Abstract
The rodent olfactory epithelium (OE) is capable of prolonged neurogenesis, beginning at E10 in the embryo and continuing throughout adulthood. Significant progress has been made over the last 10 years in revealing the signals that drive induction, differentiation and survival of its Olfactory Receptor Neurons (ORNs). Our understanding of the identity of specific progenitors or precursors that respond to these signals is, however, less well developed, and the search is still on for the elusive, definitive multipotent neuro-glial OE “Stem cell”. Here, we review several lines of evidence that support the existence of a heterogeneous population of neural and glial progenitors in the olfactory mucosa, and highlight the differences in the identity and activity of progenitors found in the embryonic and adult OE. In particular, we show how recent advances in mouse transgenesis, and in the development of in vitro assays of progenitor activity, have helped to demonstrate the existence of multiple classes of olfactory mucosa-based progenitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
To understand the mechanisms that drive nervous system (NS) development and regeneration, we must first demonstrate when and where specific cell types originate, establish the lineage contribution of each progenitor, and how the interaction of sequentially generated cells impacts the patterning of the NS. To develop a thorough understanding of how region-specific neural stem cells are regulated within their different niches, during development or regeneration, we must first establish their distinct identities (Shihabuddin et al. 1999; Weiss 1999; McKay 2000). In the adult NS, the re-establishment of functional circuitry, and adult neurogenesis, is restricted to exclusive niches, one of which is the rodent OE (Graziadei and Graziadei 1979; Schwob 2002). In the OE, newly differentiated olfactory receptor neurons (ORNs) re-integrate into the existing circuitry and sustain the sense of smell for the lifetime of the organism (Farbman 1990; Roskams et al. 1996). The main central nervous system (CNS) target for ORNs, the olfactory bulb (OB), adapts to changing input by also supporting adult neurogenesis (Altman 1969; Lledo et al. 2002), and is reviewed elsewhere in this edition.
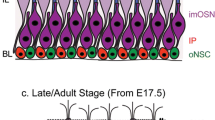
Despite its long history as a hotspot of adult neurogenesis (Altman 1969; Graziadei and Graziadei 1979; Farbman 1990; Carr and Farbman 1992; Luskin 1993; Roskams et al. 1996; Calof et al. 1998a; Huard et al. 1998; Schwob 2002; Bauer et al. 2003), we still know relatively little about the cellular identity of different progenitors that fuel the development and turnover of over several million rat/mouse ORNs, or how the embryonic OE is patterned in time and space. In the mouse OE, neurogenesis occurs in three distinct phases: (1) embryonic establishment (E10–P0); (2) postnatal expansion (P1–P30); and (3) adult maintenance (P30 - death). Each neurogenic phase occurs in distinct, spatiotemporal patterns, and within very different cellular and extracellular environments (Fig. 1). Embryonic and early postnatal OE development is characterized by extensive ORN production, concurrent with the primary organization of OE zones and projections (Fig. 1A, C). Postnatal development terminates with the organization of the OE into a fixed laminar structure that mediates chemosensation, and undergoes maintenance neurogenesis, based on demand (Fig. 1B, D).
Organization and structure of embryonic and adult OE (A, C) The embryonic OE contains apical and basal PCNA-expressing progenitors with processes that span the height of the OE. Immature olfactory receptor neurons (iORN) are flanked by apical and basal dividing progenitors. The developing lamina propria contains olfactory ensheathing cell (OEC) precursors and immature (i)OECs, which ensheathe olfactory receptor neuron axons. (B, D) The apical layer of adult OE contains sustentacular cell (Sus) bodies with progenitors segregated to the basal layers below layers of increasingly differentiated ORNs. The (C) E16.5 OE contains proliferating progenitors (PCNA+; red; arrowheads) that flank immature Dcx+ ORNs (green), that in the (D) adult are reduced in frequency and located basally (arrowhead). Nuclear stain DAPI (blue); dotted line denotes basal lamina; OE, olfactory epithelium; LP, lamina propria. Scale bar represents 50 μm in C,D
Identifying different progenitors in the embryonic or adult OE has been historically challenging because of an inability to antigenically distinguish between distinct embryonic and adult OE progenitors, and expand them in vitro, at the same time as demonstrating their developmental potential or repopulation activity in vivo. The development of sophisticated transgenic mouse technologies, employing site-specific recombinases has, however, recently revolutionized genetic fate mapping studies in the mouse CNS (Joyner and Zervas 2006). Specifically, the relationship between progenitor gene expression and the fate of its daughters (genetic lineage) can now be accurately demonstrated. Moreover, inducible genetic lineage tracing allows us to temporally map the progeny derived from a progenitor in fine developmental time windows. Our current understanding of OE progenitor lineage contribution has largely been established in adult lesion-induced adult neurogenesis and many studies (Roskams et al. 1994; Roskams et al. 1996; Schwob 2002; Beites et al. 2005), including ours, have suggested that mechanisms regulating adult and embryonic neurogenesis may be equivalent. However, it has yet to be established if the mechanisms that mediate ORN development in embryogenesis are recapitulated in adult regeneration, or whether quite different regulatory signals or progenitors may be at work in adult OE. Here, we review the evidence for different classes of progenitor in the adult and embryonic OE, and highlight how recent studies using cre-lox fate mapping have opened up new avenues into our understanding of OE progenitor lineage contribution.
Structure of the adult and embryonic OE
The postnatal OE is a pseudostratified epithelium composed primarily of three main cell types arranged in a developmentally hierarchical manner atop a defined basal lamina, which separates the OE proper from its underlying lamina propria (LP). The OE proper contains (1) Basal cell progenitors, (2) Immature (i)ORNs, (3) Mature ORNs and (4) Sustentacular (Sus; Supporting) cells, whereas the LP contains axon bundles, olfactory ensheathing cells (OECs; Fig. 1B), connective tissue, blood vessels and Bowman’s glands. Each cell type can be distinguished by location, morphology, and antigen expression profile (summarized in Table 1).
