Abstract
Transformer 2β (Tra2β), a member of the serine/arginine-rich-like protein family, is an important RNA-binding protein involved in alternative splice. Deregulation of Tra2β has been observed in several cancers. However, the detailed role of Tra2β in non-small cell lung cancer (NSCLC) has not been elucidated. In this study, the contribution of Tra2β to NSCLC development was investigated. On histological level, the expression of Tra2β was determined by Western and immunohistochemistry assays. It demonstrated that Tra2β was expressed higher in NSCLC tumor tissues compared with adjacent non-tumor tissues. In addition to confirm the association of Tra2β expression with histological differentiation and clinical stage (p < 0.05), we also confirmed significant positive correlation between the expression level of Tra2β and that of Ki67 (p < 0.05, r = 0.446) by Spearman rank correlation test. Moreover, high expression of Tra2β predicted poor prognosis by Kaplan–Meier survival analysis. And Tra2β among with other clinicopathologic variables was an independent prognostic indicator for patients’ overall survival by multivariate analysis. On cellular level, Tra2β expression was demonstrated to promote proliferation of NSCLC cells through a series of assays, including serum starvation and release assay, Western blot assay and flow cytometry analysis. Moreover, knockdown of Tra2β was confirmed to inhibit proliferation and to induce apoptosis of NSCLC cells through flow cytometry analysis, western analysis, cell counting kit-8 assay and Tunnel assay. Our results indicated that Tra2β was involved in the tumorigenesis of NSCLC and might be a potential therapeutic target of NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the most common causes of cancer death in the United States (Siegel et al. 2013). Among all lung cancer cases, non-small cell lung cancer (NSCLC) accounts for more than 80 %, which including adenocarcinoma, squamous cell carcinoma, adenosquamous cell carcinoma, and large cell carcinoma (Buyukcelik et al. 2004; Zhang et al. 2014). Despite advances achieved in lung cancer treatment and tumor molecular biology, prognosis of this disease remains still poor. Comprehensive analysis of NSCLC with all stages and subtypes revealed that the overall 5-year survival rate of NSCLC remains less than 15 % (Cai et al. 2013; Jemal et al. 2011). Therefore, it is urgent to identify new molecular targets of lung cancer which will benefit both diagnosis and treatment of NSCLC (Kim et al. 2006).
Transformer 2β (Tra2β), also known as RA301 and SFRS10, is a serine/arginine-rich (SR)-like protein. It was originally cloned as a novel RNA-binding protein in rat astrocytes exposed to hypoxia followed by reoxygenation (Matsuo et al. 1995). Tra2β belongs to the SR-like protein family and has an RRM and two RS domains (Best et al. 2013). Tra2β protein is encoded by the TRA2B gene on human chromosome 3. The TRA2B gene consists of 10 exons and 9 introns, and generates 5 transcripts (Tra2β1–5) by alternative splicing (Nayler et al. 1998). Alternative splicing of pre-mRNAs is a powerful mechanism to regulate gene function, and the majority of protein-coding genes undergo alternative splicing (Tazi et al. 2009). As a RS domain-containing splicing factor, Tra2β is structurally and functionally related to the classical SR proteins, and it has been reported to influence alternative splicing of transcripts essential for the proper functions of multiple tissues (Roberts et al. 2014). Tra2β was shown to play an important role in normal development and is essential for the development of normal mouse embryonic and brain. Tra2β deficiency in mice resulted in early embryonic lethality (Grellscheid et al. 2011; Mende et al. 2010). Besides, Tra2β has been reported to be associated with several pathologic conditions: including stroke, tumorigenesis, silicosis, nerve injury and arteriosclerosis (Best et al. 2013; Daoud et al. 2002; Kiryu-Seo et al. 1998; Segade et al. 1995; Tsukamoto et al. 2001). Tra2β is now considered as one of the important splicing regulators that are involved in the progression of several diseases including cancer (Takeo et al. 2009).
