has been applied to repair MI as an ideal choice (Roura et al. 2016). It provides a biomechanical support for cardiomyocytes until they are able to produce their own extracellular matrix (Guo et al. 2011). Furthermore, it can effectively improve the microenvironment surrounding cardiomyocytes, induce angiogenesis after MI, and provide excellent conditions for regeneration of cardiomyocytes (Christman et al. 2004; Xiu et al. 2014). Therefore, it was hypothesized that transplanting rAAV9-CyclinA2 combined with injectable FG had a protective effect on the infarcted myocardium of Sprague–Dawley (SD) rats. The aim was to explore whether more cardiomyocytes could be promoted to reenter the cell cycle and whether the mitosis of cells could be promoted further to decrease the infarction area and improve cardiac function after infarction based on the aforementioned combination.
Materials and methods
Construction of adenovirus vectors
The adeno-associated virus serotype 9 vector carrying the green fluorescent protein (GFP) or CyclinA2 gene was packaged and synthesized by Virovek (Hayward, CA, USA). The cytomegalovirus was used as the promoter. The fragments of murine CyclinA2 were P1 (F): 5′-ATATGAATTCCACCATGCCGGGCACCTCGAGGCA-3′ and P2 (R): 5′-GGCCGTCGACTCACACACTTAGTGTCTCTG-3′. After polymerase chain reaction amplification, the viruses were packaged. The obtained recombinant virus concentration was 2.39 × 1013 vg/ml.
Fibrin glue
The fibrin (RAAS. Shanghai, China) is a two-component system. One component consists of fibrinogen and aprotinin, and another comprises thrombin and CaCl2. These components were mixed in an injector to rapidly form a gelatinous product. The ratio of fibrinogen to thrombin in the mixture was 1:1.
Myocardial infarction and intramyocardial injection
A total of 90 male, clean-grade SD rats (weighing 200–250 g) were selected for this study. The animals were purchased from the Experimental Animal Center of Xinjiang Medical University [permission number: SYXK (Xin) 2010-0002]. They were housed in comfortable conditions with an illumination time of 12 h and free access to food and water. All of the experimental procedures were performed according to the requirements of the ethics committee of the Xinjiang Medical University, China.
The rats were randomly divided into 6 groups (15 in each group): sham, MI, rAAV9-GFP + MI, rAAV9-CyclinA2 + MI, FG + MI, and rAAV9-CyclinA2 + FG + MI. The rats were anesthetized with ketamine (80 mg/kg), xylazine (20 mg/kg), and atropine (0.006 mg/kg), and the anterior descending branch of the left coronary artery was ligated with a 7-0 silk suture, as described previously (Miyahara et al. 2006), after thoracotomy. The sham group underwent the same surgical procedure without coronary ligation.
After establishing MI models, 5 × 1011 vg/ml packed virus of rAAV9-GFP and rAAV9-CyclinA2 was diluted in 150 μl of normal saline or FG, respectively; the solution was then transfected into myocardial cells through an intramyocardial injection in the rAAV9-GFP + MI (rAAV9-GFP in normal saline), rAAV9-CyclinA2 + MI (rAAV9-CyclinA2 in normal saline), and rAAV9-CyclinA2 + FG + MI (rAAV9-CyclinA2 in FG) groups. The sham and MI groups were injected with an equal volume of normal saline at the same sites, while the FG + MI group was injected with FG (Fig. 1I).
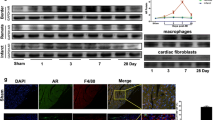
I Methods of intramuscular injection. After MI, the solution containing viruses or normal saline or FG was injected into myocardial tissue at five points (each 30 µL) at the junction of infarcted and normal tissues, which was divided into five equal sections. II Immunofluorescence staining of CyclinA2 in the myocardium of FG + CyclinA2 group. It shows a section containing papillary muscle after 5 weeks of myocardial injection. Arrows show the positive expression of CyclinA2. (a–d) Blue DAPI; red α-SMA; green CyclinA2. Scale bar 5 µm. III Immunohistochemical staining showed the expression of CyclinA2 and PCNA in each group. (e) The arrows showed that CyclinA2 express positively in the cytoplasm of myocardial cells without in the nucleus. (f) The arrows show the positive expression of PCNA in the nuclei of myocardial cells (×200, 5 µm). (Color figure online)
Echocardiography
Five weeks after the operation, echocardiography was performed on five to seven rats from each group. The echocardiography was spread with a 14-Hz ultrasound probe (Hewlett Packard Sonos 5500, USA). The left ventricular 2D image-guided M-mode curves were collected for the measurement, and three to six cardiac cycles were recorded. To obtain relevant data, heart rate, left ventricular end-systolic diameter (LVESD), and left ventricular end-diastolic dimension (LVEDD) were measured. The left ventricular short-axis ejection fraction (EF) and fractional shortening (FS) were calculated using the following formulae: EF = [(EDD3 − ESD3)/EDD3] × 100 and FS = [(EDD − ESD)/EDD] × 100. The average values were obtained for analyses.