Sus cells span the height of the OE and form the apical OE interface with the nasal cavity, with nuclei aligned in a single apical row comprising approximately 15% of OE cells. Sus cells, which express the Sus4 antigen (Goldstein and Schwob 1996) are often referred to as “olfactory glia” but are distinct from the axon-ensheathing OECs of the LP, and function in detoxification, degradation of olfactory stimuli, regulating ionic composition, and enhancing ORN function. Beneath Sus cells, the middle-upper layers of the OE contain bipolar ORN cell bodies, where the most mature ORNs express olfactory marker protein (OMP) (Farbman and Margolis 1980) and comprise 75–80% of the adult OE. The basal cell layer (5–10%) lies between the lamina propria and the ORN layer and contains 2 major cell types: globose (GBCs) and horizontal basal cells (HBCs). Cells within this layer are responsible for the regenerative capacity of the adult OE, however, the lineage contribution of different basal cells is a matter of continuing debate, and is discussed in detail below. GBCs are polyhedral, they frequently divide (Fig. 1B) and differentiate chiefly into ORNs. Directly below GBCs are flattened HBCs that rarely divide and rest on the basement membrane directly adjacent to the LP (Mackay-Sim and Kittel 1991). In contrast, the embryonic OE at each stage of its development is a highly dynamic and changing structure that is largely devoid of Sus cells and HBCs. The developing OE contains clusters of both apical and basal progenitors, with regions that are pre-neurogenic (quiescent, 2-cells thick), neurogenic (apical and basal layers of PCNA + cells with iORNS sandwiched between) and fully developed OE within the same turbinate. (Fig. 1A, C). Labeling for mitotic cells indicates “bursts” of embryonic neurogenesis can produce bulges in the embryonic OE throughout development that can measure 30–40 cells high (Farbman 1992; Schwob 2002; Cowan and Roskams 2004).
Candidate adult OE progenitors
Manipulating adult OE neurogenesis in vivo
The fact that ORN dendrites are directly exposed to a potentially toxic external environment is the major rationale provided for the increased vulnerability of ORNs to loss, and the justification for the retention of progenitors capable of fuelling their replacement to preserve the sense of olfaction (Farbman 1990). Because the death and replacement of ORNs is tightly coupled (Costanzo and Graziadei 1983; Schwob et al. 1992), ORNs that are inhibited from environmental exposure and stimulation by naris occlusion, demonstrate enhanced lifespan, and a net reduction in ORN turnover and progenitor division. In the rat following naris occlusion, basal cell proliferation and overall OE thickness is reduced, but the number of OMP-expressing ORNs remains unchanged (Farbman et al. 1988). Reversal of naris occlusion, stimulates progenitor activity and the OE restores its ORN population within 6–10 days (Cummings and Brunjes 1997). Although sensory deprivation can be used to manipulate the kinetics of “normal” turnover, the most revealing demonstrations of OE progenitor activity have come from a series of lesion models that each carry a different repopulation demand. In different lesion models, the time course and pattern of ORN replacement depends both on the type and rate of ORN death, and the extent of cell types lost (Carter et al. 2004).
Bulbectomy: In rodents, surgical removal of the olfactory bulb (bulbectomy), induces a retrograde wave of apoptosis in ORNs within 72 h of lesion (Cowan et al. 2001), stimulating mitosis in local progenitors (Graziadei and Graziadei 1979). Several million ORNs are then generated from adult OE-residing progenitors by 2–3 weeks after bulbectomy (Costanzo and Graziadei 1983; Schwob et al. 1992). Although a clean lesion for studying repopulation of ORNs only (where the unlesioned OE serves as a built-in control), the removal of the OB means that the bulbectomy is of limited use in testing mechanisms of axon re-targeting.
Chemical/pharmacological lesion: Alternatively, an intranasal chemical lesion applied directly to the nasal cavity leaves the olfactory bulb available for re-targeting, but also destroys multiple cell types. Detergent (Triton X-100), zinc sulphate (ZnSO4) (Harding et al. 1978), methylbromide (MeBr) gas (Schwob et al. 1995) and the thyroid drug, methimazole (Bergman et al. 2002; Bergstrom et al. 2003) have all demonstrated efficacy in inducing widespread OE cell loss, and stimulating regeneration of multiple lineages. If the damage is too excessive, reconstitution can be incomplete (ZnSO4), or respiratory epithelium will replace the OE (Schwob 2002). After MeBr treatment, proliferation occurs 1–2 days following lesion, peaking after 1 week and continuing for up to 4 weeks, with BrdU, a thymidine analog, being incorporated into Sus, HBCs, and GBCs during the regeneration response. The OE is almost fully restored to its pre-lesion state by 6 weeks. Since MeBr is a highly toxic gas and regulations restrict its widespread usage, Methimazole (Mx; 1-methyl-2-mercaptoimidazol), a drug used to treat hyperthyroidism, provides an excellent alternative. Upon binding of Mx to Sus and Bowman’s gland (BG) cells, OE destruction occurs via the formation of toxic metabolites in the olfactory mucosa, mediated by cytochrome P450 (Brittebo 1995; Bergman et al. 2002), multiple cell types in the OE are lost, and the olfactory bulb is left intact for re-targeting. Although there appears to be some variability in strain sensitivity to methimazole, in our hands, two sequential intraperitoneal injections of Mx, 3 days apart, causes the OE to thin to 52 ± 0.7% 3 days after the final injection in both CD-1 outbred and C57/Bl6 inbred mouse strains.
The replacement of ORNs in the adult OE following lesion results from the division of GBCs, HBCs, or a combination of both (Graziadei and Graziadei 1979; Caggiano et al. 1994; Schwob et al. 1994). It is clear that the ORN replacement program is not uniform across all turbinates (Weiler and Farbman 1997; Cowan et al. 2001; Carter et al. 2004), and lesion-induced ORN neurogenesis appears to be governed by a balance between the loss rate of mature ORNs (demand for replacement) and the degree of readiness of the local endogenous progenitor population (in active versus quiescent zones). During post-bulbectomy neurogenesis, rather than undergoing uniform neuronal replacement following the loss of mature ORNs (Moulton 1974; Camara and Harding 1984; Carr and Farbman 1992; Gordon et al. 1995; Huard et al. 1998; Schwob 2002), adjacent regions of OE in a given turbinate demonstrate a highly patchy pattern of neurogenesis. This is likely due to (i) a dynamic spatiotemporal retrograde ORN apoptosis leading to localized changes in feedback induction/repression signaling (Cowan et al. 2001; Bauer et al. 2003); (ii) a change in lateral inhibition from neighboring cells that relieve inhibitory mechanisms that normally permit only a subset of basal cells to respond to a mitotic stimulus (Shou et al. 2000; Morrison 2001; Watt 2001) and (iii) Stimulation of neurogenesis from underlying OECs that are no longer in contact with axons. In the contralateral OE, a delayed (but surprising) significant increase in local basal cell mitosis also suggests that a feedback loop could exist from the central nervous system that senses the loss of ipsilateral ORN input and instructs the contralateral OE to compensate (Carter et al. 2004).