Deregulation of Tra2β has been observed in several cancers, including breast cancer, cervical cancer, endometrial cancer, gastric cancer cells and colon cancer cells (Gabriel et al. 2009; Kajita et al. 2013; Ouyang et al. 2011; Watermann et al. 2006). Tra2β1 was over-expressed in breast cancer and it could facilitate alternative splicing of the CD44 gene via binding to CD44 exons v4 and v5 (Watermann et al. 2006). In endometrial cancer, Tra2β1 protein levels were elevated in poorly differentiated cases and it was an independent prognostic factor for endometrial cancer (Ouyang et al. 2011). Tra2β was regulated by Ets1 and heat shock factor1 in colon cancer cells. Silencing of Tra2β inhibited proliferation of HCT116 cells and caused apoptotic cell death (Kajita et al. 2013). By genome-scale co-expression network analysis, Tra2β was identified to be involved in lung cancer (Bidkhori et al. 2013). However, the detailed role of Tra2β in NSCLC has not been elucidated.
In this study, we aim to investigate the role of Tra2β in NSCLC’s progression. Expression level of Tra2β in eight paired tumor and adjacent non-tumor tissues were assessed by Western blot analysis. Immunohistochemistry (IHC) assay was performed in 83 NSCLC samples. We also investigated the association of Tra2β expression with clinical and pathologic parameters, as well as its implication for clinical prognosis. Moreover, Tra2β siRNA transfection was used to explore the effects of on NSCLC cell proliferation and apoptosis.
Materials and methods
Patients and specimens
NSCLC cancer specimens were collected of 83 patients who underwent salvage ectomy in Affiliated Hospital of Nantong University from the period of 2006–2009. This study was approved by the ethics committee of Affiliated Hospital of Nantong University and written informed consent was obtained from all patients. Patients who had received chemotherapy or radiotherapy prior to surgery were excluded. All samples were classified according to the World Health Organization classification guidelines. The main clinical and pathologic variables of patients were shown in Table 1. In addition, eight paired tumor and adjacent non-tumor specimens were snap-frozen in liquid nitrogen and stored at −80 °C for specific analysis.
Western blotting
Tissue and cell protein were immediately homogenized in a in RIPA buffer [50 mM Tris–Cl (pH 7.5), 120 mM NaCl, 10 mM NaF, 10 mM sodium pyrophosphate, 2 mM EDTA, 1 mM Na3VO4, 1 mM PMSF, and 1 % NP-40] containing a protease inhibitor cocktail (Roche, Basel, CH), and then centrifuged at 12,000g for 20 min to collect the supernatant. The protein content of the lysates was measured with a Bio-Rad protein assay (BioRad, Hercules, CA, USA). The supernatant diluted in 2× SDS loading buffer and boiled for 15 min. Proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride filter (PVDF) membranes (Millipore, Bedford, MA, USA). Membranes were blocked in 5 % non-fat milk. The primary antibodies used included anti-Tra2β (1:1,000, Abcam), anti-PCNA (1:1,000, Santa Cruz Biotechnology), anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, 1:1,000 Santa Cruz Biotechnology.), anti-Cyclin A (1:1,000, Santa Cruz Biotechnology), anti-caspase 3 (1:500, Santa Cruz Biotechnology). The peroxidase-linked species-specific antibody (Amersham, Arlington Heights, IL, USA) was used as secondary antibody.
Immunohistochemistry
All samples were fixed in 10 % formalin, then embedded in paraffin, and sectioned consecutively at 4 μm. All sections were deparaffinized and dehydrated. Antigen retrieval was performed by heating for 20 min at 121 °C in citrate buffer (0.01 mmol/L, pH 6. 0). After antigen retrieval, the slides were washed with phosphate-buffered saline (PBS) and incubated with normal goat serum to block nonspecific staining. Tissue sections were then incubated with Tra2β and Ki-67 primary antibody at 4 °C overnight. Following washing in PBS, the slides were treated with goat anti-rabbit antibody (Zhongshan Jinqiao Biotechnology Co., Ltd.). After rinsing in PBS, the sections were visualized with diaminobenzidine solution (DAB). Then the slides were counterstained with hematoxylin, dehydrated, and coverslipped. Immunohistochemical evaluation was performed as previously described (Lv et al. 2014).
