Abstract
Baicalein is a natural flavonoid that possesses notable anti-inflammatory effects. In this study, we detected whether baicalein protects against inflammatory response in unilateral ureteral obstruction mice model to ameliorate tubulointerstitial fibrosis. Baicalein treatment significantly attenuated tubulointerstitial fibrosis by markedly reducing fibronectin and collagen-I. The downregulation of alpha-smooth muscle actin and upregulation of E-cadherin indicated that the epithelial–mesenchymal transition process was suppressed. Furthermore, baicalein administration blocked the infiltration of macrophages and lymphocytes, as evidenced by the significantly reduced CD68 and CD3 positive cells. Meanwhile, the mRNA expression of the pro-inflammatory cytokines tumor necrosis factor-α, interleukin-1β, and monocyte chemotactic protein in baicalein-treated groups was markedly reduced compared with the vehicle-treated group. More importantly, unilateral ureteral obstruction induced the activation of NF-κB and mitogen-activated protein kinase signal pathways to switch on inflammatory response to aggravate kidney fibrosis, but these effects were mitigated by baicalein. These data demonstrate that baicalein could inhibit inflammatory process via inactivation of NF-κB and MAPK signal pathways to execute its anti-fibrotic actions in obstructive kidney disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) is a global health issue and considered as an irreversible process that leads to poor prognosis. Renal fibrosis, especially renal interstitial fibrosis, is a final common outcome caused by progressive loss of renal function in varied CKDs, as well as a major force leads CKDs to end stage renal disease (Ni et al. 2013). Unilateral ureteral obstruction (UUO) is a common animal model widely used to mimic the pathological changes of chronic obstructive nephropathy. This model can reflect inflammatory responses and fibrosis in human CKD. UUO is characterized by increased intraluminal pressure, interstitial inflammation, immediate macrophage and lymphocyte infiltration, evaluated cytokine levels, activation of myofibroblasts, and accumulation of interstitial extracellular matrix (ECM) (Eddy 2014). Previous studies demonstrated that inflammatory cells are recruited to the renal interstitium after UUO; these cells generate numerous cytokines and growth factors, which sustain and enhance inflammatory response (Crisman et al. 2001; Schreiner et al. 1988). Chronic interstitial inflammation, followed by functional renal parenchyma loss and interstitial fibrosis, mainly contributes to the deprivation of the renal function (Klahr and Morrissey 2002). Therefore, suppressing inflammatory response could attenuate renal fibrosis.
Baicalein (5,6,7-trihydroxyflavone) is a natural flavonoid extracted from the Chinese herb Scutellaria baicalensis (Hou et al. 2011). It has been widely used as a therapeutic agent for microbial infection in East Asian countries. Moreover, baicalein possesses many biochemical and pharmacological benefits, including anti-tumor, anti-fibrogenic (Oh et al. 2012; Shimizu 2001), anti-inflammatory (Liu et al. 2014; Zhang et al. 2014), and cardiovascular protective effects. Baicalein has been increasingly used against renal (Wang et al. 2012), myocardial (Kong et al. 2011), pulmonary (Gao et al. 2013), and hepatic (Inoue and Jackson 1999; Shimizu 2000; Sun et al. 2010) fibroses as a potential antioxidant. Intriguingly, several studies have also revealed the extensive anti-inflammatory effects of baicalein as a protective agent in various diseases. For example, Liu et al. (Liu et al. 2014) reported that baicalein can attenuate liver injury induced by polymicrobial sepsis by inhibiting inflammation. Furthermore, baicalein reduces airway inflammation in allergen and IL-1β-induced asthma model. Baicalein also exhibits protective properties in kidney injury (Wu et al. 2014) and fibrosis (Wang et al. 2012). It promotes the recovery of renal function, alleviates kidney injury in ischemia–reperfusion (I/R) model, and ameliorates kidney fibrosis through downregulated TGF-β1 and Smad-2 in UUO mice model. However, whether baicalein has an effect on inflammation in obstructed kidneys and the mechanisms involved in this response is not clearly elucidated. Given these data, we hypothesize that baicalein may have a potential to inhibit inflammation in UUO model to ameliorate renal fibrogenesis.