Candidate adult multipotent progenitors in vivo and in vitro
Globose basal cells: Several lines of evidence suggest that the olfactory mucosa contains more than one kind of progenitor in vivo and in vitro (Calof et al. 1998a; Schwob 2002; Carter et al. 2004). ORN progenitors are largely localized to the basal cell compartment of the OE, and GBCs in particular have been identified as neuronal precursors for ORNs (Graziadei and Graziadei 1979; Calof and Chikaraishi 1989; Caggiano et al. 1994; Schwob et al. 1994; Calof et al. 1998b; Huard et al. 1998). GBCs are neurogenic and bipotential in that they can produce both ORNs and Sus cells. Some GBCs are Mash1-expressing neuronal precursors, their mitosis and differentiation is stimulated by lesion, is regulated by BMP4 and FGF2, and they appear to have a limited ability to divide (Calof and Chikaraishi 1989; DeHamer et al. 1994; Shou et al. 1999). Mash-1 + or FoxG1-expressing GBCs are the only staged precursor that has been identified in both embryonic and adult OE (Regad et al. 2007). Although some GBCs are clearly neuronal precursors, there is little evidence to suggest that GBCs can self renew or demonstrate the multipotency required for regenerating all the cell types in the OE, and are therefore not likely to be long-term repopulating (“stem”) cells (Jang et al. 2003; Beites et al. 2005; Schwob 2005).
Retroviral vector (RV) labeling of mitotic cells and their progeny after MeBr lesion has revealed localized clones of cells containing both neuronal and non-neuronal cell types, including ORNs, Sus, HBCs and GBCs (Huard et al. 1998). Although highly supportive of the existence of a GBC bipotential progenitor (the majority of dividing cells after MeBr are GBCs), HBCs also divide after MeBr, thus making identification of the infected basal cell type, and progenitor of origin of these diverse progeny, difficult. Following bulbectomy, mitotic OE cells, include HBCs but are mostly GBCs, (Holcomb et al. 1995; Carter et al. 2004). Retrovirally labeled GBCs transplanted into one of two differentially conditioned rat hosts generate only retrovirally tagged ORNs in a bulbectomy model, whereas in MeBr-treated hosts, marked neurons, sustentacular and basal cells were found (Goldstein et al. 1998). GBC bi-potentiality has also been further confirmed by the transplantation of GBC-2 + FACS-selected cells into MeBr lesioned murine hosts that were demonstrated to repopulate GBCs, ORNs and Sus cells (Chen et al. 2004). These results indicate that the differentiation of OE progenitors is regulated by signals within the host environment (Goldstein et al. 1998), in which GBC (and potentially HBC?) precursors, can produce neuronal and non-neuronal cell types.
HBC multipotent progenitors: Although bipotential progenitors clearly reside among GBCs, it is evident that other basally situated multipotent neuro-glial and/or glial OE progenitors may exist. The location of HBCs atop the OE basal lamina, and their reluctance to divide in vivo is more indicative of quiescent “stem-like” cell behavior (Carter et al. 2004). The existence of cells with a morphology between HBCs and GBCs suggests that HBCs could be GBC precursors (Graziadei and Graziadei 1979; Holbrook et al. 1995), and the co-expression of some HBC and GBC antigens following MeBr lesion suggests a close relationship between the two BCs. The expression of GBC antigens preceding HBC antigens in ventral rat OE following MeBr (Schwob et al. 1995), and because GBCs can be identified earlier in development than HBCs (Holbrook et al. 1995), has been suggested as evidence that GBCs could be hierarchical to HBCs. However, ontogenic time of emergence is not evidence for hierarchical progenitor lineage, because adult progenitor or stem cells may shift into an “adult” state just prior to the establishment of a mature tissue, hence embryonic and adult stem cells that repopulate the same tissue can appear at different times (van der Kooy and Weiss 2000).
In contrast with GBCs, HBCs are relatively quiescent, and both in vivo and in explant or mixed culture are EGF-and TGFα-responsive (Mahanthappa and Schwarting 1993; Farbman and Buchholz 1996; Getchell et al. 2000). In earlier work from our lab, we found that rarely dividing HBCs may contribute to neurogenesis in vivo, and can demonstrate limited self-renewal and multipotency in vitro. To do this, we built on the established biology of adult stem cells in the bone marrow, colon and epidermis where progenitors, transit amplifying cells and mature, terminally differentiated cells are organized in discrete laminae, similar to the OE (Booth and Potten 2000; Watt 2001). The HBC niche utilizes integrin combinations that are now established as the most highly conserved adhesion receptors shared between different primitive stem cells and SVZ neural stem cells, in both functional and gene expression studies (Hurley et al. 1995; Papayannopoulou et al. 1995; Hirsch et al. 1996; Hurley et al. 1997; Voura et al. 1997; Levesque and Simmons 1999; Potocnik et al. 2000; Suzuki et al. 2000; Lemischka 2001; Ivanova et al. 2002; Ramalho-Santos et al. 2002; Campos et al. 2004). HBC adhesion receptor expression profile enabled us to develop in vitro conditions to positively select HBCs, and also to control their behavior to demonstrate that a HBC can exhibit multipotency in vitro. In tracking the receptor expression profiles of HBCs, we found that, rather than possessing a singular identity, HBCs are a heterogeneous population of cells, some of which can generate colonies containing multiple olfactory and non-olfactory neural lineages, as well as glia and GBC-like precursors, from single cells. These data do not argue against the existence of a GBC-like progenitor in the OE, but reveal the possibility that heterogeneous populations of GBC and HBC progenitors exist. Although HBCs remain relatively quiescent, and let GBCs lead the repopulation effort when only ORNs are required after bulbectomy, the multi-lineage potential of HBCs during extensive OE regeneration following MeBr lesion in vivo has now been confirmed using inducible Cre-mediated lineage tracing in Keratin 5-Cre transgenic reporter mice (Leung et al. 2007). However, in both this study and ours, only a subpopulation of HBCs was either labeled (in vivo) or selected (in vitro), leaving open the possibility that these HBCs may still represent a multipotent daughter of a more primitive stem cell in the OE that has yet to be identified.