Cell culture and transfections
The human lung cancer cell line A549 was purchased from China Academy of Science cell library. A549 was maintained in Nutrient Mixture F-12 Ham (SIGMA,USA) supplemented with 10 % fetal bovine serum (FBS), 2 mM l-glutamine and 100 U/mL penicillin–streptomycin mixture (GibCo BRL, Grand Island, NY) at 37 °C and 5 % CO2. Human bronchial epithelial cell line BEAS-2B was gifted from Department of Pathology of Nantong University, and cells were cultured in high-glucose DMEM (GibCo BRL, Grand Island, NY, USA) supplemented with 10 % fetal bovine serum, 100-U/mL penicillin–streptomycin mixture (GibCo BRL, Grand Island, NY, USA) at 37 °C and 5 % CO2 The shRNA targeting Tra2β sequences were: 5′-AGCTAAAGAACGTGCCAAT-3′, 5′-CCGATGTGTCTATTGTATA-3′, 5′-ACGCCAACACCAGGAATTT-3′ and 5′-GAGTATTTGGGCTGAGCTT-3′. Cell transfections were performed as previously described (Wang et al. 2013).
Cell viability assay
After treatment according to the protocols, cells were seeded at 2 × 104 per well in 100 μL medium in 96-well plates and incubated overnight. Then Counting Kit-8 reagents (Dojindo, Japan) was added to each well, and incubated at 37 °C for 2 h. The absorbance was recorded at 450 nm.
Flow cytometric analysis
For cell cycle analysis, cells were collected and fixed with 70 % cold ethanol at −20 °C overnight. Then cells were incubated with 1 mg/mL RNase A for at 37 °C 30 min. Subsequently, cells were stained with propidium iodide (50 µg/mL PI) in PBS, 0.5 % Tween-20, and analyzed using a Becton–Dickinson flow cytometer BD FACScan (San Jose, CA, USA).
Terminal deoxynucleotidyl transferase-mediated biotinylated-dUTP nick-end labeling
A549 cells were seeded in 24-well plates on coverslips for 24 h incubation, and then transfected with control-shRNA and Tra2β-shRNA for 48 h. Then cells were subjected to TUNEL staining by using an In Situ Cell Death Detection Kit (Roche, Mannheim, Germany) according to the manufacturer’s protocols. Cells were fixed in 4 % paraformaldehyde at 4° C for 30 min, then treated with 0. 1 % TritonX-100, and labeled with fluorescein-12-dUTP using terminal deoxynu-cleotidyl transferase. Photographs were detected by fluorescence microscopy (Leica, DM 5000B; LeicaCTR 5000; Germany).
Statistical analysis
To analyses the association between Tra2β expression and clinicopathological features χ2 test was used. Survival curves were calculated by the Kaplan–Meier method, and the log-rank test was used. Multivariate analysis was performed using Cox’s proportional hazards model (Hu et al. 2014). Relationship between Tra2β and Ki-67 expression in NSCLC was measured using the Spearman rank correlation test. Other data were analyzed with Student´s t test. p < 0.05 was considered to be statistically significant. All statistical analyses were performed using SPSS 13.0 software.
Results
Tra2β was over-expressed in human NSCLC tissues and NSCLC cell line
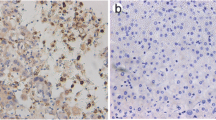
Western blot analysis was performed to detect different expression level of Tra2β in eight paired tumor and adjacent non-tumor tissues of NSCLC, and in two cell lines including NSCLC cell line A549 and normal human bronchial epithelial cell line BEAS-2B. As shown in Fig. 1a, in most cases, Tra2β expression was significantly higher in tumor tissues than in peritumoral tissues. Moreover, the expression pattern of Tra2β in NSCLC was similar to that of PCNA, which is a proliferation marker. Consistent with its expression pattern in NSCLC tissues, Tra2β expression was higher in A549 cells than in BEAS-2B cells (Fig. 1b). To further evaluate Tra2β expression and its clinical significance in NSCLC, IHC assay was performed to determine the expression of Tra2β and Ki-67, which is also a cell proliferation index, in 83 NSCLC samples. Immunoreactivity of Tra2β and Ki-67 was seen predominantly in the nucleus (Fig. 2). Moreover, the expression of Tra2β and Ki-67 also showed the same stain tendency. Both of their expression was up-regulated in NSCLC tissues compared with non-tumor tissues, which showed rare or almost none expression. In addition, less differentiated tumor tissues showed much significant higher expression of Tra2β (Fig. 2).
