Abstract
Adiponectin receptor ADIPOR1 activates the intracellular second messenger AMP-activated protein kinase (AMPK) that participates in the control of the oxidative stress and apoptosis. This study reveals the presence of a functional ADIPOR1 receptor in all the cells of the renal glomeruli. Isolated glomeruli were incubated in vitro with adiponectin and proteins analysed by western blot. Electron microscopy using immunogold labeling was carried out on kidney sections. ADIPOR1 and catalytic AMPK sub-units α1 and α2 were revealed in normal rat glomeruli and incubation of freshly isolated rat glomeruli with either adiponectin or AICAR led to the activation by phosphorylation of catalytic AMPK. Electron microscopy localized with high resolution these proteins at the plasma membrane of the three glomerular cells, namely the endothelial, the mesangial and the podocyte cells, as well as on Bowman’s capsule epithelial cells. It is concluded that glomerular cells express a functional adiponectin receptor ADIPOR1 which, through activation of AMPK, may play important roles in the control of oxidative stress and cell survival within the glomerulus.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adiponectin, also known as ACRP30, AdipoQ, or GBP28, is an adipokine produced almost exclusively by white adipocytes and encoded by the APM1 gene (Adipose most abundant gene transcript 1) in human and rodents (Scherer et al. 1995; Hu et al. 1996; Maeda et al. 1996). Its structure is similar to the C1q protein, with a C-terminal globular domain and N-terminal collagenous tail (Tsao et al. 2002). It circulates in the blood as trimers of truncated (globular) or full-length isoforms, or as high molecular weight complexes (HMW) (Fruebis et al. 2001). Two receptors for adiponectin, ADIPOR1 and ADIPOR2, have been recently cloned and found to be widely expressed in tissues (Yamauchi et al. 2003). ADIPOR1 has a high affinity for the globular form of adiponectin and a low one for the other isoforms, whereas ADIPOR2 has an intermediate affinity for all forms (Yamauchi et al. 2003). The two receptors also display functional differences: ADIPOR2 is associated with stimulation of the nuclear transcription factor PPARα, while ADIPOR1 activates AMP-activated protein kinase (AMPK), at least in muscle and liver (Yamauchi et al. 2002; Kadowaki and Yamauchi 2005).
AMPK is a well-known major metabolic switch and also a potent oxidative stress inhibitor. Its structure is composed of three sub-units, one catalytic (α) and two regulatory (β and γ) (Stapleton et al. 1996). Binding of adiponectin to the ADIPOR1 receptor leads to activation of α sub-units by phosphorylation of a threonine 172 (Hawley et al. 1996). Once activated, catalytic AMPK phosphorylates proteins involved in the control of energy metabolism, increasing the activity of ATP-producing pathways while shutting down energy-consuming processes (Stapleton et al. 1996; Bruce et al. 2005; Ceddia et al. 2005). On the other hand, its roles in the control of reactive oxygen species generation (ROS) and apoptosis (Rakatzi et al. 2004; Ouedraogo et al. 2006; Park et al. 2006; Davis et al. 2006), as well as in the regulation of cell polarity and mitosis (Motoshima et al. 2006; Lee et al. 2007) are primordial.
ADIPOR1 and AMPK are expressed in kidney but their roles in renal physiology have not been studied (Yamauchi et al. 2003). In particular, their localization in the different cell types along the nephron has yet to be determined. Nevertheless, numerous clinical studies have revealed that several pathologies associated to variations in adiponectin plasma levels are linked to renal pathologies (Ohashi et al. 2007; Schalkwijk et al. 2006). Interestingly, adiponectin is present in urine of healthy individuals (Shimotomai et al. 2005) which shows that cells within the glomerulus and along the proximal tubules are exposed to this hormone from their luminal as well as basolateral fronts. These observations led us to topographically dissect the glomerulus in terms of cell expression of ADIPOR1 and catalytic AMPK.
Materials and methods
Chemicals
Antibodies were acquired from various companies: rabbit anti-ADIPOR1 (Affinity BioReagents, Golden, CO, USA), rabbit anti-AMPK α1 and rabbit anti-AMPK α2 (Bethyl Lab, Montgomery, TX, USA), rabbit anti-phosphorylated AMPK α (thr 172) (Cell Signaling, Boston, MA, USA) and mouse anti-β-actin (clone AC15) (Sigma-Aldricht, St-Louis, MO, USA). AICAR (5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside) was from Sigma-Aldricht. Rat globular adiponectin was obtained from Biovision (Mountain View, CA, USA). Protein A-gold (10 nm gold particles) was prepared as previously described (Bendayan 1995).
