Abstract
Recent studies have reported that NF-κB mediated down-regulation of miRNA-29 and lower expression of miRNA-29 promoted the deposition of collagens in fibrotic liver. Our previous research demonstrated that the increased Hedgehog (Hh) signaling, a key regulator for hepatic fibrogenesis, induced the severe hepatic fibrosis in the livers with impaired NF-κB signaling. These findings led us to investigate the effect of Hh and miRNA-29 on the hepatic fibrosis under dysregulated NF-κB signaling. In this study, we used IKKβF/F and IKKβ-deficient IKKβΔHEP mouse model with a defective NF-κB signaling pathway, and assessed the expression of the miRNA-29 family (miRNA-29a, miRNA-29b, and miRNA-29c), Hh, and proliferation of MF-HSCs in liver from IKKβF/F mice and IKKβΔHEP mice both before and after MCDE treatment. The activation of NF-κB was significantly increased in MCDE diet-fed IKKβF/F mice compared to IKKβΔHEP mice. Expression of miRNA-29 family was greater in MCDE diet-fed IKKβΔHEP mice than IKKβF/F mice, demonstrating that the impaired NF-κB pathway was unable to suppress the expression of miRNA-29s after injury. However, expression of the Hh signaling pathway was greatly enhanced, and activation of Hh promoted the accumulation of MF-HSCs with impaired NF-κB, eventually increasing fibrogenesis in the damaged liver of IKKβΔHEP mice. Therefore, these results demonstrated that Hh signaling regulates the proliferation of MF-HSCs irrespective of the action of miRNA-29, eventually contributing hepatic fibrosis, when the NF-κB pathway is disrupted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Liver fibrosis is the primary characteristic of most chronic liver diseases, including liver cancers. Activated hepatic stellate cells (HSCs) represent one of the primary sources for the accumulation of extracellular matrix (ECM) in injured livers (Bataller and Brenner 2005). Although various cytokines and intracellular signaling pathways involved in hepatic fibrogenesis have been identified, it remains unclear how these cytokines and stimulated signaling pathways provoke HSC activation leading to fibrosis. The Hedgehog (Hh) signaling pathway orchestrates tissue reconstruction in the damaged adult liver. Hh signaling acts in paracrine and autocrine manners and regulates the proliferation of Hh-responsive cells, such as HSCs and hepatic progenitor cells (Hooper and Scott 2005; Omenetti et al. 2011). In chronic liver conditions, massive apoptosis occurs in hepatocytes, resulting in the release of Hh ligands into the extracellular environment (Jung et al. 2010; Rangwala et al. 2011). Released Hh ligands bind to the Hh receptors of Hh-responsive cells and activate the Hh signaling pathway. Hh-responsive HSCs undergo a transition into myofibroblastic HSCs (MF-HSCs) and contribute to fibrogenesis (Choi and Diehl 2009).
The nuclear factor-κB (NF-κB) signaling pathway regulates the expression of genes responsible for immune responses and inflammation and plays an important role in mediating cell survival by inhibiting p53-dependent apoptosis, up-regulating anti-apoptotic Bcl-2 family members, and caspase inhibitors (Grimm et al. 1996; Stehlik et al. 1998; Ryan et al. 2000; Mitsiades et al. 2002). NF-κB is normally sequestered in the cytoplasm by inhibitors of κB (IκB). Phosphorylation of IκBs by IκB kinase (IKK), including subtypes IKKα, IKKβ, and IKKγ, allows NF-κB to translocate to the nucleus (Gilmore 2006; Perkins 2007). Recent studies demonstrated that NF-κB signaling pathway was implicated in liver fibrogenesis (Wang et al. 1998; Elsharkawy et al. 2005; Muriel 2009).
MicroRNAs (miRNAs), small non-coding RNAs with a length of 18–22 nucleotides, negatively regulate gene expression through binding to the complementary sequences on 3′-untranslated regions of target mRNAs to degrade the target mRNA or inhibit its translation (Lewis et al. 2003; Guo et al. 2009). Growing evidence demonstrates that miRNAs control a variety of cellular processes in plants and animals, and this regulation differs in a wide spectrum of pathological states and types of tissues and cells. Dysregulation of miRNAs is related with liver diseases in human and animal models (Varnholt et al. 2008; Ura et al. 2009). Recently, it was reported that NF-κB down-regulated the expression of anti-fibrotic miRNA-29 family members, and this down-regulation promoted collagens production, leading to liver fibrosis (Roderburg et al. 2011; Kwiecinski et al. 2010; Ogawa et al. 2010). Accumulating evidence emphasizes the association of miRNAs with NF-κB signaling and the various effects of this association on liver diseases, including liver fibrosis. In a previous study, we employed transgenic mice (IKKβΔHEP mice) in which the IKKβ allele was removed specifically in albumin-expressing cells, and thereby, NF-κB signaling was disrupted in these cells. We demonstrated that the severe hepatic fibrosis observed in this mouse model was induced by increased Hh signaling. Therefore, we investigated whether Hh signaling influenced the anti-fibrotic effect of miRNA-29 under dysregulated NF-κB signaling.