Tra2β was over-expressed in human NSCLC tissues and NSCLC cell line. a Western blot was performed to detect expression of Tra2β and PCNA in eight representative paired NSCLC tumor tissues (T) and adjacent non-tumor tissues (N). GAPDH was used as a loading control. The bar chart demonstrates the relative levels of Tra2β protein to GAPDH by densitometry. The data are mean ± SEM (*p < 0.05, compared with adjacent tumor tissues). b Western blot was performed to determine Tra2βexpression in NSCLC cell line (A549) and human lung epithelial cell line (BEAS-2B). The bar chart demonstrates the relative level of Tra2β protein to GAPDH by densitometry. The data are mean ± SEM. The same experiment was repeated at least three times (*p < 0.05, compared with BEAS-2B cells)
Immunohistochemical stain of Tra2β and Ki-67 in NSCLC tissues. Paraffin-embedded tissue sections were stained for Tra2β and Ki-67. Tra2β and Ki-67 expression in adjacent non-tumor tissues (a, b), well differentiated (c, d), moderate differentiated (e, f), and poor differentiated (g, h) NSCLC tissues (×200)
Correlation of Tra2β expression with clinicopathologic variables in NSCLC
To further explore the role of Tra2β in NSCLC, Pearson χ2 test was performed to analyze the association of Tra2β expression with clinicopathologic variables. Clinicopathologic data of the patients were summarized in Table 1. Consistent with its expression results showed in Fig. 2, the expression level of Tra2β was associated with histological differentiation and clinical stage (p < 0.01, Table 1). Moreover, there was significant positive correlation between the expression level of Tra2β and that of Ki67 (p = 0.003, Table 1). In addition, the correlation between Tra2β and Ki67 expression in NSCLC was further evaluated by Spearman rank correlation test (p < 0.05, r = 0.446, Fig. 3). However there was no significant relation between Tra2β expression and other clinical factors (Table 1).
High expression of Tra2β predicted poor prognosis of NSCLC patients
To evaluate the prognostic significance of Tra2β expression, Kaplan–Meier analysis was performed. The result showed that high expression of Tra2β was significantly associated with poor overall survival rate of NSCLC patients (p = 0.034, Fig. 4). Additionally, multivariate analysis using the Cox proportional hazards model demonstrated that Tra2β expression (p = 0.041), histological differentiation (p = 0.003), clinical stage (p = 0.009), lymph node status (p = 0.03) and Ki-67 expression (p = 0.029) were independent prognostic factors of overall survival (Table 2).
Tra2β expression promoted proliferation of A549 cells
It was reported that Tra2β was associated with cell proliferation and survival (Takeo et al. 2009). Therefore, we further evaluated the expression of Tra2β during cell cycle progression in A549 cells. A549 cells were cultured in serum-deprived condition for 72 h and then recovered serum refeeding. Flow cytometry analysis was performed to analyze the cell cycle progression of A549 cells. It showed that after serum starvation, cells were arrested in the G0/G1 phase. The percentage of cells in the G0/G1 phase was more than 70 %. Then upon serum refeeding, cells in the G0/G1 phase decreased, with concomitant increase of cells in the S phase (Fig. 5a). Next, Western blot assay were performed to analyze the expression of Tra2β, PCNA and Cyclin A. As expected, Tra2β expression was increased as early as 4 h and reached the highest level 12 h after serum re-addition, which was consistent with PCNA (a marker of proliferation) and Cyclin A (a marker of cell cycle) (Fig. 5b, c). Thus, these results indicated that Tra2β might have an impact on the proliferation of NSCLC cells in a cell cycle-dependent pathway.