Animals
Sprague-Dawley male rats (300 g) were from Charles River Co. (St-Constant, QC, Canada) and handled following the guidelines of the Canadian Council on Animal Care (CCAC). Animals were kept in individual cages with 12:12 h light–dark cycles with free access to water and food. They were anaesthetized by intraperitoneal injection of urethane (1 mg/Kg) prior sampling the tissues. All protocols were approved by the Committee of Deontology on Animal Experimentation of the University of Montreal.
Electron microscopy
Samples of renal cortex were rapidly fixed by immersion for 2 h at 4°C in a 4% paraformaldehyde-lysine-periodate solution. They were rinsed in 0.1 mol/l phosphate buffer, dehydrated in methanol solutions at decreasing temperatures till −20°C and embedded in Lowicryl K4 M resin (CANEMCO, St-Laurent, QC, Canada). Ultrathin sections (70 nm thick) were mounted on Parlodion-carbon-coated nickel grids. The immunogold labelling protocol was carried out as described previously (Bendayan 1995). Briefly, tissue sections were exposed to a saturated sodium metaperiodate solution for 10 min, then incubated with glycine (0.15 mM in PBS pH 7.4) for 10 min, followed by ovalbumin (1% in PBS) for 15 min. Incubation with the primary antibody (anti-ADIPOR1 1/20, anti-AMPK α1 or α2 1/10) was carried out overnight at 4°C. The following morning, grids were washed with PBS, incubated for 5 min with 1% ovalbumin and then with the protein A-gold complex for 30 min at room temperature. After washing, uranyl acetate was used to counter-stain the tissue sections. Morphological observation was carried out with a Philips 410LS electron microscope (FEI Systems Canada Inc, St-Laurent, QC, Canada). Controls of specificity were performed by omitting the primary antibody from the labelling protocol.
In vitro incubation of isolated glomeruli
Glomeruli were isolated by the sieve technique as previously described (Regoli and Bendayan 1999). Briefly, minced kidneys were sequentially pushed through three sieves of respectively, 125, 180 and 106 μm. Glomeruli were then resuspended in a Krebs–Henseleit bicarbonate Buffer (KHB). Purity of the suspension was confirmed by light microscopy; glomeruli appeared deprived of Bowman’s capsule. This ensures free access of globular adiponectin to all cell types. Isolated glomeruli were incubated in fresh KHB buffer without (basal) or with globular adiponectin (10 μg/ml final) or AICAR (2 mM) at 37°C for 60 min, with gentle shaking. At the end of the incubation, glomeruli were homogenized in RIPA buffer volume/volume and left on ice for 1 h. Samples were then sonicated and insoluble material removed by centrifugation at 10,000g for 3 min. Supernatants were frozen and kept at −80°C until use.
Western blot
Protein concentration was measured with a Non-Interfering Protein Assay (MJS BioLynx Inc., Brockville, ON, Canada). Samples (20 μg proteins) heated in Laemmli buffer at 95°C for 5 min were loaded on a 10% SDS-polyacrylamide gel. Transfer to nitrocellulose membrane was carried out at 4°C in the presence of methanol. Incubations with primary antibodies were performed overnight at 4°C in TBST (10 mM Tris pH 7.5, 150 mM NaCl, 0.1% Tween-20) containing 1% non fat dry milk (for anti-ADIPOR1 1/200, anti-AMPK α1 1/1,000, anti-AMPK α2 1/1,000 and anti-β-actin 1/2,500) or bovine serum albumin (5%) and fluoride sodium (5 mM) (for AMPK α-thr172-Pi 1/100). After incubation with secondary antibodies, proteins were revealed using a Lumi-light Plus kit (Roche Diagnostics, Laval, QC, Canada). Band intensities were evaluated using Scion Image software (Scion Corporation, Frederick, MD, USA). Statistics (Student t-test) were determined using Microsoft Excel software.