Materials and methods
Animal model
Forty male C57/BL6 mice (aged 6–8 weeks) were provided by Wuhan University Center for animal experiment (Wuhan, China). The Institutional Animal Care and Use Committee of Wuhan University approved the animal work protocol, which was performed in accordance with the Principles of Laboratory Animal Care (NIH publication Vol. 25, No. 28, revised 1996). Left ureters of the mice were exposed and subsequently ligated to induce the UUO model as an established procedure (Eddy et al. 2012). Mice were then randomly divided into four groups (n = 10), namely, sham surgery, UUO plus vehicle (UUO + V), UUO plus 50 mg/kg/day baicalein (Nanjing Dilger Medical Technology Co., Ltd (Nanjing, China), and UUO plus 100 mg/kg/day baicalein. Baicalein was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich Company Ltd, Dorset, UK), and the mice were administered with baicalein daily by oral gavage. Baicalein administration started the day after the surgery until the seventh day when the mice were sacrificed. In the vehicle-treated group, mice were administered with 300 μl of PBS in DMSO. After the experiment was completed, the obstructed kidneys of the mice were harvested.
Western blot analysis
Tissue samples were collected as described and homogenized in lysis buffer (Biyuntian, Haimen, China) with a polytron homogenizer (IKA GmbH, Königswinter, Germany) on ice. The lysates were then denatured in sodium dodecyl sulfate (SDS) loading buffer and subsequently separated by SDS–polyacrylamide gel electrophoresis. Proteins were transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA) for 1 h. The membranes were blocked with 5 % non-fat milk in tris-buffered saline (TBS) and incubated with primary antibodies against collagen-1, fibronectin, alpha-smooth muscle actin (α-SMA), NF-κB P65 (Abcam, USA); E-cadherin, IκBα, p38, p-p38, p-extracellular receptor kinase (ERK), ERK, p–c-Jun N-terminal kinase (JNK), and JNK (Cell Signaling Technology, USA). Incubation was conducted overnight at 4 °C in a blocking buffer at the dilutions specified by the preliminary experiment. The membranes were then rinsed three times in TBST and incubated with a secondary antibody (LICOR, USA; 1:1000 dilution) conjugated to horseradish peroxidase for 1 h. Finally, the membranes were scanned under a two-color infrared imaging system (Odyssey, LICOR, USA). Membranes were probed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an additional loading control.
Histological and immunohistochemical studies
Kidneys were embedded in paraffin and sectioned at 4 µm using an established procedure. Masson’s trichrome staining was performed for histomorphometric analysis. For immunohistochemical studies, kidney sections were incubated in 3 % H2O2 to block endogenous peroxidase activity for 10 min. Five percent bovine serum albumin was used for blocking non-specific binding for about 1 h. Tissues were subsequently incubated with primary rabbit anti-mouse antibodies (collagen-1, fibronectin, CD3, and CD68; Abcam) overnight at 4 °C. Tissues were then incubated with goat anti-rabbit secondary antibody for another 1 h. Peroxidase–streptavidin–biotin complex (Boshide Biotechnology Co., Ltd., Wuhan, China) and diaminobenzidine (Sigma) were used to visualize the proteins. Ten random fields were selected (200 magnifications), photographed, and measured with Image Pro-Plus 6.0 software.