Sustentacular progenitors? Fully differentiated Sus cells are highly elongated, demonstrate a low rate of mitosis in most lesion studies, and may not seem attractive candidate progenitors. However, their retained expression of the established neural progenitor transcription factors Pax6 (Davis and Reed 1996), Sox 2 and Otx-2 in the adult OE (Larouche and Roskams unpublished), concurrent with the c-kit ligand, Steel (Murray et al. 2003), suggests their potential contribution should not be overlooked. Despite this intriguing expression profile, however, Sus4-expressing cells transplanted into MeBr-lesioned OE produce only sustentacular and duct cells after transplantation, making them an unlikely functional progenitor (Chen et al. 2004).
Adult models of OE regeneration have thus played a key role in establishing the potential lineage contribution of some OE progenitors. Similarly, gene expression assays following OE lesions have provided clues to genetic and epigenetic factors regulating the progenitor regenerative response (Getchell et al. 2002; Iwema et al. 2004; Shetty et al. 2005). The subsequent application of transgenic mouse and in vitro models (reviewed below) has enabled us to understand the contribution of some of these factors to regulating ORN genesis.
Molecular regulation of OE neurogenesis
OE precursors clearly possess an extensive capacity for proliferation and differentiation, and must be tightly regulated, spatially and developmentally, to maintain the integrity of the OE over a lifetime. ORN genesis is thus controlled by the balance of positive regulatory factors from cells (e.g. apoptotic ORNs, macrophages, OECs) that sense a need for more ORNs and stimulate mitosis and differentiation, and a reduction in negative feedback from mature ORNs to inhibit additional ORN production (Shou et al. 2000; Bauer et al. 2003; Wu et al. 2003).
Factors that positively regulate ORN genesis: Leukemia inhibitory factor (LIF) is a mitogen that contributes to proliferation in embryonic and postnatal OE in vitro and in vivo (Satoh and Yoshida 1997; Nan et al. 2001; Getchell et al. 2002; Bauer et al. 2003; Carter et al. 2004). LIF and its receptor transcripts are also up-regulated and required acutely following bulbectomy for an adequate proliferation response, and subsequent ORN production (Getchell et al. 2002; Bauer et al. 2003).
Fibroblast growth factors are secreted proteins that signal via four tyrosine kinase receptors (FGFr1–4), which are stabilized by a proteoglycan receptor (Yayon et al. 1991). FGF receptor transcripts are expressed in the E15 OE, but the specific cells expressing these receptors have not been identified in vivo (DeHamer et al. 1994). GBC-like cells proliferate in response to FGF2 in E14.5 OE explants or when dissociated, allowing for 1–2 divisions prior to differentiation into NCAM + ORNs (DeHamer et al. 1994; Mumm et al. 1996) or βIII tubulin positive neurons in the adult OE (Newman et al. 2000). These studies suggest a role for FGF2 in the OE as a mitogen of neuronal precursors, likely GBCs, without inducing immediate neuronal differentiation. The source of FGF2 in the OE, however, has yet to be definitively established, and appears to vary with species and detection approach (Goldstein et al. 1997; Chuah and Teague 1999; Hsu et al. 2001).
Epidermal growth factor (EGF) and TGF-α bind to, and signal through, the EGF receptor (EGFR), part of the EGF receptor family of tyrosine kinases (Schlessinger 2000). The EGFR is most prominently expressed in HBCs and Sus cells while TGF-α and EGF stimulate mitosis of basal cells in vitro, and in vivo (Holbrook et al. 1995; Farbman and Buchholz 1996; Ezeh and Farbman 1998; Carter et al. 2004). HBCs demonstrate a 6 fold increase in proliferation, shown by cytokeratin + BrdU incorporation, without any proliferative effect on GBCs, when TGF-α expression is directed specifically to HBCs via the regulation of a human keratin-14 promoter/enhancer in transgenic mice (Getchell et al. 2000). These results suggest that HBCs are the primary EGF/ TGF-α-responsive OE cell.
Factors that negatively regulate ORN genesis: The TGF-β superfamily of growth factors includes TGF-β, activins and BMPs. They are highly conserved proteins that play active roles during differentiation and development in a variety of tissues, and signal via a serine-threonine kinase receptor complex (Attisano and Wrana 2002). Transcripts for growth and differentiation factor 11 (GDF11), a TGF-β family member, and its receptors can be detected in E12.5 to adult ORNs and their progenitors (Wu et al. 2003). GDF11 can inhibit neurogenesis through division arrest in FGF2-stimulated neurogenin1 + INPs, via up-regulation of the cyclin dependent kinase inhibitor p27Kip1, and proliferative arrest is reversed by either genetic loss or antagonism of GDF11 (Wu et al. 2003). GDF11 therefore appears to act as a negative regulator of OE neurogenesis, in parallel with a similar role in retinal development (Kim et al. 2005).