Results
Expression of ADIPOR1 and activation of catalytic AMPK α1, α2 by globular adiponectin and AICAR
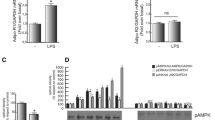
Western blots were carried out on glomerular homogenates from three different animals (G1, G2 and G3). ADIPOR1 appears as a single band close to 42 kDa (Yamauchi et al. 2003) while catalytic AMPK α1 and α2 sub-units were revealed at 64 kDa (Fig. 1A) (Hawley et al. 1996). ADIPOR2 receptor was not detected in glomerular homogenates while present in liver samples as a single band of 34 kDa (results not shown), which indicates that ADIPOR2 is not expressed to a significant extent by glomerular cells. To assess the functionality of ADIPOR1 receptors, glomeruli were freshly isolated and incubated in the absence (basal) or presence of globular adiponectin (10 μg/ml) (AdipoN) or AICAR (2 mM) for 1 h at 37°C. Globular adiponectin is the main ligand of ADIPOR1 (Yamauchi et al. 2003). AICAR is a permeable substance that is intracellularly converted into ZMP (AICA-Ribotide) that allosterically activates AMPK without affecting levels of AMP (Sullivan et al. 1994). It is used as a positive control. At the end of the incubation, glomeruli were homogenized and analysed by western blots for the detection of AMPK α1 and α2, their phosphorylated (activated) form and β-actin. The antibody against phosphorylated AMPK recognizes a threonine 172 within a conserved sequence (SDGEFLRTSCGSPNYA) that is identical in both AMPK α1 and α2, in rats and humans (Stapleton et al. 1996; Hawley et al. 1996). Levels of AMPK α1 and α2, and β-actin were unchanged after 1 h of incubation with adiponectin or AICAR (Fig. 1B). On the other hand, phosphorylation of catalytic AMPK (expressed as the ratio phospho-AMPK/total AMPK corrected for β-actin levels) was potently increased by globular adiponectin (1.13 ± 0.31) and AICAR (1.21 ± 0.15) when compared to basal (0.27 ± 0.07) (Fig. 1B), demonstrating the functionality of ADIPOR1.
Western blot analysis of ADIPOR1, AMPK α1, α2 in freshly isolated glomeruli (A) and effects of globular adiponectin and AICAR on AMPK activation (B). ADIPOR1, AMPK α1 and α2 are present in glomerular homogenates (three different animals G1, G2 and G3) (A). Freshly isolated glomeruli were incubated without (basal) or with globular adiponectin (AdipoN) (10 μg/ml) or with AICAR (2 mM) for 1 h. Immunoblots were carried out for the detection of phosphorylated AMPK (Pi-AMPK), AMPK α1, α2, and β-actin (B). Activation of AMPK is expressed as the ratio Pi-AMPK/Total AMPK (means ± SEM) (n = 4) and significant differences between basal and stimulating agents were found ** P ≤ 0.01. β-actin remained unchanged
Localization of ADIPOR1, AMPK α1 and α2 by immunogold cytochemistry
To identify the glomerular cells that express these proteins, immunogold labelling was carried out on rat renal tissue sections. ADIPOR1 (Figs. 2A and 3A), AMPK α1 (Figs. 2B and 3B) and α2 (Figs. 2C and 3C) were found at the level of the plasma membrane of podocyte foot processes, endothelial cells and mesangial cell processes. Bowman’s capsule epithelial cells displayed a similar staining (results not shown). Controls carried out in parallel by omitting primary antibodies from the protocol displayed no significant labelling (results not shown). Urinary space, capillary lumen and glomerular basement membrane were almost devoid of labelling.
Immunogold cytochemistry of rat glomeruli. ADIPOR1 (A), AMPK α1 (B) and α2 (C) were detected on podocyte foot processes and endothelial cells at the level of their plasma membranes. In A, the glomerular wall is viewed in tangential section, the gold particles associated with the luminal membrane of the endothelial cells are located at the fenestration sites. CL, capillary lumen; END, endothelial cell; GBM, glomerular basement membrane; M, mesangial cell; P, podocytes; US, urinary space
Immunogold cytochemistry of rat glomeruli. ADIPOR1 (A), AMPK α1 (B) and α2 (C) were detected on podocyte foot processes and endothelial cells as well as in mesangial cell processes at the level of their plasma membranes. CL, capillary lumen; END, endothelial cell; GBM, glomerular basement membrane; M, mesangial cell; P, podocytes; US, urinary space
Discussion
Low adiponectin plasma levels have been linked to inflammation in various pathologies, including cardiovascular diseases, metabolic syndromes and type 2 diabetes (Funahashi and Matsuzawa 2006). Similarly, knock-out mice for adiponectin display exacerbation of albuminuria, glomerular hypertrophy and tubulointerstitial fibrosis which are reversed by adiponectin treatment (Ohashi et al. 2007). On the other hand, clinical studies on diabetes type 1 nephropathies, end-stage-renal failure and nephritic syndromes are characterized by increases in plasma adiponectin levels, that have been considered as attempts to limit renal inflammation (Zoccali et al. 2003; Saraheimo et al. 2005; Shen et al. 2007). All studies suggest a protective role of adiponectin on renal tissue.