Real-time PCR
Total RNA was isolated from the renal cortex using Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. A cDNA copy was created with the PrimeScriptTM RT reagent kit (Takara, Japan). Subsequently, gene expression was analyzed with one-step real-time (RT) PCR, and mouse GAPDH was used to normalize the relative value of different genes. The reactions were conducted in a 20 µl volume. RT-PCR was performed on the resulting cDNA with the SYBR Green Mix and the AB7500 RT-PCR detection system. The sequences of the primer were as follows: forward 5′-TCCCCAAAGGGATGAGAAG-3′, reverse 5′-CACTTGGTGGTTTGCTACGA′ for mouse tumor necrosis factor-α (TNF-α); forward 5′-GCAACTGTTCCTGAACTCAACT-3′, reverse 5′-ATCTTTTGGGGTCCGTCCAACT-3′ for mouse IL-β; forward 5′-TTTTGTCACCAAGCTCAAGAGA-3′, reverse 5′-ATTAAGGCATCACAGTCCGAGT-3′ for mouse monocyte chemotactic protein (MCP-1); and forward 5′-AGTGGCAAAGTGGAGATT-3′, reverse 5′-GTGGAGTCATACTGGAACA-3′ for mouse GAPDH. PCR was performed under normal conditions. The threshold cycle (Ct) values of each sample were calculated with the 2−ΔΔCT data analysis method.
Statistical analysis
All experiments were repeated three times independently. Data were expressed as mean ± SD. One way ANOVA was used for the statistical analysis by using SPSS 19.0 software. P < 0.05 was considered statistically significant.
Results
Baicalein attenuates renal fibrogenesis after UUO
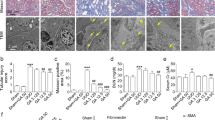
After 7 days of ureteral obstruction, the mice showed typical features of renal tubulointerstitial fibrosis in ligated kidneys, as revealed by Masson’s staining. Immunohistochemistry staining also showed that early fibrosis markers, such as collagen-1 and fibronectin, were accumulated in obstructive kidneys. Compared with the vehicle-treated group, baicalein treatment (50 and 100 mg/kg/day) ameliorated collagen accumulation and resulted in reduced renal cortical expressions of fibronectin and collagen-1 in renal interstitium (Fig. 1a). This finding is confirmed by the results of quantitative immunohistochemical determination of fibronectin and collagen-1 (Fig. 1b). In addition, the same results were also observed using Western blot analysis (Fig. 1c, d). These data suggest that baicalein could inhibit renal fibrogenesis in UUO model.
Baicalein attenuates interstitial matrix disposition in the obstructed kidney at 7 days after UUO. a Representative images of Masson’s staining (green signal) and immunohistochemistry staining (brown signal) of collagen-1 and fibronectin, original magnification ×200. b Semi-quantitative analysis of positive area for immunohistochemistry staining in different groups. c Representative immunoblotting of fibronectin and collagen-1. d Quantitative evaluation of fibronectin and collagen-1 protein expression. + P < 0.01 versus sham group, *P < 0.01 versus UUO with vehicle-treated group, # P < 0.01 versus UUO with 50 mg/kg/day baicalein-treated group, and T P < 0.05 versus UUO with 50 mg/kg/day baicalein-treated group. Scale bars, 100 µm. (Color figure online)
Baicalein prevents inflammation in the kidney during obstruction
Baicalein has numerous effects on the infiltration of inflammatory cells in chronic fibrotic diseases; thus, the expressions of CD68 (a macrophage marker) and CD3 (a lymphocyte marker) were examined at 7 days after UUO. Compared with the vehicle group, baicalein significantly reduced the CD68 and CD3 expressions. This significant decrease in expressions indicates that the number of infiltrative macrophage and lymphocyte was markedly inhibited after baicalein treatment. Quantitative immunohistochemical analysis demonstrated the decrease of CD68 and CD3 positive cells after baicalein treatment (Fig. 2).
Baicalein suppresses the infiltration of macrophage and lymphocytes in the obstructed kidneys. a CD68 and CD3 positive cells stained with immunohistochemistry as indicated in the figure. b The corresponding group data show the number of positive staining cells. *P < 0.01 versus UUO with vehicle-treated group, and T P < 0.05 versus UUO with 50 mg/kg/day baicalein-treated group. Scale bars, 100 µm
To verify the anti-inflammatory effect of baicalein on the obstructed nephropathy, we tested the mRNA levels of TNF-α, IL-1β, and MCP-1 on day 7 after the UUO method. These pro-inflammatory cytokines were significantly induced in obstructed kidneys compared with those in non-obstructed kidneys, whereas baicalein administration suppressed the expression of these pro-inflammatory cytokines compared with the UUO group (Fig. 3).