In this study, we demonstrated reduced expression of miRNA-29 family members upon NF-κB activation after liver injury. This was ameliorated in the damaged livers of IKKβΔHEP mice despite the increased fibrosis. Hh-responsive HSCs that could not respond to NF-κB signaling contributed to collagen accumulation in the injured livers of IKKβΔHEP mice. Therefore, our results demonstrated that the up-regulation of Hh signaling resulted in the activation of HSCs, which promoted fibrogenesis by attenuating the effects of miRNA-29 on fibrosis, when NF-κB signaling was impaired.
Materials and methods
Experimental animals
IKKβF/F and IKKβΔHEP mice were provided from Dr. Michael Karin (University of California, San Diego, CA, USA). IKKβΔHEP mice were generated by Cre-LoxP system. Albumin (Alb)-expressing cells are IKKβ-deficient (IKKβΔHEP) as a result of breeding between IKKβF/F mice and Alb-Cre mice. To induce oxidative liver injury, IKKβF/F and IKKβΔHEP mice were fed a methionine/choline-deficient diet supplemented with 0.15 % ethionine (MCDE). Surviving mice were sacrificed after being fed MCDE diets for 1 week (IKKβF/F mice n = 7, IKKβΔHEP mice n = 5). Chow-fed IKKβF/F (n = 6) and IKKβΔHEP mice (n = 6) were also sacrificed at the same time point (Jung et al. 2010).
Animal care and surgical procedures were approved by the Duke University Medical Center Institutional Animal care and Use Committee as set forth in the ‘Guide for the Care and Use of Laboratory Animals’ published by the National Institutes of Health.
Isolation of primary hepatic stellate cells (HSCs) from mice liver
HSCs were isolated from livers of mice as previously described (Choi et al. 2009). Briefly, HSCs were isolated by density gradient method, 5.8 % Larcoll (Sigma-Aldrich) and 15.6 % Histodenz (Sigma-Aldrich), and purity of HSCs was >98 %. Purity and viability were determined by phase-contrast microscopy examining autofluorescence and propidium iodide exclusion (50 μg/ml:Roche). HSCs were culture in 10 % serum-supplemented DMEM (Invitrogen, Carlsbad, CA, USA) with streptomycin-penicillin.
RNA analysis
Analysis of mRNA expression was performed as described previously (Roderburg et al. 2011) with modifications. Total RNA from quick frozen liver tissues that had been stored at −80 °C was extracted using TRIzol (Invitrogen) or miRNeasy Mini kit (Qiagen). After determining quantity and quality of the RNA, total RNA (5 μg) was used to synthesize cDNA using the SuperScript First-strand Synthesis System (Invitrogen) or miScript Reverse Transcriptase Kit (Qiagen). mRNAs were quantified by quantitative RT-PCR analysis using Power SYBR Green Master Mix (Applied Biosystem) or miScript SYBR Green PCR Kit (Qiagen) per the manufacturer’s specifications (Eppendorf, Mastercycler Real-Time PCR). Samples were analyzed in triplicate. Data were analyzed according to the ∆∆Ct method. The sequences of primers for mice are summarized in Table 1. Conventional RT-PCR was performed as previously described (Bär et al. 2004).