BMPs are morphogenetic proteins whose effects can vary according to their concentration and target cell (Mehler et al. 2000). Although multiple BMPs can be found in the OE during development (Peretto et al. 2002), BMP4 is the predominant transcript detected in the mid-late gestation embryonic OE and ORN layers in the adult (Shou et al. 2000). BMPs can inhibit OE neurogenesis at high concentrations and stimulate neurogenesis at low concentrations. High concentrations (10–20 ng/ml) of BMP 2, 4 or 7 added to in vitro colony assays from E14.5–15.5 OE, inhibits progenitor proliferation and the formation of neuronal colonies, without affecting non-neuronal subtypes (Shou et al. 1999). BMPs block ORN genesis by targeting Mash 1 for proteolysis via the proteasome pathway (Shou et al. 1999). Alternatively, BMPs at low concentrations (0.1–0.2 ng/ml) promote the survival (but not the proliferation) of newly generated ORNs (Shou et al. 2000). Thus BMPs exert opposing effects on cells at different stages of the ORN lineage, and different TGF-β subfamily members regulate OE progenitors using differential regulatory pathways. During OE homeostasis, concentrations of negative regulatory molecules produced by mature ORNs remains, thus inhibiting additional neurogenesis. Following the lesion-induced removal of adult ORNs, the concentration of negative regulators drops to a critically low level, thus relieving inhibitory feedback and allowing positive regulatory molecules of neurogenesis to stimulate basal progenitors to proliferate and generate replacement ORNs (Calof et al. 1998a; Shou et al. 2000; Bauer et al. 2003).
In vitro assays for OE-based progenitors and regulators: “neurosphere” assays
Stem cells are largely defined by their function—a combination of multipotency with the ability to self-renew—whilst retaining their plasticity (van der Kooy and Weiss 2000). The “neurosphere assay” applied under highly defined conditions is commonly used to assess the presence of potential neural stem cells in the CNS, and how their activity may change under altered growth factor conditions, or following the ablation of regulatory genes (Reynolds and Weiss 1992; Shingo et al. 2001; Vanderluit et al. 2004). Self-renewal of neural stem cells is usually assessed as the ability of single neurospheres to reform spheres while retaining the production of neurons and glia over several passages (Reynolds and Weiss 1996). There is clear evidence that GBCs and HBCs contain subpopulations with varying degrees of multipotency, but do either of them self-renew? Attempts to reproduce the neurosphere assay in the murine OE have not succeeded at recapitulating the primary and subsequent vast expansion capacity obtained from SVZ progenitors (Othman et al. 2003; Murdoch and Roskams 2007). Some investigators have produced OE-derived “neurospheres” from mixed, non-selected OE cells, after a period of starvation or expansion in serum-containing media in vitro. Human and mouse OE-derived cells with significant proliferative potential and capacity to make multiple cell types have emerged after several weeks of culture, but the primary origin of these cells is unknown, and the extended time in culture may have enabled epigenetic events to occur that underlie this enhanced plasticity (Roisen et al. 2001; Morshead et al. 2002). After extended time in culture, OE-derived neurospheres may not accurately represent their true abundance or in vivo mechanisms of regulation (Murrell et al. 2005; Othman et al. 2005).
Because both the abundance and phenotype of OE-based progenitors appears to change significantly from early embryonic to adult life (Fig. 1), we tested if neurosphere assays could provide a read-out of progenitor activity (including potency) or abundance in mouse OE from different ages. Cells isolated from P5 or adult OE and cultured in EGF and/or FGF2 under the same serum-free conditions as those used for SVZ cells of the lateral ventricle (LV) produce non-adherent cell clusters that appear similar to CNS neurospheres, together with adherent colonies, reminiscent of those produced from a subpopulation of ICAM-1 + HBCs (Fig 2A–C) (Carter et al. 2004). Sphere-forming cells from the P5 and adult OE however, have a very low frequency—less than 0.1% of those from the SVZ of the same mice, under identical conditions (Murdoch and Roskams 2007). P5 spheres and adherent colonies demonstrate a very limited capacity to serially passage under defined conditions, where they can reproduce more spheres or adherent colonies, but this capacity rapidly declines with successive passages, unlike CNS neurospheres that can expand extensively (Reynolds and Weiss 1996; Halpern and Martinez-Marcos 2003). Adult OE-derived spheres and colonies are 4- and 9- fold less abundant, respectively, than their P5 counterparts. P5 and adult spheres/colonies are, in turn, significantly less abundant than those obtained from the E13.5 OE, and also differ significantly in their time course of generation, morphology and passaging activity. Thus, when a simple biological assay is applied, progenitor activity in the OE not only changes over developmental time, but is also significantly different than that from age-matched CNS neurogenic regions.
In vivo and in vitro correlates of postnatal and adult OE spheres and adherent colonies Cells plated at clonal density in serum-free medium supplemented with FGF and/or EGF from postnatal day 5 (P5) or adult mice, produce (A) SVZ-derived non-adherent neurospheres (B) OE-derived non-adherent spheres and (C) adherent OE colonies. (D–F) At postnatal day 5 (P5) nestin (green) is found in rare cells within the OE (F, arrowheads), but mainly localizes to olfactory ensheathing cells of the lamina propria, some of which co-express S100β (F, arrows, red). Nestin expression does not coincide with (D) ICAM-1 + basal cells (arrowhead, red), and (E) immature neurons (Dcx+, red). (G–I) Sectioned P5 OE-derived spheres and (J–L) adherent colonies retain these cellular relationships and expression profiles where they contain cells expressing (G, J) nestin (green) distinct from ICAM-1 + basal cells (red), (H, K) Nestin + cells (green) adjacent to βIII tubulin + neurons (NST; red) and glia (S100β; red). (I, L) Nestin + cells (green); S100β + cells (red). (L) Inset shows nestin + S100β + /− expressing cells migrating away from a colony. Nuclear stain DAPI (blue); dotted line denotes basal lamina; OE, olfactory epithelium; LP, lamina propria; ORN, olfactory receptor neuron; Ax, axon bundle; SVZ, subventricular zone. Scale bar represents: in A-100 μm (A, C); B-50 μm; in E-50 μm (D–F); in I-50 μm (G–I); J-50 μm; in K-100 μm (K, L)
The cellular expression of genes associated with CNS progenitors and neuroblasts, is also different in the postnatal OE. In P5 OE, the putative neural stem cell marker, nestin, is not expressed in candidate HBC progenitors, but is found largely in a subpopulation of S100β-expressing OECs of the LP (Fig. 2D, F). The microtubule-associated protein, Doublecortin, is not found on neuroblasts, as in the CNS (Gleeson et al. 1999), but is expressed in immature ORNs and their axons (Fig. 2E). Immunocytochemical analysis of sectioned neurospheres from P5 OE reveals largely lineage-negative cells at the core of OE-derived spheres and colonies (Fig. 2G–L). Nestin is found in cells predominantly on the periphery of spheres and colonies, in close apposition to ICAM1 + HBCs (Fig. 2G), and near immature ORNs expressing neuron-specific (βIII) tubulin, NST (Fig. 2H). Glia expressing S100β are usually found closer to the centre of spheres, in a similar arrangement to that seen in CNS neurospheres (Fig. 2I) (Campos et al. 2004). Subpopulations of cells expressing ICAM-1 or nestin are found in adherent colony cores, or more often at their periphery where they are usually clustered adjacent to S100β + cells (Fig. 2J–L). NST + ORNs are usually on the surface of adherent colonies, and in the periphery of spheres, adjacent to nestin + and/or S100β + cells (Fig. 2H, I, K, L). With passaging of primary spheres, the production of neurons decreases (similar to CNS neurospheres), and glial production predominates. Thus, although the production and passaging of postnatal spheres and colonies is limited under serum-free conditions, the organization of the cells produced in vitro closely mimics the in vivo environment, and may serve well as a limited model system for testing mechanisms regulating OE neuro- and glio-genesis.