Baicalein reduces the release of the pro-inflammatory cytokines TNF-α, IL-1β, and MCP-1 at day 7 after UUO. TNF-α, IL-1β, and MCP-1 mRNA levels were measured using real-time PCR after various treatments. + P < 0.01 versus sham group, *P < 0.01 versus UUO with vehicle-treated group, △ P > 0.05 versus UUO with 50 mg/kg/day baicalein-treated group, and # P < 0.01 versus UUO with 50 mg/kg/day baicalein-treated group
Baicalein blocks the epithelial–mesenchymal transition (EMT) during renal fibrosis
In fibrotic kidneys, tubular epithelial cells lose their marker E-cadherin and transform to activated myofibroblasts, which are characterized with positive α-SMA or vimentin. This process is called EMT. To investigate whether baicalein modulates the accumulation of myofibroblastic fibrogenic cells, we measured the renal expressions of α-SMA and E-cadherin on postoperative day 7. As shown in Fig. 3a, bolts indicate the decreased α-SMA expression and retention of E-cadherin expression after baicalein treatment, indicating that the EMT process was inhibited by baicalein administration (Fig 4).
Baicalein inhibits the EMT process in the obstructed kidneys at day 7 after UUO operation. a Western blot analysis for α-SMA and E-cadherin in different treatment groups. b Statistical analysis of relative expression of α-SMA and E-cadherin. + P < 0.01 versus sham group, *P < 0.01 versus UUO with vehicle-treated group, and T P < 0.05 versus UUO with 50 mg/kg/day baicalein-treated group
Baicalein abolishes the activation of NF-kB and MAPK to decrease inflammation in obstructed kidney
Mitogen-activated protein kinase (MAPK) and NF-κB P65 are important inflammatory response pathways in kidney fibrosis development. Thus, we assessed the influence of baicalein on the activation of these two signal pathways. Figure 5a shows that NF-κB P65 activation and degradation of inhibitory subunit, IκBα, were observed on day 7 post-operation. However, this response was dramatically suppressed after baicalein treatment.
Seven days of ureteral obstruction triggered the phosphorylation of p38, JNK, and ERK. Meanwhile, the phosphorylation proteins were significantly attenuated in baicalein-treated groups, both in 50 and 100 mg/kg/day groups (Fig. 5b)
Discussion
This study is the first to demonstrate that baicalein ameliorates inflammation response in the progression of renal interstitial fibrosis. We found that baicalein inhibited the accumulation of the ECM components fibronectin and collagen in the obstructed kidney model. Interestingly, baicalein could block the EMT process, paralleled with suppressing the infiltration of macrophages and T lymphocytes, as well as inhibiting cytokine release (TNF-α, IL-1β, and MCP-1), via inactivation of the NF-κB and MAPK signal pathways. These findings suggest that baicalein plays a critical anti-inflammatory role in renal fibrosis and could be a therapeutic candidate against renal fibrogenesis.
In fibrotic kidneys, renal tubular epithelial cells lose their hallmark E-cadherin and acquire the phenotype of α-SMA, transforming to activated myofibroblasts. These myofibroblasts are the major source of ECM and contribute to the progression of kidney fibrosis. The EMT process plays an essential role in the pathogenesis of renal fibrosis (Liu 2011). In this study, our data demonstrate that baicalein reduced the expression of α-SMA, whereas E-cadherin was preserved in the fibrotic mice model. This result suggests that the role of baicalein in stabilizing the epithelial phenotype is partially due to TGF-β1/Smad3 signaling blockade.