Tra2β expression promoted proliferation of A549 cells. a Flow cytometry quantitation of cell cycle progress in A549 cells. Cells were synchronized at G1 after serum starvation for 72 h, then progressed into cell cycle by adding medium containing 10 % FBS for the indicated times (R4 h, R8 h, R12 h, R24 h). b The cells were harvested and analyzed for Tra2β, PCNA and cyclin A expression by western blot. c The bar graph indicates density of Tra2β/PCNA/Cyclin A versus GAPDH at each time point. Data are presented as mean ± SEM of three independent measurements (*, #, ^ p < 0.05, compared with control cells serum starved for 72 h)
Knockdown of Tra2β inhibited proliferation and induced apoptosis of A549 cells
To further investigate the effects of Tra2β expression on NSCLC cell proliferation, A549 cells were transiently transfected with Tra2β-shRNA and control-shRNA. The efficiency of Tra2β-shRNA was confirmed by Western blot assay 48 h after transfecting. As shown in Fig. 6a, Tra2β protein level decreased maximally in Tra2β-shRNA#4 transfected A549 cells. So Tra2β-shRNA#4 got the best interference efficiency. Therefore we used Tra2β-shRNA#4 for subsequent experiments. Following Tra2β-shRNA#4 transfection, expression of PCNA and cleaved-caspase3 was determined by Western analysis. It revealed that knockdown of Tra2β resulted in decrease of PCNA with concomitant increase of cleaved-caspase3 (Fig. 6b). Cell vitality was determined by CCK-8 assay at indicated times, and the data showed that cell proliferation was inhibited due to down-regulation of Tra2β (Fig. 6c). Furthermore, flow cytometry analysis of cell cycle distribution revealed a significant increase of cells in the G0/G1 phase (from 58.29 to 78.24 %), with a concomitant decrease of cells in S phase (from 32.72 to 14.64 %) compared with control-shRNA#4 (Fig. 6d). Finally, to evaluate the effect of Tra2β expression on cell apoptosis, Tunnel assay was performed. The result showed that knockdown of Tra2β induced apoptosis of A549 cells ( Fig. 6e).
Knock down of Tra2β expression inhibited A549 cell proliferation and induced apoptosis. a Tra2β expression was determined by Western blot following Tra2β-shRNA transfecting in A549 cells. The bar chart below demonstrated the ratio of Tra2β to GAPDH by densitometry. The data are mean ± SEM (*p < 0.05 compared with the control). b The expression of Tra2β, PCNA, caspase3 and cleaved-caspase3 was determined following control-shRNA and Tra2β-shRNA#4 transfection. c CCK-8 assay was performed to determine cell vitality of A549 cells transfected with Tra2β-shRNA#4 exhibited significantly weakened proliferation. d Flow cytometric analysis of cell cycle distribution following control-shRNA and Tra2β-shRNA#4 transfection. e Tunnel assay was performed to evaluate the effect of Tra2β expression on cell apoptosis following control-shRNA and Tra2β-shRNA#4 transfection. The experiment details were described in “Materials and methods”. All these data are representative of at least three independent experiments
Discussion
The initiation and progression of NSCLC is a comprehensive pathologic process involving complex alterations in oncogenes and tumor suppressor genes that play roles in cell proliferation and cell apoptosis. In spite of the development in therapy methods such as surgical resection, chemotherapy and radiation therapy, this disease is rarely curable and prognosis is poor (Sun et al. 2007). A deeper understanding of the genes associated with NSCLC development is of great necessity. In this study, we aimed to investigate the role of Tra2β, a SR-like splicing factor, in the development of NSCLC.