We demonstrate herein that adiponectin receptor ADIPOR1 and catalytic AMPK sub-units are in fact expressed by the four cell types constituting the glomerulus, namely endothelial cells, podocytes, mesangial cells and Bowman’s capsule epithelial cells. Localization of ADIPOR1 at the plasma membrane of cells in contact with the urinary space is consistent with the presence of adiponectin in urine of healthy patients (Shimotomai et al. 2005). Globular adiponectin in its trimeric form has indeed a molecular weight small enough to cross the glomerular filtration barrier while other isoforms are too voluminous (Fruebis et al. 2001). On the other hand, the localization of catalytic AMPK at the level of plasma membranes is in accordance with the presence of a myristic acid in the N-terminal portion of the β sub-units which is bound to the α sub-units (Hawley et al. 1996).
Subsequently, we observed increases in phosphorylation of catalytic AMPK when tissues were exposed to globular adiponectin, confirming the functionality of ADIPOR1. Two other adiponectin receptors, T-cadherin and ADIPOR2, are present on endothelial cells (Yamauchi et al. 2003; Hug et al. 2004) but could not be responsible for the activation of AMPK. T-cadherin which binds only the HMW isoforms has no intracellular domain (Hug et al. 2004) and is not associated to an intracellular messenger. ADIPOR2 was not detected in glomerular homogenates. Furthermore, even in liver, ADIPOR2 does not stimulate AMPK (Yamauchi et al. 2007). Indeed, in ADIPOR1-knockout mice, AMPK activation by adiponectin is completely abolished, at least in liver and skeletal muscles (Yamauchi et al. 2007). We can therefore reasonably conclude that in our in vitro conditions, ADIPOR1 is the activator of catalytic AMPK.
It is well-demonstrated that adiponectin and AMPK have protective effects on endothelial cells: neutralization of reactive oxygen species (ROS), decrease of adhesion molecules synthesis (ICAM-1, VCAM-1 and E-selectin), inhibition of TNFα-mediated activation of NFκB as well as suppression of cell proliferation (Rakatzi et al. 2004; Ouedraogo et al. 2006; Park et al. 2006; Davis et al. 2006; Motoshima et al. 2006; Lee et al. 2007). Most interestingly, high levels of ROS, elevated expression of adhesion molecules and hypercellularity are all markers of glomerulonephropathies (Lee et al. 2007). These observations suggest that adiponectin crossing the glomerular filtration barrier can control oxidative stresses and cell division within glomerular cells through ADIPOR1-mediated activation of AMPK.
Cell culture models are now required to determine the roles adiponectin and AMPK play in each glomerular cell type. Particularly, their action in the presence of stress factors such as high glucose concentrations and TNF-α will have to be studied at the level of intracellular pathways, oxidative stress generation and cell survival. Demonstrating that adiponectin, through ADIPOR1 and AMPK, protects glomerular cells against inflammatory injuries may help find new avenues in the treatment of glomerulopathies.
References
Bendayan M (1995) Colloidal gold post embedding immunocytochemistry. Prog Histochem Cytochem 29:1–163
Bruce CR, Mertz VA, Heigenhauser GJ et al (2005) The stimulatory effect of globular adiponectin on insulin-stimulated glucose uptake and fatty acid oxidation is impaired in skeletal muscle from obese subjects. Diabetes 54:3154–3160. doi:10.2337/diabetes.54.11.3154
Ceddia RB, Somwar R, Maida A et al (2005) Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia 48:132–139. doi:10.1007/s00125-004-1609-y
Davis BJ, Xie Z, Viollet B et al (2006) Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55:496–505. doi:10.2337/diabetes.55.02.06.db05-1064
Fruebis J, Tsao TS, Javorschi S et al (2001) Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98:2005–2010. doi:10.1073/pnas.041591798
Funahashi T, Matsuzawa Y (2006) Hypoadiponectinemia: a common basis for diseases associated with overnutrition. Curr Atheroscler Rep 8:433–438. doi:10.1007/s11883-006-0042-8
Hawley SA, Davison MD, Woods A et al (1996) Characterization of the AMP-activated protein kinase from rat liver and identification threonine-172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 271:27879–27887. doi:10.1074/jbc.271.44.27879