Liver histology and stain
Liver specimens were fixed in formalin, embedded in paraffin and cut into 4 μm sections. For immunohistochemistry, specimens were deparaffinized, hydrated and incubated for 10 min in 3 % hydrogen peroxide to block endogenous peroxidase. Heat-induced antigen retrieval was performed in 10 mM sodium citrate buffer (pH 6.0) for 10 min. Sections were blocked in Dako protein block (X9090; Dako, USA) for 30 min and incubated with primary antibody, NF-κB p65 (ab7970, 1:5,000; Abcam, Cambridge, MA, USA) at 4 °C overnight. Other sections were also incubated at 4 °C overnight in non-immune sera. Polymer-horseradish peroxidase (HRP)-anti rabbit (K4003; Dako) was used as secondary antibody. 3,3′-Diaminobenzidine (DAB) was employed in the detection procedure. Omitting primary antibody from reaction eliminated staining, demonstrating staining specificity. Sirius red staining on livers was performed as previously described (Jung et al. 2010). To identify whether certain proteins are co-expressed in cells, we performed double immunohistochemistry on liver sections. Polymer-HRP anti-rabbit (K4003; Dako) and the MACH3 mouse AP polymer kit (MP530; Biocare Medical, Concord, CA, USA) were used as secondary antibodies. Gli2 protein was identified by DAB (Dako) to show a brown color, and HSP47 protein was identified by the Ferangi Blue chromogen kit (FB812S; Biocare Medical) to generate a blue color.
Statistical analysis
QRT-PCR results of miRNAs are expressed as median and range to show distributions and Mann–Whitney U test was used to analysis. Differences were considered significant when p ≤ 0.05. The other analyzed data from QRT-PCR and Western blot are expressed as mean ± SD, and student t test was performed. Differences were considered significant when p < 0.05.
Results
Decreased expression of miRNA-29 family in the injured liver with NF-κB-dependent manner
Recent studies demonstrated the effects of miRNAs on hepatic apoptosis and fibrosis. In particular, activation of NF-κB signaling inhibited the transcription of miRNA-29 family members both in the whole liver and primary HSCs and thereby enhanced hepatic fibrosis (Roderburg et al. 2011). Another study revealed that miRNA-29b suppressed the expression of genes involved in ECM synthesis and down-regulated miRNA-29b under chronic oxidative stress, thus contributing to the deposition of ECM in trabecular meshwork cells (Luna et al. 2009). Therefore, we examined whether the activation of NF-κB signaling was related with the expression of miRNA-29 family members in IKKβF/F mice exhibiting normal NF-κB signaling after oxidative liver injury. As examined by IHC, MCDE diet induced the translocation of NF-κB p65 into the nucleus of hepatocytes in IKKβF/F mice (MCDE), whereas these activated NF-κB p65s were not detected in chow-fed IKKβF/F mice (CON) (Fig. 1a). The expression of miRNA-29 family members a, b, and c was significantly decreased in the livers of MCDE diet-fed IKKβF/F mice compared to their expression in chow-fed IKKβF/F mice (25 % decrease on average) (Fig. 1b–d). These data indicated that activated NF-κB might be associated with the down-regulation of miRNA-29 in the damaged liver.
Reduced expression of miRNA-29 family with NF-κB activation in IKKβF/F mice after liver injury. a Immunochemical staining for NF-κB p65 in liver from representative IKKβF/F mice before (CON) and after MCDE diet (MCDE) for 1 week (×40). b–d QRT-PCR analysis of hepatic expressions of miRNA-29 family including miRNA-29a in b, miRNA-29b in c and miRNA-29c in d from chow-fed (white box) and MCDE diet-fed (grey box) IKKβF/F mice (n ≥ 3 mice/treated group). Medians and ranges of results are graphed (*p ≤ 0.05 vs chow-fed IKKβF/F control group)
Regulation of miRNA-29 in the mouse model with disrupted NF-κB signaling in the liver
To investigate the effects of NF-κB on the expression of miRNA-29 in the liver, we employed IKKβΔHEP mice in which the IKKβ allele was specifically truncated in albumin-expressing cells, such as hepatocytes. When IKKβ is defective, NF-κB cannot translocate to the nucleus, resulting in impaired NF-κB signaling in the livers of these mice. Liver injury stimulated the activation of NF-κB subunit p65 in the livers of IKKβF/F mice (Fig. 1a, right panel), whereas nuclear localization of p65 was rarely detected in the livers of MCDE diet-fed IKKβΔHEP mice (Fig. 2a). NF-κB p65 was localized predominantly in the cytosol of both hepatocytic and ductular cells in both chow-fed and MCDE-fed IKKβΔHEP mice.