Measurement of infarct size
After echocardiography, the rats were sacrificed by injecting excessive pentobarbital (200 mg/kg). The maximum infarction area was obtained through resection along the horizontal axis of the heart. The tissues were fixed with optimum cutting temperature fixative (Sakura Finetek, CA, USA) and sliced into 10-μm sections using a freezing microtome. Eight to ten frozen sections from each group were randomly selected, and the A, B, C, and D solutions in the Masson reagent kit (Invitrogen, USA) were sequentially used according to the instructions. The staining results were observed under a microscope to avoid excessive staining. The infarct area (blue area), as determined by percent of the left ventricular mass, was measured after staining. The Image-Pro Plus 6.0 image analysis software was used to calculate the percentage of areas in the same field of view in the immunohistochemistry analysis.
Western blot
The myocardial tissues were lysed using 600–700 μl of tissue protein lysis buffer (Beyotime Institute of Biotechnology, China). Also, the phosphatase and proteinase inhibitors were added to the tissues at a ratio of 1:100. The tissues were placed in a homogenizer for ultra-speed rotational grinding into a paste form. The rotationally ground tissues were placed on ice for lysis for approximately 1 h. The protein concentration was determined using the bicinchoninic acid method. All the manipulation procedures were performed according to the instructions. A total of 80 μg of protein was loaded for electrophoresis and transferred onto a 0.45-μm polyvinylidene difluoride membrane. After blocking in 5% nonfat milk for 1 h, the membrane was incubated with CyclinA2 (1:500, Santa Cruz, sc53227), PCNA (1:1000, Cell Signaling, 2586), and H3P (1:1000, Abcam, ab115152) antibodies that were diluted at the proper dilution ratio in 5% bovine serum albumin (BSA) on a shaker at 4 °C overnight. The membrane was washed with 0.05% Tris-buffered saline with Tween 20. The corresponding secondary antibodies were added and incubated at room temperature for 1 h. The expression of the target proteins was detected using the enhanced chemiluminescence method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the internal control. Optical density (OD) values of the target bands and the internal control were measured, and the ratio of OD values between these two measurements was used as the expression level of the target protein.
Immunohistochemistry
The tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm sections. The sections were deparaffinized at 65 °C for 40 min, dehydrated in gradient alcohol, blocked with 3% hydrogen peroxide, and rehydrated in 0.01 mmol/L citrate buffer at 92–98 °C for 10 min for antigen retrieval. After cooling down to room temperature, the sections were washed with phosphate-buffered saline (PBS) three times, and nonspecific antigens were blocked with 5% goat serum for 15 min. The CyclinA2, PCNA, and H3P antibodies were added. After the primary antibodies were diluted in BSA following the instruction, the sections were incubated overnight at 4 °C with primary antibodies. The sections were washed with PBS, followed by a 1-h incubation with a biotinylated goat anti-mouse immunoglobulin G antibody. The sections were washed with PBS three times after incubation, and the diaminobenzidine (DAB) developing solution was added. The development time was controlled under a microscope. The sections were placed in hematoxylin to stain the nuclei. Images were acquired using a Leica inverted fluorescence microscope.
Immunofluorescence
The tissues were fixed in 4% paraformaldehyde for 8 h and dehydrated in 20% sucrose overnight. The tissues were sectioned in a freezing microtome after they were embedded in OCT compound. The sections were fixed in acetone, washed with PBS three times, and added to goat serum for blocking for 1 h, followed by incubation with the properly diluted primary α-smooth muscle actin (SMA) (1:100; Proteintech; 551351-AP) and c-TnT (1:200; Abcam; ab8295) antibodies overnight at 4 °C. The sections were removed at room temperature for 30 min, washed with PBS, and incubated with fluorescein isothiocyanate (1:1000; Abcam; ab6717) and Alexa Fluor (1:1000; Abcam; ab150077) in a 37 °C incubator for 1 h. After being washed with PBS. The sections were added to a DAPI (1:500, Santa Cruz Biotechnology; sc-3598) dilution solution after washing with PBS and incubated in the dark for 7 min. The sections were washed with a formaldehyde solution for 5 min and placed in PBS. They were photographed under a Leica inverted fluorescence microscope; five regions were randomly selected to observe the expression of the corresponding factors.