Hu E, Liang P, Spiegelman BM (1996) AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271:10697–10703. doi:10.1074/jbc.271.18.10697
Hug C, Wang J, Ahmad NS et al (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101:10308–10313. doi:10.1073/pnas.0403382101
Kadowaki T, Yamauchi T (2005) Adiponectin and adiponectin receptors. Endocr Rev 26:439–451. doi:10.1210/er.2005-0005
Lee JH, Koh H, Kim M et al (2007) Energy-dependent regulation of cell structure by AMP-activated protein kinase. Nature 447:1017–1020. doi:10.1038/nature05828
Maeda K, Okubo K, Shimomura I et al (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221:286–289. doi:10.1006/bbrc.1996.0587
Motoshima H, Goldstein BJ, Igata M et al (2006) AMPK and cell proliferation—AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol 574:63–71. doi:10.1113/jphysiol.2006.108324
Ohashi K, Iwatani H, Kihara S et al (2007) Exacerbation of albuminuria and renal fibrosis in subtotal renal ablation model of adiponectin-knockout mice. Arterioscler Thromb Vasc Biol 27:1910–1917. doi:10.1161/ATVBAHA.107.147645
Ouedraogo R, Wu X, Xu SQ et al (2006) Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway. Diabetes 55:1840–1846. doi:10.2337/db05-1174
Park IJ, Hwang JT, Kim YM et al (2006) Differential modulation of AMPK signaling pathways by low or high levels of exogenous reactive oxygen species in colon cancer cells. Ann N Y Acad Sci 1091:102–109. doi:10.1196/annals.1378.059
Rakatzi I, Mueller H, Ritzeler O et al (2004) Adiponectin counteracts cytokine- and fatty acid-induced apoptosis in the pancreatic beta-cell line INS-1. Diabetologia 47:249–258. doi:10.1007/s00125-003-1293-3
Regoli M, Bendayan M (1999) Expression of β1 integrins in glomerular tissue of streptozotocin-induced diabetic rats. Biochem Cell Biol 77:71–77. doi:10.1139/bcb-77-1-71
Saraheimo M, Forsblom C, Fagerudd J et al (2005) Serum adiponectin is increased in type 1 diabetic patients with nephropathy. Diabetes Care 28:1410–1414. doi:10.2337/diacare.28.6.1410
Schalkwijk CG, Chaturvedi N, Schram MT et al (2006) Adiponectin is inversely associated with renal function in type 1 diabetic patients. J Clin Endocrinol Metab 91:129–135. doi:10.1210/jc.2005-1117
Scherer PE, Williams S, Fogliano M et al (1995) A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem 270:26746–26749. doi:10.1074/jbc.270.45.26746
Shen YY, Charlesworth JA, Kelly JJ et al (2007) Up-regulation of adiponectin, its isoforms and receptors in end-stage kidney disease. Nephrol Dial Transplant 22:171–178. doi:10.1093/ndt/gfl552
Shimotomai T, Kakei M, Narita T et al (2005) Enhanced urinary adiponectin excretion in IgA-nephropathy patients with proteinuria. Ren Fail 27:323–328. doi:10.1081/JDI-200056597
Stapleton D, Michellhill KI, Gao G et al (1996) Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271:611–614. doi:10.1074/jbc.271.45.28445
Sullivan JE, Brocklehurst KJ, Marley AE et al (1994) Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett 353:33–36. doi:10.1016/0014-5793(94)01006-4
Tsao TS, Lodish HF, Fruebis J (2002) ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol 440:213–222. doi:10.1016/S0014-2999(02)01430-9
Yamauchi T, Kamon J, Minokoshi Y et al (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8:1288–1295. doi:10.1038/nm788
Yamauchi T, Kamon J, Ito Y et al (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423:762–769. doi:10.1038/nature01705
Yamauchi T, Nio Y, Maki T et al (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13:332–339. doi:10.1038/nm1557
Zoccali C, Mallamaci F, Panuccio V et al (2003) Adiponectin is markedly increased in patients with nephrotic syndrome and is related to metabolic risk factors. Kidney Int Suppl 84:S98–S102. doi:10.1046/j.1523-1755.63.s84.49.x
Acknowledgments
This work was supported by grants from the CIHR, the FRSQ and Diabete Quebec. The authors would like to thank Dr. Irene Londono for sharing her knowledge on renal physiology and Diane Gingras for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cammisotto, P.G., Bendayan, M. Adiponectin stimulates phosphorylation of AMP-activated protein kinase α in renal glomeruli. J Mol Hist 39, 579–584 (2008). https://doi.org/10.1007/s10735-008-9198-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-008-9198-6