The activation of inflammatory cascade is an early feature of CKD. This feature is speculated as one of the detrimental contributors to the occurrence and development of tubulointerstitial fibrosis. The classical concept on the connection between inflammation and fibrosis is that infiltrated inflammatory cells release profibrotic cytokines, and then act on renal tubular cells and resident fibroblasts to facilitate renal fibrogenesis via a paracrine fashion (Liu 2011). This hypothesis is conformed experimentally, as activated mononuclear cells release cytokines, which induce matrix production and EMT (Nightingale et al. 2004). At the beginning of the kidney injury, T lymphocytes and macrophages were recruited to the damaged site (Lee and Kalluri 2010), and secrete fibrogenic cytokines, including TNF-α, MCP-1, IL-1β and 8β, which are the markers of inflammation (Vielhauer et al. 2010). In addition, Macrophages are a major source of transforming growth factor-β1 (TGF-β1) in fibrotic organs (Ricardo et al. 2008), which is considered to be a major mediator in fibrosis to promote EMT. Emerging evidence show an close connection at the molecular level exists between inflammatory signal pathways and fibrosis within the same cells. For example, IL-1β (Fan et al. 2001) and IL-8β (Bani-Hani et al. 2009) are profibrogenic cytokines capable of inducing EMT and ECM accumulation through activation of TGF-β1 signal pathway, and inhibition of IL-1β ameliorates early experimental renal interstitial fibrosis (Jones et al. 2009). Thus, the decreasing the recruitment of macrophage in renal interstitium and reducing the inflammatory cytokines TNF-α, IL-1β, and MCP-1 induced by baicalein may possesses an anti-fibrotic property.
In addition, experimental data prove that snail is stabilized by the tumor necrosis factor via the activation of NF-κB (Wu et al. 2009). Because snail is a crucial transcription factor inducing pathological EMT, and progression of later fibrosis (Boutet et al. 2006), this finding sets up a molecular link between inflammation and fibrosis. NF-κB is activated in renal fibrosis (Esteban et al. 2004) and upregulates many cytokines and chemokines, contributing to kidney inflammation in obstructed kidneys (Panzer et al. 2009; Tashiro et al. 2003). Thus, inhibiting the activation of NF-κB alleviates renal tubular cell apoptosis and renal fibrosis in obstructed kidneys (Tashiro et al. 2003). Furthermore, blocking the nuclear translocation of NF-κB p65 attenuates pro-inflammatory cytokines (TNF-α, IL-1β) and chemokines (MCP-1), which dissolve inflammation (Zheng et al. 2013). Baicalein possesses a protective effect in radiation-induced injury by modulating NF-κB-mediated inflammatory response via the MAPKs and Akt pathways. Based on these findings, our study reveals NF-κB p65 activation induced by p65 phosphorylation after UUO was reduced by baicalein treatment, which is a potential mechanism for the anti-inflammatory effect of baicalein on renal fibrosis.
MAPK signal pathway is involved in modulating inflammatory action response to various stresses in progressive renal fibrosis. MAPK family consists of three major members, namely, the ERKs, JNKs, and p38 MAPKinases. In chronic kidney disease, P38 MAPK pathway is activated after UUO, and blockade of this signal reduces ECM accumulation and inflammation (Stambe et al. 2004). JNK is upregulated in response to kidney inflammation and subsequently increases MCP-1 expression to recruit inflammatory cells toward the damaged tubulointerstitium (de Borst et al. 2009). Meanwhile, MAPK-ERK1/2 is induced by ureteral obstruction, and inhibition of this pathway contributes to prevent progression of renal fibrosis (Rodriguez-Pena et al. 2008). Baicalein has been proven to attenuate inflammatory responses by inactivating p38 MAPK, JNK1/2, and NF-κB P65 signal pathways. In agreement with these studies, we demonstrated that baicalein prevented the ERK, JNK, and p38 MAPK activation in the obstructed kidneys. These results suggest that MAPKs and NF-κB inhibition are involved in the protective effects of baicalein in renal fibrosis.
In conclusion, this study identified direct anti-fibrotic effects of baicalein in obstructed nephropathy. The anti-fibrotic mechanisms of baicalein may involve its anti-inflammatory effects, mainly via blockade of NF-κB and MAPK signaling. These findings indicate that baicalein could be used as a therapeutic alternative for early intervention of renal fibrosis.