Increased expression of miRNA-29 in IKKβΔHEP mice after liver injury. a Immunochemical staining for NF-κB p65 in liver from representative IKKβΔHEP mice before (CON) and after MCDE diet (MCDE) for 1 week (×40). b–d QRT-PCR analysis of hepatic expressions of miRNA-29 family including miRNA-29a in b, miRNA-29b in c and miRNA-29c in d from chow-fed (diagonal lined white box) and MCDE diet-fed (diagonal lined grey box) IKKβΔHEP mice (n ≥ 3 mice/treated group). Medians and ranges of results are graphed (*p ≤ 0.05 vs chow-fed IKKβΔHEP control group)
To determine whether these changes in NF-κB localization were associated with differences in miRNA-29 expression, we examined the expression of miRNA-29 family members in IKKβΔHEP mice before and after being fed the MCDE diet. As opposite to the down-regulation of miRNA-29s in IKKβF/F mice, the expression of miRNA-29b and c increased with 8.07 ± 3.56- and 3.06 ± 0.92-fold more, respectively, in MCDE-fed IKKβΔHEP than chow-fed IKKβΔHEP mouse livers (Fig. 2c, d). The expression of miRNA-29a also showed an increase trend in the damaged liver of IKKβΔHEP mice (Fig. 2b). The up-regulation of miRNA-29 family was equivalent to the expression levels of miRNA-29 family in chow-fed IKKβF/F mice (Fig. 3). Interestingly, the basal level of miRNA-29s expression was significantly lower in IKKβΔHEP than IKKβF/F mice.
Expression levels of miRNA-29 family over all in IKKβF/F and IKKβΔHEP mice before and after liver injury. a–c QRT-PCR analysis of hepatic expressions of miRNA-29 family including miRNA-29a in a, miRNA-29b in b and miRNA-29c in c from chow- (white box) or MCDE diet-fed (grey box) IKKβF/F and IKKβΔHEP mice (n = 3 mice/treated group). Medians and ranges of results are graphed (*p ≤ 0.05 vs chow-fed IKKβF/F control group)
Because miRNA-29 was demonstrated to inhibit hepatic fibrosis (Roderburg et al. 2011), we assessed the degree of hepatic fibrosis in MCDE-fed IKKβΔHEP mice. Severe hepatic fibrosis was observed in the livers of MCDE diet-fed IKKβΔHEP mice despite the higher level of expression of miRNA-29 family members (Fig. 4). Western blot analysis showed that the level of α-SMA protein, a fibrotic marker, was significantly greater in the livers of MCDE diet-fed IKKβΔHEP mice (Fig. 4a, b). In addition, Sirius red staining revealed the pericellular and sinusoidal deposition of collagen fibrils in IKKβΔHEP mice, as indicated by the red color, and none of these collagen depositions was observed in IKKβF/F mice (Fig. 4c). These results suggested that other critical signaling pathways neutralize the anti-fibrotic role of the miRNA-29 family and promote fibrogenesis in livers with impaired NF-κB signaling.
Enhanced hepatic fibrosis under defective NF-κB signaling. a, b Western blot analysis of α-SMA (β-actin was used as an internal control). Data shown represent one of three experiments with similar results (a immunoblot/b band density of α-SMA, n ≥ 3 mice/group). White and black bar indicate chow-fed and MCDE-fed mice, respectively. Mean ± SD results that were obtained by measuring the band density of three different blots are graphed (*p < 0.05 vs IKKβF/F mice). c Sirius red staining in liver sections from representative MCDE diet-fed IKKβF/F and IKKβΔHEP mice (×20)
Stimulation of HSCs to undergo the epithelial-to-mesenchymal transition (EMT) by Hh signaling, thus promoting fibrogenesis
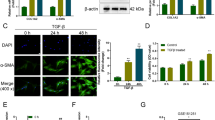
Quiescent HSCs (Q-HSCs) are activated and transformed into MF-HSCs, which are known to generate collagen fibrils and up-regulate genes related to ECM formation (Reeves and Friedman 2002; Olsen et al. 2011). Several signaling molecules, such as TGF-β and Hh, promote the transformation of Q-HSCs into MF-HSCs through EMT. Roderburg et al. (2011) demonstrated that miRNA-29 decreased collagen expression in HSCs, and miRNA-29 expression was down-regulated in activated HSCs in a TGF-β- and NF-κB-dependent manner. To determine the effect of miRNA-29 on NF-κB signaling in HSCs, we used IKKβΔHEP mice in which the nuclear accumulation of NF-κB was blocked, because of IKKβ mutation (Maeda et al. 2005). We isolated HSCs from IKKβΔHEP and IKKβF/F mice and examined NF-κB signaling by RT-PCR. A higher level of albumin expression was observed in Q-HSCs, and this expression was significantly decreased in HSCs after 7 days in culture (Fig. 5a). When albumin mRNA was expressed, cre gene and mutant IKKβ allele expression was detected in HSCs from IKKβΔHEP mice, demonstrating that these HSCs had defective NF-κB signaling. Therefore, there was a possibility that miRNA-29 in