Statistical analysis
All data are presented as the mean ± standard deviation. The comparison among multiple groups was analyzed using one-way analysis of variance, while pairwise comparisons were analyzed by a least significant difference test (P < 0.05). The Image-Pro Plus 6.0 image analysis software was used to calculate the percentage of areas in the same field in the immunohistochemistry analysis.
Results
Specific expression of CyclinA2 in cardiac muscles
The Western blot showed that after 5 weeks of treatment, the expression of CyclinA2 in the CyclinA2 group increased significantly compared with that in the GFP group (50.13 ± 3.80 vs. 14.07 ± 1.64%, P < 0.001). Also, the expression in the rAAV9-CyclinA2 + FG group increased significantly compared with that in the FG and CyclinA2 groups (70.1 ± 1.86% vs. 14.74 ± 2.02%, P < 0.01; and vs. 50.13 ± 3.80%; P < 0.01, respectively) (Fig. 2a, b).
Expressions of Cyclin-A2, PCNA, and H3P in the myocardium of the six groups using Western blot. a The representative Western blot of the expression of CyclinA2, PCNA, and H3P in each group. b The expression level of CyclinA2 in each group. c The expression level of PCNA showed a difference between the FG + CyclinA2 and other groups. d The expression of H3P showed a significant difference in the six groups. The size of proteins: CyclinA2: 49 kDa; PCNA: 36 kDa; H3P: 17 kDa; GAPDH: 36 kDa. # P < 0.05, *P < 0.01, **P < 0.001
Immunohistochemistry and immunofluorescence analyses showed that after CyclinA2 was transfected into the cardiac muscles for 5 weeks, the expression of CyclinA2 in the cytoplasm of the myocardial cells of SD rats was observed, whereas low expression was noted in the cell nuclei (Fig. 1II,III). Positive results were not observed in the sham group.
Expression of proliferation-related antigens
After the transfection of CyclinA2 combined with FG into the cardiac muscles for 5 weeks, the expression of PCNA increased significantly compared with that in the other groups. The comparison between the GFP + MI and rAAV9-CyclinA2 + MI groups (0.142 ± 0.0092 vs. 0.16 ± 0.0095%, P < 0.001), and the rAAV9-CyclinA2 + MI and rAAV9-CyclinA2 + FG + MI groups (0.16 ± 0.0095 vs. 0.179 ± 0.0145%, P < 0.05) both displayed significant differences (Fig. 2c). The immunohistochemistry showed that the expression of PCNA increased significantly in the rAAV9-CyclinA2 + FG group (Fig. 2d).
Cardiac function
FS and EF are indicators of cardiac systolic function. FS showed a statistically significant increment in the rAAV9-CyclinA2 + FG + MI group compared with that in the FG + MI and rAAV9-CyclinA2 + MI groups (39.2 ± 3.91% vs. 35.69 ± 3.21%, P < 0.01 and 26.07 ± 2.51%, P < 0.05, respectively). FS between the GFP and CyclinA2 groups was significantly different (13.97 ± 1.91 vs. 35.69 ± 3.21%, P < 0.05) (Fig. 3a). EF showed a statistically significant increment in the rAAV9-CyclinA2 + FG group compared with that in the FG and CyclinA2 groups (75.37 ± 4.69% vs. 56.88 ± 4.07%, P < 0.01 and 70.57 ± 3.76%, P < 0.05, respectively) (Fig. 3b).