Embryonic OE progenitors
Although HBCs and Sus cells exhibit molecular and cellular expression profiles characteristic of a potential multipotent progenitor, neither cell type is found (with its unique gene expression profile, Table 1) until late in embryonic development (Murdoch and Roskams 2007). Adult ORNs (and OECs) are thus likely to be produced from regionally restricted adult-residing precursors derived from embryonic neuro-glial progenitors, but the establishment of their specific identity has been stalled by the paucity of identifiable genes we can use to distinguish, and assay the potential of, individual candidate progenitors, similar to other stem cell-containing tissues (Weissman et al. 2001). Early placode-derived embryonic progenitors clearly demonstrate the highest multipotency of all OE-based progenitors, generating peripheral (olfactory, vomeronasal) and central (hypothalamic LHRH+) neurons, and glia, but this multipotency doesn’t appear to continue in the adult, despite the persistence of active progenitors (Wray et al. 1989; De_Carlos et al. 1995; Schwanzel-Fukuda 1999). This may be because of a progressive restriction in potential in olfactory or vomeronasal neuron development (similar to other CNS regions like retina and cerebral cortex) (Cayouette et al. 2003; Cayouette and Raff 2003; Fishell and Kriegstein 2003; Fishell and Kriegstein 2005), or due to differences in the local environment of the embryonic and adult OE (Fig. 1).
Embryonic ORNs originate from a subset of cells within the olfactory placode, paired oval-shaped epithelial patches in the anterolateral region of the head. The placode becomes first evident at embryonic day 9.5 (E9.5) in the mouse, and invaginates to form a nasal pit by E10.5 (Cuschieri and Bannister 1975), concurrent with formation of the medial and lateral nasal processes (Farbman 1992). Induction mediated by local molecular signals orchestrates axis formation, patterned gene expression, morphogenesis, and cellular differentiation during the initial formation of the OE and nerve (LaMantia et al. 2000). Axial signaling is established by interactions between the fronto-nasal mesenchyme and developing OE in a similar manner to other bilaterally symmetrical non-axial structures such as the limbs, branchial and aortic arches (reviewed in Balmer et al. 2005). The classic neuronal inducers retinoic acid (RA; lateral), bone morphogenetic protein 4 (BMP4; posterior), fibroblast growth factor 8 (FGF8; medial), and sonic hedgehog (SHH; medial) are all implicated in ORN induction (LaMantia et al. 2000), and are reviewed elsewhere in this edition. FGF8 is also critical for the normal development of the OE, as FGF8 null mice exhibit a loss of virtually all OE neuronal subtypes (Kawauchi et al. 2005). Underlying these morphological cellular changes in the olfactory primordium are discrete spatial patterns of gene expression ascribed to primitive neural progenitors, where cells in the ventral rim of the invaginating nasal pit induce transcription of SOX2, Pax6, Dlx5 (Kawauchi et al. 2005). The proneural basic helix-loop-helix (bHLH) transcription factors Mash1 and Ngn1 are also detected in a pattern of successive dorsal expression in the olfactory pit, designating a progressively more committed neural progenitor phenotype (Kawauchi et al. 2005).
Mash1-expressing precursors: key intermediates in ORN production
Mash1 is a basic helix-loop-helix (bHLH) transcription factor found in progenitors throughout the embryonic OE, but becomes more restricted to basal OE progenitors as development proceeds (Cau et al. 1997; Cau et al. 2000; Tietjen et al. 2003). Concurrent with the relocation of Mash1 + cells over time, PCNA + proliferating cells are redistributed from the apical to basal OE, as cells in the apical OE begin to express the sustentacular cell marker, Sus4 (Fig. 3A) (Smart 1971; Murdoch and Roskams 2007). Apical embryonic progenitors are thought to be the precursors of basal progenitors (Cau et al. 1997) and are distinguished by their expression of distinct transcripts, such as Notch signaling components, or other bHLH transcription factors (like Ngn1), that drive neuronal differentiation (Cau et al. 1997; Cau et al. 2000; Cau et al. 2002; Tietjen et al. 2003; Carson et al. 2006).