HSCs from IKKβΔHEP mice could not contribute to hepatic fibrosis, and instead, there might be other signaling pathways that compromised the anti-fibrotic effect of miRNA-29. In previous research, we demonstrated that Hh up-regulation promoted fibrogenesis in IKKβΔHEP mice with chronically increased hepatocyte apoptosis (Jung et al. 2010). In addition, emerging evidence demonstrated that Hh plays an important role in the transition of Q-HSCs into MF-HSCs, contributing to hepatic fibrogenesis (Sicklick et al. 2005; Yang et al. 2008). Hence, we hypothesized that Hh signaling could compromise the effects of miRNA-29 and promote fibrosis. To evaluate this hypothesis, we examined whether NF-κB-defective HSCs responded to Hh signaling. Double immunohistochemical staining revealed the expression of Hh target gene Gli2 (brown) in collagen-synthesizing HSCs (blue) in the livers of MCDE-fed IKKβΔHEP mice (Fig. 5b). Co-expressing cells for Gli2 and HSP47 were rarely detected in other groups (Fig. 5b). In addition, NF-κB-defective HSCs exhibited decreased expression of the epithelial cell marker bmp7 and increased expression of mesenchymal cell markers, including α-smooth muscle actin and vimentin, during culture (Fig. 5c). These results demonstrated that HSCs from IKKβΔHEP mice were more vulnerable to transition into MF-HSCs and suggested that Hh signaling compromised the action of miRNA-29 and contributed to fibrogenesis when NF-κB signaling was impaired.
Hepatic stellate cells (HSCs) with the impaired NF-κB pathway are Hh-responsive and undergo EMT, triggering fibrogenesis. a Primary HSCs were harvested from IKKβF/F and IKKβΔHEP mice (n ≥ 8 mice/group). RT-PCR analysis of RNA expressions of the mutant IKKβ allele, albumin, cre and gapdh (glyceraldehyde phosphate dehydrogenase; used as an internal control) in representative mice. These experiments were repeated with 3 times and we sacrificed n ≥ 8 mice/group at every experiment. b Double immunochemical staining for Gli2 (brown) and HSP47 (activated HSC marker, blue colored) in MCDE-fed IKKβΔHEP mice (original magnification ×63). Gli2-positive HSCs are indicated by arrows. c QRT-PCR analysis of HSC RNA from IKKβF/F and IKKβΔHEP mice for EMT markers including bmp7, α-sma and vimentin. Mean ± SD of results are graphed (*p < 0.05, **p < 0.005 vs HSC from IKKβF/F)
Discussion
In the present study, we demonstrated that activated NF-κB signaling was associated with the reduced expression of miRNA-29s, whereas miRNA-29 expression was increased, altering its effect on liver fibrosis, when NF-κB signaling was impaired. In the livers of MCDE-fed IKKβF/F mice, the expression of α-SMA was down-regulated, even though the expression of miRNA-29s was reduced by the activated NF-κB (Figs. 1, 4a). The reason for this inconsistency also found in other studies remains unclear. Lipopolysaccharide (LPS) also induced the decreased expression of miRNA-29s, but did not lead to collagen production (Seki et al. 2007). NF-κB activated by interleukin-1 had a different effect on miRNA-29s (Roderburg et al. 2011). Regulation of miRNAs varies according to the pathological condition or cell status, and their functions and expression change consequently. In addition, the same miRNA can exert different effects according to cell type, cell condition, or stimulus type. For example, miRNA-29 has both anti-fibrotic and pro-apoptotic effects. These effects vary depending on which upstream signaling pathways are engaged (Mott et al. 2007; Xiong et al. 2010; Santanam et al. 2010). Therefore, it is possible that the effect of miRNA-29s and regulation of miRNA-29s expression may be different according to which signaling pathway is triggered in response to the damages. We observed the decreased baseline of miRNA-29 expression in IKKβΔHEP, compared to that in IKKβF/F mice. It was demonstrated that stress-related signaling pathways other than NF-κB control the expression of miRNA and oxidative stress lessens the expression of miRNA-29 (Roderburg et al. 2011). Jung et al. (2010) reported more hepatic injuries, such as increased number of apoptotic hepatocytes and progenitors in the livers from IKKβΔHEP mice. In the current study, we provided the increased expression of α-SMA in these mice. It seems that other signaling pathways may be stimulated in response to hepatic injury, and influence on the down-regulation of miRNA-29. Therefore, miRNA-29s could not be modulated by NF-κB in IKKβΔHEP mice, suggesting that there might be other signaling pathways that could supplement or inhibit the role of miRNA-29 in fibrosis.