References
Bani-Hani AH et al (2009) IL-18 neutralization ameliorates obstruction-induced epithelial–mesenchymal transition and renal fibrosis. Kidney Int 76:500–511. doi:10.1038/ki.2009.216
Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA (2006) Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO J 25:5603–5613. doi:10.1038/sj.emboj.7601421
Crisman JM, Richards LL, Valach DP, Franzoni DF, Diamond JR (2001) Chemokine expression in the obstructed kidney. Exp Nephrol 9:241–248
de Borst MH et al (2009) c-Jun NH2-terminal kinase is crucially involved in renal tubulo-interstitial inflammation. J Pharmacol Exp Ther 331:896–905. doi:10.1124/jpet.109.154179
Eddy AA (2014) Overview of the cellular and molecular basis of kidney fibrosis. Kidney Int Suppl 4:2–8. doi:10.1038/kisup.2014.2
Eddy AA, Lopez-Guisa JM, Okamura DM, Yamaguchi I (2012) Investigating mechanisms of chronic kidney disease in mouse models. Pediatr Nephrol 27:1233–1247. doi:10.1007/s00467-011-1938-2
Esteban V et al (2004) Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway, regulates the inflammatory response in unilateral ureteral obstruction. JASN 15:1514–1529
Fan JM, Huang XR, Ng YY, Nikolic-Paterson DJ, Mu W, Atkins RC, Lan HY (2001) Interleukin-1 induces tubular epithelial-myofibroblast trans differentiation through a transforming growth factor-beta1-dependent mechanism in vitro. Am J kidney Dis 37:820–831
Gao Y, Lu J, Zhang Y, Chen Y, Gu Z, Jiang X (2013) Baicalein attenuates bleomycin-induced pulmonary fibrosis in rats through inhibition of miR-21. Pulm Pharmacol Ther 26:649–654. doi:10.1016/j.pupt.2013.03.006
Hou YC, Lin SP, Tsai SY, Ko MH, Chang YC, Chao PD (2011) Flavonoid pharmacokinetics and tissue distribution after repeated dosing of the roots of Scutellaria baicalensis in rats. Planta Med 77:455–460. doi:10.1055/s-0030-1250433
Inoue T, Jackson EK (1999) Strong antiproliferative effects of baicalein in cultured rat hepatic stellate cells. Eur J Pharmacol 378:129–135
Jones LK et al (2009) IL-1RI deficiency ameliorates early experimental renal interstitial fibrosis. Nephrol Dial Transplant 24:3024–3032. doi:10.1093/ndt/gfp214
Klahr S, Morrissey J (2002) Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol 283:F861–F875. doi:10.1152/ajprenal.00362.2001
Kong EK, Yu S, Sanderson JE, Chen KB, Huang Y, Yu CM (2011) A novel anti-fibrotic agent, baicalein, for the treatment of myocardial fibrosis in spontaneously hypertensive rats. Eur J Pharmacol 658:175–181. doi:10.1016/j.ejphar.2011.02.033
Lee SB, Kalluri R (2010) Mechanistic connection between inflammation and fibrosis. Kidney Int Suppl 78:S22–S26. doi:10.1038/ki.2010.418
Liu Y (2011) Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 7:684–696. doi:10.1038/nrneph.2011.149
Liu A et al (2014) Baicalein protects against polymicrobial sepsis-induced liver injury via inhibition of inflammation and apoptosis in mice. Eur J Pharmacol 748C:45–53. doi:10.1016/j.ejphar.2014.12.014
Ni H, Chen J, Pan M, Zhang M, Zhang J, Chen P, Liu B (2013) FTY720 prevents progression of renal fibrosis by inhibiting renal microvasculature endothelial dysfunction in a rat model of chronic kidney disease. J Mol Histol 44:693–703. doi:10.1007/s10735-013-9521-8
Nightingale J, Oncostatin M et al (2004) A cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. JASN 15:21–32