Proliferating OE progenitors change location and frequency between embryonic and adult development (A) Proliferating (PCNA+) cells become relocated over time from predominantly apical to basal OE with age (n = 3–5 mice/group). Immunohistochemistry of E13.5 OE labeled with (B, E) PCNA (red), (C, E) DAPI (blue) and (D, E) nestin (green). Cells undergoing cytokinesis are found at the apical OE (arrowheads), and (F) express the neural progenitor marker, Prominin-1 (red) at their extreme apical membrane, alongside developing dendrites of immature NST+ (green) ORNs. Inset shows larger version of boxed area. P5-postnatal day 5; Adult-2 months of age. OE-olfactory epithelium; LP-lamina propria; NC-nasal cavity. Dotted line indicates basal lamina. Size bar represents: in E-50 μm (B–E); F-100 μm
Mash1 is thus thought to be a pro-neural determination gene, required in a subpopulation of ORN progenitors during early ORN specification to activate Notch signaling and initiate neuronal differentiation. Different combinations of Notch receptors, ligands and effectors have been implicated in regulating both ORN and OEC development (Manglapus et al. 2004; Carson et al. 2006), and are reviewed elsewhere in this edition. Mash1 expression peaks during embryogenesis, when neurogenesis is extensive, and is down-regulated in the adult OE. Mash1 induces the expression of later bHLH proteins, like Ngn1 followed by NeuroD, to drive eventual exit from the cell cycle and ORN differentiation. Mash1-/- mutants have a significantly reduced ORN population, fail to express Notch signal transducers, neurogenin 1 (Ngn1) and NeuroD, and lose mitotic basal, but retain apical, progenitors (Guillemot et al. 1993; Cau et al. 1997; Cau et al. 2000; Cau et al. 2002). Mash1 and Ngn1 may transmit distinct responses to environmental cues such as mitogens, survival and differentiation factors, serving as checkpoints at successive stages of ORN development to ultimately control neuron production (Cau et al. 1997; Cau et al. 2002). Following the induction of lesion-induced neurogenesis, the number of mitotic Mash1 + cells is increased. This precedes the appearance of differentiated ORNs and immediate neuronal precursors—cells that divide one or two times before becoming postmitotic neurons, which express Ngn1 and/or NeuroD (Gordon et al. 1995). Mash1 thus appears to be a common hierarchical regulator of both embryonic and adult OE neurogenesis. Other factors that we have identified that demonstrate a unique embryonic expression pattern in transgenic reporter mice (from the GENSAT database; (Gong et al. 2003)) that indicate a potential role in the regulation of ORN or OEC development, are summarized in Table 2.
CNS-like precursors in the embryonic OE
Beyond the central role of Mash1-expressing precursors, relatively little is known about the identity of other ORN progenitors that pattern the OE, compared with other CNS regions (Corbin et al. 2001; Yoshida et al. 2006). From E10–E15, the OE contains proliferating progenitors, lacking expression of neuronal, glial and sustentacular lineage-specific antigens that undergo massive expansion to lay down the initial turbinates that will contain the zones containing ORNs of different classes (Farbman 1992; Iwema et al. 2004). The PCNA + nuclei of proliferating cells are equally distributed between the apical and basal OE during early embryonic development, but by E16.5 and later, become largely located at the basal OE (Fig. 3A). Mitotic cells actively undergoing cytokinesis in embryonic OE are often clustered at the OE apical surface, and frequently grouped on apposing apical membranes (Fig. 3B–E). Progenitors in S-phase are localized to the basal OE, distinct from apical cytokinetic progenitors, in an distribution highly reminiscent of the organization of embryonic CNS ventricular zone progenitors (Gotz et al. 2005), and very different from the pattern in adult OE. Like neuro-epithelial cells of the VZ, cells clustered at the apical embryonic OE express the transmembrane protein, Prominin-1, a putative stem/progenitor marker in neural and non-neural tissues, which may signal nearby progenitor proliferation by releasing prominin-containing particles into the adjacent fluid filled cavity (Fig. 3F) (Gotz et al. 2005; Marzesco et al. 2005; Corti et al. 2007). If these PCNA + apical cells are a distinct class of progenitors, then their preferential localization in apical OE suggests that basal neurogenesis in adult rodent OE must be derived from neural progenitors different to those in the embryo (reviewed in (Brazel and Rao 2004; Rao 2004)).
Given the striking parallels between the organization of cortical and OE embryonic neurogenic zones, we recently tested if embryonic OE contains progenitors that morphologically or antigenically resemble multipotent CNS radial glia. Previously, investigators have searched for cells in adult OE that may express Nestin, an intermediate filament protein characteristic of CNS neuroepithelial stem cells (Hockfield and McKay 1985), during the neurogenic response following OE lesion. Nestin in the OE has previously been localized to the end-feet of adult sustentacular (Sus) cells in vivo (Doyle et al. 2001), in OECs (Pixley 1996; Au and Roskams 2003), in a cytokeratin + neurogenic basal cell line (Satoh and Yoshida 2000) and human OE-derived neurospheres, in vitro (Othman et al. 2003; Zhang et al. 2004). These studies all consistently show that the predominant Nestin-expressing cells of the adult olfactory mucosa are OECs (Au and Roskams 2003). However, improved detection methods using multiple Nestin antibodies have revealed that Nestin expression can be detected at E10.5 in cells with a radial glia-like morphology (termed radial glial-like progenitors; RGLPs) (Murdoch and Roskams 2007), adjacent to columns of immature ORNs expressing neuron-specific βIII tubulin (NST) (Fig. 4A–C). These RGLPs appear similar to those found in the developing forebrain and embryonic OB (Fig. 4A), and are most abundant at the in-fold of each developing turbinate. Within the embryonic OE and developing VNO, the majority of embryonic nestin + RGLPs co-express PCNA and are anchored at the OE basement membrane and apical surface, with processes spanning the OE (Figs. 3E, 4B, C) (Murdoch and Roskams 2007). In contrast to the CNS, the neuroblast marker Doublecortin (Dcx) is not found in migrating, mitotic neuroblasts, but is first found in post-mitotic NST-expressing immature ORNs (Fig. 4C, D). Interestingly, we have also found Doublecortin-expressing mitotic neuroblasts encased within the olfactory nerve from E10–E17 (Carson et al. 2006). The co-expression of NST and Dcx in iORNs begins at E10.5 and continues postnatally, but in regions of the OE that are more developed, NST is expressed by immediately post-mitotic, new iORNs, where iORNs migrating through the OE co-express NST and Dcx, prior to the onset of OMP expression (Fig. 4D,E).