Tra2β is an important splicing factor that involved in alternative splice. Splicing activation by Tra2β protein is a concentration dependent. Thus, Tra2β must be maintained at a proper level (Kajita et al. 2013). Previously, it has been reported that dysregulation of Tra2β is closely linked to various human diseases, including cancer. However, the role Tra2β in NSCLC is still unclear. In this study, we first analyzed Tra2β expression in lung caner tissues and cell lines by Western blot analysis. It showed that Tra2β expression was up-regulated in NSCLC tumor tissues and NSCLC cell line compared with adjacent non-tumor tissues and normal bronchial epithelial cell line (Fig. 1). In addition, we performed IHC assay in 83 paraffin-embedded NSCLC samples and analyzed the association of Tra2β expression with clinicopathological variables as well as clinical prognosis. We found that Tra2β expression was associated with histological differentiation and clinical stage of NSCLC. Multivariate analysis indicated that Tra2β could be an independent prognostic factor for the survival of NSCLC patients and Survival curve revealed that high expression of Tra2β was associated with poor prognosis of NSCLC samples.
Previous studies have shown that Tra2β participated in various cellular processes such as cell proliferation, diversification and apoptosis (Kajita et al. 2013; Roberts et al. 2014; Shukla and Fisher 2008). However, the effect of Tra2β expression on NSCLC is still unknown. We wonder whether Tra2β could influence proliferation and apoptosis of A549 cells. We knocked down the expression of Tra2β by shRNA, the data showed that knockdown of Tra2β inhibited proliferation of A549 and induced cell apoptosis with concomitant decreased expression of PCNA and increased expression of cleaved-caspase3. Alternative splicing is considered as an important mechanism in regulating gene expression and associated with tumorigenesis and metastasis of a wide variety of human cancers (Ouyang et al. 2011; Stickeler et al. 1999). Tra2β belongs to SR-like protein family, it binds to the highly degenerated purine-rich sequence motif (GAARGARR) and influences various alternatively spliced exons (Mende et al. 2010). Tra2β is related to multiple biological processes and various diseases by alternative splicing of target mRNA (Best et al. 2013). Target mRNAs of Tra2β including CD44 (Takeo et al. 2009; Watermann et al. 2006), liver scavenger receptor class B (SRB) (Zhang et al. 2007), tau (Kondo et al. 2004), homeodomain-interacting kinase 3 (HipK3) (Venables et al. 2005), fibroblast growth factor receptor 2 (FGFR2) (Chen et al. 2004), glutamate receptor subunit B (GluR-B) (Chen et al. 2004), calcitonin/calcitonin gene-related peptide (CGRP) (Tran et al. 2003), survival motor neuron 2 (SMN2) (Hofmann et al. 2000), and nuclear autoantigenic sperm protein (Nasp-T) (Grellscheid et al. 2011). CD44, HipK3 and Nasp-T are known pro-oncogenic splicing targets. Tra2β enhances the inclusion of CD44 exons v4 and v5 and acts synergistically with YB-1 in breast cancer (Watermann et al. 2006). Tra2β is up-regulated in several different cancers, but the detailed mechanism is unknown. Keisuke et al. reported that in colon cancer cells transcription of Tra2β was regulated by heat shock factor 1 and proto-oncogene Ets1 (Kajita et al. 2013). Our results suggested that high expression of Tra2β might play an important role in the development and progression of NSCLC. However, further studies are necessary to elucidate the molecular mechanisms of Tra2β in NSCLC pathogenesis.
In summary, our studies showed that Tra2β was up-regulated in NSCLC and associated with poor prognosis. Downregulation of Tra2β inhibited proliferation and induced apoptosis of A549 cells. Therefore, Tra2β might serve as a novel molecular target for the diagnosis and treatment of NSCLC.