The decreased expression of miRNA-29s by the activated NF-κB didn’t lead to liver fibrosis in MCDE-fed IKKβF/F mice, although Roderburg et al. reported that liver fibrosis in the chronic liver disease including human and mouse was caused by the reduced expression of miRNA-29 which was partially regulated by NF-κB. It is possible that MCDE treatment for 1 week may not be enough for inducing liver fibrosis, because cells having the normal NF-κB signaling pathway participate in reconstituting liver, and can protect the liver from cell death in response to apoptotic stimuli. Jung et al. previously demonstrated the lower expression of Hh, TGF-β, and extracellular matrix genes and less injury in the livers from MCDE-fed IKKβF/F than IKKβΔHEP mice (Jung et al. 2010). In line with our explanation, Roderburg group suggested that the anti-fibrotic action of miRNA-29s might require a previous strong induction of the extracellular matrix genes by TGF-β (Roderburg et al. 2011).
We provided evidence that apoptotic hepatocytes produced Hh ligands, and these released Hh ligands induced EMT, promoting hepatic fibrosis in the livers of IKKβΔHEP mice (Jung et al. 2010). It was reported that the release of the Hh ligand, shh, from ballooned hepatocytes stimulated the proliferation of Hh-responsive myofibroblasts, which promoted fibrosis in samples from patients with NASH (Rangwala et al. 2011). Hh signaling is known to stimulate the transition of Q-HSCs into myofibroblasts through EMT (Choi et al. 2009). Although Q-HSCs express relatively high levels of Hh-interacting proteins (HIPs, Hh antagonist) in healthy livers, HIP expression in HSCs is suppressed by competitive Hh ligands in injured livers. Hence, Q-HSCs undergo EMT and simultaneously transform into MF-HSCs in an Hh-dependent manner (Choi et al. 2009). Expression of albumin, cre, and mutant Ikkβ in isolated primary HSCs suggested that HSCs had defective NF-κB signaling, which altered the regulation of miRNA-29 in HSCs. Albumin expression in RNA from isolated primary HSCs could be considered as the results of the hepatocytes contamination. However, Kim et al. reported the albumin gene expression in isolated HSCs from the normal rats. Moreover, they demonstrated the albumin production in isolated HSCs and liver section (De Minicis et al. 2007; Kim et al. 2009; Choi et al. 2012). Therefore, it was assumable that increased Hh signaling might influence other upstream signaling pathways that affect the function of miRNA-29s in the liver, especially when NF-κB signaling was impaired. Subsequent experiments used double IHC for the Hh target gene Gli2 and HSP47 collagen-synthesizing cells, and greater numbers of Gli2-positive HSCs were observed in the liver of MCDE-fed IKKβΔHEP mice. These results indicated that NF-κB-defective HSCs were stimulated by Hh in IKKβΔHEP mice. In further study, we demonstrated that these HSCs up-regulated the expression of EMT markers, suggesting that they readily transitioned into mesenchymal types, inducing more severe hepatic fibrosis in IKKβΔHEP mice.
It was reported that miRNA-29b had a binding site for Gli1, and an interaction between miRNA-29 and Gli1 was demonstrated in a cholangiocarcinoma cell line (Mott et al. 2010). In this study, Hh decreased the expression of miRNA-29b and protected cholangiocytes from apoptosis, eventually leading to the survival of cancer cells. Although the Hh-dependent reduction of miRNA-29 expression in cholangiocytes is different from our results revealing the restoration of miRNA-29 expression in IKKβΔHEP mice, this is not applicable to our observation. As previously explained, the regulation of miRNA is highly cell type- and physiological condition-specific. Further studies are needed to identify the interaction between miRNA-29s and Hh in HSCs. Emerging evidence has demonstrated that the production of Hh ligands by dying hepatocytes stimulated Q-HSCs, suggesting a mechanism that links hepatocyte apoptosis to fibrogenesis. Prior reports of the effect of NF-κB-dependent miRNA-29s expression on liver fibrosis led us to investigate which other signaling pathways induce severe fibrosis in livers with defective NF-κB signaling. In this study, we demonstrated that increased Hh signaling covered the anti-fibrotic effect of miRNA-29s and stimulated HSCs, contributing to hepatic fibrogenesis, when NF-κB signaling was impaired. However, further studies are needed to assess the specific regulation of miRNA-29 by Hh and the relevance of the interaction of Hh with NF-κB on the modulation of miRNA-29 in various types of liver injury models.