Cardiac function indicators in each group 5 weeks after intramyocardial injection. a, b Left ventricular fractional shortening and ejection fraction after MI was measured by echocardiography, respectively. c The levels of left ventricular end-systolic diameter in the FG + CyclinA2, CyclinA2, and FG groups were significantly smaller than the MI group. d Left ventricular end-diastolic dimension showed no significant differences among the six groups after injection. # P < 0.05, *P < 0.01, **P < 0.001
Furthermore, LVESD and LVEDD are indicators of cardiac diastolic function (Fig. 3c, d). The LVESD values were as follows: 4.37 ± 5.18 mm in the rAAV9-CyclinA2 + FG group, 5.14 ± 1.11 mm in the CyclinA2 group, 5.68 ± 0.79 mm in the FG group, and 6.56 ± 0.67 mm in the MI group. The LVESD in the rAAV9-CyclinA2 + FG group was significantly higher than that in the CyclinA2 (P < 0.05) and FG groups (P < 0.01). The LVEDD values did not differ significantly among all groups (P > 0.05).
Infarction area
Masson staining was performed to evaluate the content of collagen fibers in the infarction region so as to measure the infarct size in the left ventricle. Infarct size in the MI group was 28.93 ± 2.40%, which did not differ significantly from that in the GFP group (P = 1.01). However, the infarction area was significantly less in the CyclinA2 + FG (16.63 ± 2.12%), FG (23.3 ± 11.97%), and CyclinA2 (19.88 ± 1.20%) groups than that in the MI and GFP groups (all P < 0.01). The combination of CyclinA2 and FG was more beneficial for decreasing myocardial fibrosis in the infarction region and preventing left ventricular remodeling compared with the single use of each (P < 0.05). (Fig. 4 I, II).
Infarct size and arteriole density in the infarct area 5 weeks after injection. I Infarct area (blue area) of different groups were measured 5 weeks after injection. II The infarct size of the FG + CyclinA2 group was markedly smaller than other groups after measuring. III New blood vessels were formed in the myocardium of FG + CyclinA2 group 5 weeks after injection. The green ones are the newborn vessels. IV Arteriole density of the FG + CyclinA2 group was much higher than the CyclinA2 group. # P < 0.05. (Color figure online)
Neovascularization
Neovasculature was evaluated by immunofluorescence staining using α-SMA antibody and labeled and measured as previously described (Rybinski et al. 2015). After 5 weeks, the extent of neovasculature in the marginal zone of the infarction region with different interventions was determined (Fig. 4III). The results showed 46.73 ± 3.87/mm2 neovasculature in the sham group, 12.27 ± 1.88/mm2 in the MI group, 12.62 ± 1.05/mm2 in the GFP group, 28.35 ± 3.17/mm2 in the FG + MI group, 13.07 ± 1.82/mm2 in the CyclinA2 group, and 27.91 ± 3.91/mm2 in the FG + CyclinA2 group. No significant difference was found between the FG and FG + CyclinA2 groups (P = 0.057). A significant difference in vessel density was observed between the FG + CyclinA2 and CyclinA2 groups (P < 0.05). The difference between CyclinA2 and MI groups was not significant (P > 0.05) (Fig. 4IV).
Discussion
The present study showed that rAAV9-CyclinA2 combined with FG promoted the mitosis of myocardial cells, slowed down ventricular wall remodeling, reduced infarction areas, and promoted the regeneration of microvessels of the infarct area, finally improving left ventricular function after MI. The low rate of cell survival and the rapid loss of molecules in the injection area are the key issues that must be addressed (Roura et al. 2015). Using different treatment methods to increase the number of survived myocardial cells and improve the environment in cardiac muscles after infarction has become the focus of current studies.
It was shown that myocardial cells could be regenerated after establishing the MI model in CyclinA2 transgenic mice, which was consistent with the findings of Shapiro et al. (2014). This regeneration was considered to be associated with the restart of the cell cycle after persistent expression of CyclinA2 (Cheng et al. 2007). CyclinA2 plays the role of cell cycle promotion in the G1/S and G2/M phases (Li et al. 2013). CyclinA2 activates CyclinA2-dependent kinase-2 (CDK2) by binding with it. Activated CDK2 phosphorylates a series of substrates, thus promoting DNA synthesis and mitosis regulation. The expression levels of CyclinA2, PCNA, and H3P in the combined group were higher than those in the CyclinA2 group. It might be due to FG can slow the release of cytokines (Melhem et al. 2015). This further shows that the presence of FG limits the rapid loss of rAAV9-CyclinA2, inducing the synthesis of DNA in the cell cycle after cytoplasmic aggregation. Therefore, the expression of PCNA (DNA synthesis marker) and H3P (mitotic marker) increases.