References
Best A, Dagliesh C, Ehrmann I, Kheirollahi-Kouhestani M, Tyson-Capper A, Elliott DJ (2013) Expression of Tra2 beta in cancer cells as a potential contributory factor to neoplasia and metastasis. Int J Cell Biol 2013:843781. doi:10.1155/2013/843781
Bidkhori G, Narimani Z, Hosseini Ashtiani S, Moeini A, Nowzari-Dalini A, Masoudi-Nejad A (2013) Reconstruction of an integrated genome-scale co-expression network reveals key modules involved in lung adenocarcinoma. PLoS One 8:e67552. doi:10.1371/journal.pone.0067552
Buyukcelik A, Yalcin B, Utkan G (2004) Multidisciplinary management of lung cancer. N Engl J Med 350:2008–2010 (author reply 2008–2010)
Cai J et al (2013) miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res 73:5402–5415. doi:10.1158/0008-5472.CAN-13-0297
Chen X, Huang J, Li J, Han Y, Wu K, Xu P (2004) Tra2betal regulates P19 neuronal differentiation and the splicing of FGF-2R and GluR-B minigenes. Cell Biol Int 28:791–799. doi:10.1016/j.cellbi.2004.07.009
Daoud R, Mies G, Smialowska A, Olah L, Hossmann KA, Stamm S (2002) Ischemia induces a translocation of the splicing factor tra2-beta 1 and changes alternative splicing patterns in the brain. J Neurosci 22:5889–5899. doi:10.1002/cne.23405
Gabriel B et al (2009) Significance of nuclear hTra2-beta1 expression in cervical cancer. Acta Obstet Gynecol Scand 88:216–221. doi:10.1080/00016340802503021
Grellscheid S et al (2011) Identification of evolutionarily conserved exons as regulated targets for the splicing activator tra2beta in development. PLoS Genet 7:e1002390. doi:10.1371/journal.pgen.1002390
Hofmann Y, Lorson CL, Stamm S, Androphy EJ, Wirth B (2000) Htra2-beta 1 stimulates an exonic splicing enhancer and can restore full-length SMN expression to survival motor neuron 2 (SMN2). Proc Natl Acad Sci USA 97:9618–9623. doi:10.1073/pnas.160181697
Hu B, Mu HP, Zhang YQ, Su CY, Song JT, Meng C, Liu DX (2014) Prognostic significance of TBX2 expression in non-small cell lung cancer. J Mol Histol. doi:10.1007/s10735-014-9569-0
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. doi:10.3322/caac.20107
Kajita K et al (2013) Ets1 and heat shock factor 1 regulate transcription of the transformer 2beta gene in human colon cancer cells. J Gastroenterol 48:1222–1233. doi:10.1007/s00535-012-0745-2
Kim IM et al (2006) The Forkhead Box m1 transcription factor stimulates the proliferation of tumor cells during development of lung cancer. Cancer Res 66:2153–2161. doi:10.1158/0008-5472.CAN-05-3003
Kiryu-Seo S, Matsuo N, Wanaka A, Ogawa S, Tohyama M, Kiyama H (1998) A sequence-specific splicing activator, tra2beta is up-regulated in response to nerve injury. Brain Res Mol Brain Res 62:220–223
Kondo S, Yamamoto N, Murakami T, Okumura M, Mayeda A, Imaizumi K (2004) Tra2 beta, SF2/ASF and SRp30c modulate the function of an exonic splicing enhancer in exon 10 of tau pre-mRNA. Genes Cells 9:121–130
Lv L et al (2014) Nemo-like kinase (NLK) inhibits the progression of NSCLC via negatively modulating WNT signaling pathway. J Cell Biochem 115:81–92. doi:10.1002/jcb.24635
Matsuo N, Ogawa S, Imai Y, Takagi T, Tohyama M, Stern D, Wanaka A (1995) Cloning of a novel RNA binding polypeptide (RA301) induced by hypoxia/reoxygenation. J Biol Chem 270:28216–28222
Mende Y et al (2010) Deficiency of the splicing factor Sfrs10 results in early embryonic lethality in mice and has no impact on full-length SMN/Smn splicing. Hum Mol Genet 19:2154–2167. doi:10.1093/hmg/ddq094
Nayler O, Cap C, Stamm S (1998) Human transformer-2-beta gene (SFRS10): complete nucleotide sequence, chromosomal localization and generation of a tissue-specific isoform. Genomics 53:191–202. doi:10.1006/geno.1998.5471