Abbreviations
- Alb:
-
Albumin
- α-SMA:
-
Alpha smooth muscle actin
- DAB:
-
Diaminobenzidine
- ECM:
-
Extracellular matrix
- Gli:
-
Glioblastoma
- Hh:
-
Hedgehog
- HIP:
-
Hedgehog-interacting protein
- HSC:
-
Hepatic stellate cell
- IκB:
-
Inhibitor of kappa B
- IKK:
-
IκB kinase
- LPS:
-
Lipopolysaccharide
- MCDE:
-
Methionine/choline-deficient diet supplemented with 0.15 % ethionine
- MF-HSC:
-
Myofibroblastic hepatic stellate cell
- miRNA:
-
MicroRNA
- NF-κB:
-
Nuclear factor kappa B
- Q-HSC:
-
Quiescent hepatic stellate cell
References
Bär KJ, Schurigt U, Scholze A, von Banchet GS, Stopfel N, Bräuer R, Halbhuber KJ, Schaible HG (2004) The expression and localization of somatostatin receptors in dorsal root ganglion neurons of normal and monoarthritic rats. Neuroscience 127(1):197–206
Bataller R, Brenner DA (2005) Liver fibrosis. J Clin Invest 115(2):209–218
Choi SS, Diehl AM (2009) Epithelial-to-mesenchymal transitions in the liver. Hepatology 50(6):2007–2013
Choi SS, Omenetti A, Witek RP, Moylan CA, Syn WK, Jung Y, Yang L, Sudan DL, Sicklick JK, Michelotti GA (2009) Hedgehog pathway activation and epithelial-to-mesenchymal transitions during myofibroblastic transformation of rat hepatic cells in culture and cirrhosis. Am J Physiol Gastrointest Liver Physiol 297(6):G1093–G1106
Choi S, Park S, Kim S, Lim C, Kim J, Cha DR, Oh J (2012) Recombinant fusion protein of albumin-retinol binding protein inactivates stellate cells. Biochem Biophys Res Commun 418(1):191–197
De Minicis S, Seki E, Uchinami H, Kluwe J, Zhang Y, Brenner DA, Schwabe RF (2007) Gene expression profiles during hepatic stellate cell activation in culture and in vivo. Gastroenterology 132(5):1937–1946
Elsharkawy A, Oakley F, Mann D (2005) The role and regulation of hepatic stellate cell apoptosis in reversal of liver fibrosis. Apoptosis 10(5):927–939
Gilmore TD (2006) Introduction to NF-κB: players, pathways, perspectives. Oncogene 25(51):6680–6684
Grimm S, Bauer M, Baeuerle PA, Schulze-Osthoff K (1996) Bcl-2 down-regulates the activity of transcription factor NF-kappaB induced upon apoptosis. J Cell Biol 134(1):13–23
Guo CJ, Pan Q, Cheng T, Jiang B, Chen GY, Li DG (2009) Changes in microRNAs associated with hepatic stellate cell activation status identify signaling pathways. FEBS J 276(18):5163–5176
Hooper JE, Scott MP (2005) Communicating with hedgehogs. Nat Rev Mol Cell Biol 6(4):306–317
Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, Guy CD, Diehl AM (2010) Signals from dying hepatocytes trigger growth of liver progenitors. Gut 59(5):655–665
Kim N, Yoo W, Lee J, Kim H, Lee H, Kim Y-S, Kim D-U, Oh J (2009) Formation of vitamin A lipid droplets in pancreatic stellate cells requires albumin. Gut 58(10):1382–1390
Kwiecinski M, Elfimova N, Noetel A, Schievenbusch S, Strack I, Toex U, Drebber U, Steffen H, Dienes H, Odenthal M (2010) Mir-29, inhibiting synthesis of profibrogenic mediators, is released into the blood stream after chronic hepatitis C infection, indicating progression of fibrosis. Hepatology 52(4 Suppl):446A–447A
Lewis BP, Shih I, Jones-Rhoades MW, Bartel DP, Burge CB (2003) Prediction of mammalian microRNA targets. Cell 115(7):787–798
Luna C, Li G, Qiu J, Epstein DL, Gonzalez P (2009) Role of miR-29b on the regulation of the extracellular matrix in human trabecular meshwork cells under chronic oxidative stress. Mol Vis 15:2488
Maeda S, Kamata H, Luo JL, Leffert H, Karin M (2005) IKKβ couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell 121(7):977–990
Mitsiades CS, Mitsiades N, Poulaki V, Schlossman R, Akiyama M, Chauhan D, Hideshima T, Treon SP, Munshi NC, Richardson PG (2002) Activation of NF-kB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: therapeutic implications. Oncogene 21(37):5673–5683