The arteriole density in the rAAV9-CyclinA2 group did not increase significantly compared with the GFP group in this study. The result indicated that CyclinA2 could only promote cells to reenter the cell cycle but could not promote arteriole regeneration, which was consistent with the results of Shapiro et al. (2014). However, FG improved the microenvironment by promoting capillary regeneration because (1) FG contained an arginine–glycine–asparagine sequence, which could activate the vascular endothelial growth factor receptor-related α5β3 integrin (Soldi et al. 1999); (2) FG provided a better matrix environment for the migration and division (branching morphogenesis) of endothelial cells (Bach et al. 1998); (3) FG contained a large amount of fibroblast growth factors that could promote angiogenesis; fibrin fragment E, a degradation product of FG, could also promote revascularization (Bootle-Wilbraham et al. 2001).
Moreover, decreasing the infarct area is also important to improving cardiac function. The present study showed that the infarct size in the combination group was smaller than those in the CyclinA2 alone and FG alone groups, and the size in the CyclinA2 group was smaller than that in the MI group. Previous studies have demonstrated that CyclinA2 promotes myocardial cell repair and improves myocardial infarct size (Shapiro et al. 2014). CyclinA2 alone can reduce infarct size and improve cardiac function; however, its effect in combination with FG is better in this study. Because FG reduced the mechanical stretch of cardiac muscle in the infarcted area at the early stage of MI when a scar has not yet formed (Christman et al. 2004). It provided internal and mechanical support for cardiac muscles by increasing stiffness in the infarction area and infarcted border zone. These effects might occur at the early stage of MI. Matrix metalloproteinase was unregulated to cause extracellular matrix degradation, thus reducing the endurance of infarction areas (Christman et al. 2004). These results confirmed the initial echocardiographic findings that FG as well as CyclinA2 improved heart function. Unfortunately, the difference in LVESD between the experimental and control groups was not statistically significant. It might be due to the small sample size. A larger sample size may reflect a significant increase in the effect of CyclinA2 and FG treatment.
CyclinA2 promoted myocardial cell repair, and FG matrix induced myoblast proliferation. Also, as an extracellular matrix, FG could affect the local microenvironment of cardiac muscle at the molecular level and also promote the regenerated arterioles to provide nourishment for myocardial cells in infarction and border areas after MI. The combination of rAAV9-CyclinA2 and FG was chosen to treat MI in a rat MI model, which increased the survival rate of the newly generated myocardial cells and improved cardiac function. The present study indicated that rAAV9-CyclinA2 combined with FG had synergistic effects on myocardial protection, and was more effective compared with rAAV9-CyclinA2 or FG alone.
The present study had its limitations. This study chose 5 weeks as the observation time because previous studies indicated that FG degraded completely in approximately 5 weeks. If the observation time was prolonged, the advantage of rAAV9-CyclinA2 combined with FG over rAAV9-CyclinA2 or FG alone might no longer be significant. Finally, large-animal studies are required to prove the safety and feasibility of the present findings.
In summary, this study indicated that rAAV9-CyclinA2 combined with FG could promote mitosis and increase the survival of cardiac muscle cells. Hence, FG provides a more ideal microenvironment for myocardial cell regeneration, thus decreasing infarction size and improving cardiac function. Therefore, rAAV9-CyclinA2 combined with FG may be used as a new strategy for treating MI.