Ouyang YQ et al (2011) Expression levels of hnRNP G and hTra2-beta1 correlate with opposite outcomes in endometrial cancer biology. Int J Cancer 128:2010–2019. doi:10.1002/ijc.25544
Roberts JM, Ennajdaoui H, Edmondson C, Wirth B, Sanford JR, Chen B (2014) Splicing factor TRA2B is required for neural progenitor survival. J Comp Neurol 522:372–392. doi:10.1002/cne.23405
Segade F, Claudio E, Wrobel K, Ramos S, Lazo PS (1995) Isolation of nine gene sequences induced by silica in murine macrophages. J Immunol 154:2384–2392
Shukla S, Fisher SA (2008) Tra2beta as a novel mediator of vascular smooth muscle diversification. Circ Res 103:485–492. doi:10.1161/CIRCRESAHA.108.178384
Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63:11–30. doi:10.3322/caac.21166
Stickeler E, Kittrell F, Medina D, Berget SM (1999) Stage-specific changes in SR splicing factors and alternative splicing in mammary tumorigenesis. Oncogene 18:3574–3582. doi:10.1038/sj.onc.1202671
Sun S, Schiller JH, Spinola M, Minna JD (2007) New molecularly targeted therapies for lung cancer. J Clin Invest 117:2740–2750. doi:10.1172/JCI31809
Takeo K, Kawai T, Nishida K, Masuda K, Teshima-Kondo S, Tanahashi T, Rokutan K (2009) Oxidative stress-induced alternative splicing of transformer 2beta (SFRS10) and CD44 pre-mRNAs in gastric epithelial cells. Am J Physiol Cell Physiol 297:C330–C338. doi:10.1152/ajpcell.00009.2009
Tazi J, Bakkour N, Stamm S (2009) Alternative splicing and disease. Biochim Biophys Acta 1792:14–26. doi:10.1016/j.bbadis.2008.09.017
Tran Q, Coleman TP, Roesser JR (2003) Human transformer 2beta and SRp55 interact with a calcitonin-specific splice enhancer. Biochim Biophys Acta 1625:141–152
Tsukamoto Y et al (2001) Expression of a novel RNA-splicing factor, RA301/Tra2beta in vascular lesions and its role in smooth muscle cell proliferation. Am J Pathol 158:1685–1694
Venables JP, Bourgeois CF, Dalgliesh C, Kister L, Stevenin J, Elliott DJ (2005) Up-regulation of the ubiquitous alternative splicing factor Tra2beta causes inclusion of a germ cell-specific exon. Hum Mol Genet 14:2289–2303. doi:10.1093/hmg/ddi233
Wang Y et al (2013) Interaction with cyclin H/cyclin-dependent kinase 7 (CCNH/CDK7) stabilizes C-terminal binding protein 2 (CtBP2) and promotes cancer cell migration. J Biol Chem 288:9028–9034. doi:10.1074/jbc.M112.432005
Watermann DO, Tang Y, Zur Hausen A, Jager M, Stamm S, Stickeler E (2006) Splicing factor Tra2-beta1 is specifically induced in breast cancer and regulates alternative splicing of the CD44 gene. Cancer Res 66:4774–4780. doi:10.1158/0008-5472.CAN-04-3294
Zhang X, Moor AN, Merkler KA, Liu Q, McLean MP (2007) Regulation of alternative splicing of liver scavenger receptor class B gene by estrogen and the involved regulatory splicing factors. Endocrinology 148:5295–5304. doi:10.1210/en.2007-0376
Zhang T, Zhang DM, Zhao D, Hou XM, Liu XJ, Ling XL, Ma SC (2014) The prognostic value of osteopontin expression in non-small cell lung cancer: a meta-analysis. J Mol Histol. doi:10.1007/s10735-014-9574-3
Acknowledgments
Supported by grants from the National Natural Science Foundation of China (No. 81201858); Natural Scientific Foundation of Jiangsu Province Grant (No. BK2012231).
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lili Ji and Tingting Ni have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ji, L., Ni, T., Shen, Y. et al. Transformer 2β (Tra2β/SFRS10) positively regulates the progression of NSCLC via promoting cell proliferation. J Mol Hist 45, 573–582 (2014). https://doi.org/10.1007/s10735-014-9582-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-014-9582-3