Oh KS, Oh BK, Park CH, Mun J, Won SH, Lee BH (2012) Baicalein potently inhibits Rho kinase activity and suppresses actin stress fiber formation in angiotensin II-stimulated H9c2 cells. Biol Pharm Bull 35:1281–1286
Panzer U et al (2009) Resolution of renal inflammation: a new role for NF-kappaB1 (p50) in inflammatory kidney diseases American journal of physiology. Renal Physiol 297:F429–F439. doi:10.1152/ajprenal.90435.2008
Ricardo SD, van Goor H, Eddy AA (2008) Macrophage diversity in renal injury and repair. J Clin Investig 118:3522–3530. doi:10.1172/JCI36150
Rodriguez-Pena AB, Grande MT, Eleno N, Arevalo M, Guerrero C, Santos E, Lopez-Novoa JM (2008) Activation of Erk1/2 and Akt following unilateral ureteral obstruction. Kidney Int 74:196–209. doi:10.1038/ki.2008.160
Schreiner GF, Harris KP, Purkerson ML, Klahr S (1988) Immunological aspects of acute ureteral obstruction: immune cell infiltrate in the kidney. Kidney Int 34:487–493
Shimizu I (2000) Sho-saiko-to: Japanese herbal medicine for protection against hepatic fibrosis and carcinoma. J Gastroenterol Hepatol 15(Suppl):D84–D90
Shimizu I (2001) Antifibrogenic therapies in chronic HCV infection. Curr Drug Targets Infect Disord 1:227–240
Stambe C, Atkins RC, Tesch GH, Masaki T, Schreiner GF, Nikolic-Paterson DJ (2004) The role of p38alpha mitogen-activated protein kinase activation in renal fibrosis. JASN 15:370–379
Sun H, Che QM, Zhao X, Pu XP (2010) Antifibrotic effects of chronic baicalein administration in a CCl4 liver fibrosis model in rats. Eur J Pharmacol 631:53–60. doi:10.1016/j.ejphar.2010.01.002
Tashiro K et al (2003) Attenuation of renal fibrosis by proteasome inhibition in rat obstructive nephropathy: possible role of nuclear factor kappaB. Int J Mol Med 12:587–592
Vielhauer V, Kulkarni O, Reichel CA, Anders HJ (2010) Targeting the recruitment of monocytes and macrophages in renal disease. Semin Nephrol 30:318–333. doi:10.1016/j.semnephrol.2010.03.006
Wang YGM, Sun S, Dai J, Cao H, Zheng N, Fang J, Gou X, Lu X, Zhang Y (2012) The effects of baicalein on rat renal fibrosis and the experssions of TGF-β1 and Smad-2 (in Chinese). J Chang Univ Tradit Chin Med 28:383–385
Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP (2009) Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer Cell 15:416–428. doi:10.1016/j.ccr.2009.03.016
Wu K, Li H, Tian J, Lei W (2014) Protective effect of baicalein on renal ischemia/reperfusion injury in the rat. Renal Fail 1–7. doi:10.3109/0886022X.2014.991999
Zhang X et al (2014) Baicalein ameliorates inflammatory-related apoptotic and catabolic phenotypes in human chondrocytes. Int Immunopharmacol 21:301–308. doi:10.1016/j.intimp.2014.05.006
Zheng X et al. (2013) Protective effects of chronic resveratrol treatment on vascular inflammatory injury in streptozotocin-induced type 2 diabetic rats: role of NF-kappa B signaling. Eur J Pharmacol. doi:10.1016/j.ejphar.2013.10.034
Author information
Authors and Affiliations
Corresponding author
Additional information
Wei Wang and Pang-hu Zhou have contributed equally to this article.
Rights and permissions
About this article
Cite this article
Wang, W., Zhou, Ph., Xu, Cg. et al. Baicalein attenuates renal fibrosis by inhibiting inflammation via down-regulating NF-κB and MAPK signal pathways. J Mol Hist 46, 283–290 (2015). https://doi.org/10.1007/s10735-015-9621-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-015-9621-8