Mott JL, Kobayashi S, Bronk SF, Gores GJ (2007) Mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 26(42):6133–6140
Mott JL, Kurita S, Cazanave SC, Bronk SF, Werneburg NW, Fernandez-Zapico ME (2010) Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem 110(5):1155–1164
Muriel P (2009) NF-κB in liver diseases: a target for drug therapy. J Appl Toxicol 29(2):91–100
Ogawa T, Iizuka M, Sekiya Y, Yoshizato K, Ikeda K, Kawada N (2010) Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem Biophys Res Commun 391(1):316–321
Olsen AL, Bloomer SA, Chan EP, Gaça MDA, Georges PC, Sackey B, Uemura M, Janmey PA, Wells RG (2011) Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 301(1):G110–G118
Omenetti A, Choi S, Michelotti G, Diehl AM (2011) Hedgehog signaling in the liver. J Hepatol 54(2):366–373
Perkins ND (2007) Integrating cell-signalling pathways with NF-κB and IKK function. Nat Rev Mol Cell Biol 8(1):49–62
Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, Chen W, Diehl AM (2011) Increased production of sonic hedgehog by ballooned hepatocytes. J Pathol 224(3):401–410
Reeves HL, Friedman SL (2002) Activation of hepatic stellate cells-a key issue in liver fibrosis. Front Biosci 7(4):808–826
Roderburg C, Urban GW, Bettermann K, Vucur M, Zimmermann H, Schmidt S, Janssen J, Koppe C, Knolle P, Castoldi M (2011) Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology 53(1):209–218
Ryan KM, Ernst MK, Rice NR, Vousden KH (2000) Role of NF-kB in p53-mediated programmed cell death. Nature 404(6780):892–896
Santanam U, Zanesi N, Efanov A, Costinean S, Palamarchuk A, Hagan JP, Volinia S, Alder H, Rassenti L, Kipps T (2010) Chronic lymphocytic leukemia modeled in mouse by targeted miR-29 expression. Proc Natl Acad Sci 107(27):12210
Seki E, De Minicis S, Österreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF (2007) TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat Med 13(11):1324–1332
Sicklick JK, Li YX, Choi SS, Qi Y, Chen W, Bustamante M, Huang J, Zdanowicz M, Camp T, Torbenson MS (2005) Role for hedgehog signaling in hepatic stellate cell activation and viability. Lab Invest 85(11):1368–1380
Stehlik C, de Martin R, Binder BR, Lipp J (1998) Cytokine induced expression of porcine inhibitor of apoptosis protein (IAP) family member is regulated by NF-B. Biochem Biophys Res Commun 243(3):827–832
Ura S, Honda M, Yamashita T, Ueda T, Takatori H, Nishino R, Sunakozaka H, Sakai Y, Horimoto K, Kaneko S (2009) Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology 49(4):1098–1112
Varnholt H, Drebber U, Schulze F, Wedemeyer I, Schirmacher P, Dienes HP, Odenthal M (2008) MicroRNA gene expression profile of hepatitis C virus–associated hepatocellular carcinoma. Hepatology 47(4):1223–1232
Wang JY, Guo JS, Li H, Liu SL, Zern MA (1998) Inhibitory effect of glycyrrhizin on NF-κB binding activity in CC14-plus ethanol-induced liver cirrhosis in rats. Liver Int 18(3):180–185
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y, Jia WH, Zhuang SM (2010) Effects of MicroRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 51(3):836–845
Yang L, Wang Y, Mao H, Fleig S, Omenetti A, Brown KD, Sicklick JK, Li YX, Diehl AM (2008) Sonic hedgehog is an autocrine viability factor for myofibroblastic hepatic stellate cells. J Hepatol 48(1):98–106
Acknowledgments
This work was supported by the Bio-Scientific Research Grant funded by the Pusan National University (PNU, Bio-Scientific Research Grant) (PNU-2010-101-250).
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hyun, J., Choi, S.S., Diehl, A.M. et al. Potential role of Hedgehog signaling and microRNA-29 in liver fibrosis of IKKβ-deficient mouse. J Mol Hist 45, 103–112 (2014). https://doi.org/10.1007/s10735-013-9532-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-013-9532-5