References
Bach TL, Barsigian C, Chalupowicz DG et al (1998) VE-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp Cell Res 238:324–334
Bootle-Wilbraham CA, Tazzyman S, Thompson WD et al (2001) Fibrin fragment E stimulates the proliferation, migration and differentiation of human microvascular endothelial cells in vitro. Angiogenesis 42:69–275
Cheng RK, Asai T, Tang H, Dashoush NH et al (2007) Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res 100:1741–1748. doi:10.1161/CIRCRESAHA.107.153544
Christman KL, Vardanian AJ, Fang Q et al (2004) Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol 44:654–660. doi:10.1016/j.jacc.2004.04.040
Guo HD, Wang HJ, Tan YZ, Wu JH (2011) Transplantation of marrow-derived cardiac stem cells carried in fibrin improves cardiac function after myocardial infarction. Tissue Eng Part A 17:45–58. doi:10.1089/ten.TEA.2010.0124
Li Y, Hu S, Ma G, Yao Y et al (2013) Acute myocardial infarction induced functional cardiomyocytes to re-enter the cell cycle. Am J Transl Res 5:327–335
Li J, Li X, Wang Q, Hu S, Wang Y et al (2015) ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 385:441–451
Ma X, Zhao A, Yao Y, Cao W, Karmacharya U et al (2015) Therapeutic delivery of cyclin-A2 via recombinant adeno-associated virus serotype 9 restarts the myocardial cell cycle: an in vitro study. Mol Med Rep 11:3652–3658. doi:10.3892/mmr.2015.3147
Melhem M, Jensen T, Reinkensmeyer L et al (2015) A hydrogel construct and fibrin-based glue approach to deliver therapeutics in a murine myocardial infarction model. J Vis Exp 100:525–562. doi:10.3791/52562
Miyahara Y, Nagaya N, Kataoka M et al (2006) Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med 12:459–465. doi:10.1038/nm1391
Pleger ST, Shan C, Ksienzyk J et al (2011) Cardiac AAV9-S100A1 gene therapy rescues post-ischemic heart failure in a preclinical large animal model. Sci Transl Med 92:64–67. doi:10.1126/scitranslmed.3002097
Prasad KM, Smith RS, Xu Y, French BA (2011) A single direct injection into the left ventricular wall of an adeno-associated virus 9(AAV9) vector expressing extracellular superoxide dismutase from the cardiac troponin-T promoter protects mice against myocardial infarction. J Gene Med 13:333–341. doi:10.1002/jgm.1576
Roura S, Soler-Botija C, Bagó JR, Llucià-Valldeperas A et al (2015) Postinfarction functional recovery driven by a three-dimensional engineered fibrin patch composed of human umbilical cord blood-derived mesenchymal stem cells. Stem Cells Transl Med 8:956–966. doi:10.5966/sctm.2014-0259
Roura S, Gálvez-Montón C, Bayes-Genis A et al (2016) Fibrin, the preferred scaffold for cell transplantation after myocardial infarction? An old molecule with a new life. J Tissue Eng Regen Med. doi:10.1002/term.2129
Rybinski K, Imtiyaz HZ, Mittica B et al (2015) Targeting endosialin/CD248 through antibody-mediated internalization results in impaired pericyte maturation and dysfunctional tumor microvasculature. Oncotarget 28:25429–25440. doi:10.18632/oncotarget.4559
Shapiro SD, Ranjan AK, Kawase Y et al (2014) Cyclin A2 induces cardiac regeneration after myocardial infarction through cytokinesis of adult cardiomyocytes. Sci Transl Med 224:224–227. doi:10.1126/scitranslmed.3007668
Soldi R, Mitola S, Strasly M et al (1999) Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2. EMBO J 18:882–892. doi:10.1093/emboj/18.4.882
Van Aelst LN, Voss S, Carai P et al (2015) Osteoglycin prevents cardiac dilatation and dysfunction after myocardial infarction through infarct collagen strengthening. Circ Res 116:425–436. doi:10.1161/CIRCRESAHA.116.304599
White JD, Thesier DM, Swain JB et al (2011) Myocardial gene delivery using molecular cardiac surgery with recombinant adeno-associated virus vectors in vivo. Gene Ther 18(6):546–552. doi:10.1038/gt.2010.168
Woo YJ, Panlilio CM, Cheng RK et al (2006) Therapeutic delivery of cyclin A2 induces myocardial regeneration and enhances cardiac function in ischemic heart failure. Circulation 114:1206–1213. doi:10.1161/CIRCULATIONAHA.105.000455
Xiu J, Fan J, Li J, Cui G, Lei W (2014) Different angiogenic abilities of self-setting calcium phosphate cement scaffolds consisting of different proportions of fibrin glue. Biomed Res Int 2014:785146. doi:10.1155/2014/785146
Yusuf S, Rangarajan S, Teo K et al (2014) Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med 371:818–827. doi:10.1056/NEJMoa1311890
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 81660085). We are grateful to Cardiovascular Disease Institute, the First Affiliated Hospital of Xinjiang Medical University.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wen Cao and Ya-fei Chang contributed equally to this work and share the position of first author.
Xiang Ma and Yi-Tong Ma make an equal contribution.
Rights and permissions
About this article
Cite this article
Cao, W., Chang, YF., Zhao, AC. et al. Synergistic cardioprotective effects of rAAV9-CyclinA2 combined with fibrin glue in rats after myocardial infarction. J Mol Hist 48, 275–283 (2017). https://doi.org/10.1007/s10735-017-9725-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-017-9725-4