Novel markers in OE development: radial glia-like progenitors At E10.5, presumptive olfactory epithelium (pOE) contains cells expressing (A–C) nestin (green) exclusive of (A, B) neuron-specific β III tubulin (NST) or (C) Dcx (both red) in immature ORNs. Dcx (red) and NST (green) co-localize in (D) E10.5 pOE but in (E) more highly developed regions of OE E17.5, Dcx is not found in the most basal neuronal layers, where NST is induced prior to Dcx (arrowhead). (F–I) P14 Nestin-cre / Rosa YFP mice show YFP reporter expression in a subpopulation of ORNs in zone 1 (margins indicated by arrowheads), whose cell bodies and axons are independent of OCAM-expression (red) in zones 2–4. YFP + ORNs are found in the (G, I) Mature OMP + (red) and (H) immature NST + ORN layers (red). OB, olfactory bulb; VZ, ventricular zone; LV, lateral ventricle; NC, nasal cavity; OE, olfactory epithelium; LP-lamina propria; Sep, septum; Ax, axon bundles. Dotted line indicates basal lamina; “**”dorsal recess. Scale bar represents: in A-200 μm (A, F, G); in B-50 μm (B–E, H, I)
Does the embryonic OE contain radial glia?
Radial glia cannot be identified by the expression of a single antigen, but are usually characterized by a combination of markers, including nestin and RC2, and are distinguished from neuroepithelial stem cells by markers that include the glutamate transporter, NGLAST (Furuta et al. 1997). Some Nestin-expressing RGLPs in the OE and VNO co-express other radial glia proteins, such as GLAST and RC2, but do not express the neurogenic radial glia protein, Brain Lipid Binding Protein (BLBP) (Anthony et al. 2004; Murdoch and Roskams 2007). If OE-based Nestin-expressing cells are RGLPs, then lineage tracing of nestin transgene-activating cells, similar to that performed in the CNS (Tronche et al. 1999) should reveal this. By combining Nestin-Cre mice with flox’d reporters that initiate YFP expression upon excision (Novak et al. 2000; Srinivas et al. 2001), all progeny derived from cells employing CNS nestin-specific enhancers should become YFP+(Joyner and Zervas 2006). We can clearly detect nestin protein expression in the E10.5 OP (Fig. 4A), but reporter expression in Nestin-Cre mice is difficult to detect prior to E15, regardless of the reporter cross (ZEG, Rosa YFP) (Novak et al. 2000; Srinivas et al. 2001). In each of these crosses, Nestin reporter is only found in a small subpopulation of OE cells. Delay of reporter detection is common developmentally, where the transgene has to be induced sufficiently to generate sufficient Cre to excise, followed by a lag in accumulating reporter product over time (Joyner and Zervas 2006). However, at every stage of development examined, we can only detect GFP/YFP in rare OE or VNO cells that express endogenous nestin, that give rise to restricted subpopulations of Y/GFP + ORNs (Murdoch and Roskams 2007). Transgene-expressing cells not only appear to be committed neural precursors (as opposed to multipotent), but are also zonally limited in their production and distribution of chemosensory neurons. The most striking finding from examining YFP-expressing progeny derived from nestin transgene-expressing precursors indicates they produce ORNs restricted to Zone 1 and VNRNs restricted to the VR1 zone of the VNO (Fig. 4F, G) (Murdoch and Roskams 2007). Within these regions, only a sub-population of immature or mature ORNs express the reporter transgene (Fig. 4H, I). It is important to note that, although the majority of Nestin-expressing RGLPs in the OE do not drive this Nestin CNS enhancer transgene (and must therefore employ different regulatory elements), those that do reveal a highly restricted zonal pattern, that is the first of its kind reported in OE development.
GFP-expressing selected cells from VZ of Nestin:GFP mice are the predominant embryonic cortical source of multipotent embryonic neurospheres (Mignone et al. 2004). Although E13.5 SVZ-derived progenitors form only neurospheres in vitro, under identical conditions embryonic OE yields three morphologically distinct semi-adherent colony subtypes, that we termed fusiform, polygonal and spherical (Murdoch and Roskams 2007). These distinct phenotypes emerge over 10 days in vitro, contain PCNA + cells, and can produce both neurons and glia in a pattern that changes depending upon the growth factor stimulation and colony subtype (Murdoch and Roskams 2007). In colony-forming assays, cells from Nestin-cre/ZEG transgenic OE, immediately form single cells, doublets and early colonies that were largely Nestin-expressing/lineage-negative cells that formed the same colony subtypes as littermate controls and wildtype CD-1 mice (Murdoch and Roskams 2007). OE-derived colonies that contained GFP + cells (60–70% of all colonies from E13.5) were twice as likely to be neurogenic than GFP-negative colonies. GFP reporter expression was confined to ORNs generated from these colonies in vitro, suggesting that Nestin-transgene-expressing cells from the E13.5 OE cells are not multipotent, but are primarily neurogenic in vivo and in vitro.
Insights into potential OEC progenitors
In contrast to CNS radial glia, brain lipid binding protein (BLBP), a fatty acid-binding protein (FABP) expressed by radial glia in the embryo and associated with CNS neurogenesis (Feng et al. 1994; Anthony et al. 2004), was not detected at any developmental stage within the OE. Although whole mount transgenic expression data place BLBP-expressing cells in the region of the E10.5 olfactory placode (Anthony et al. 2004), BLBP expression is segregated to ensheathing cells surrounding ORN axons in the nascent olfactory nerve in the presumptive lamina propria (Fig. 5A–C). Olfactory ensheathing cells are a specialized class of glia that are unique to the olfactory nerve that exhibit a significant degree of developmental and functional plasticity (Ramon-Cueto and Nieto-Sampedro 1992). Although their origin is thought to be from the olfactory placode (Farbman 1992), evidence from zebrafish suggests at least some OECs could have a potential neural crest origin, (Whitlock 2004), a finding that has yet to be verified in the mouse or rat.
BLBP expression is exclusive to the olfactory ensheathing cell lineage (A–C). In the embryonic OE, (A) Nestin (green) expression is highest in CNS OB radial glia, but is also found in OE-based RGLPs. (B) Dcx (red) expression is highest in immature ORNs and migrating neuroblasts of the OB, whereas CNS radial glia express BLBP (green) which is not expressed in the OE, but (C) is highly expressed in the migratory mass of mitotic olfactory ensheathing cells that are aligned along Dcx + (red) ORN axons. Semi-adherent colonies formed after serum-free culture of E13.5 OE with FGF +/− EGF (D, E), whose cores can express BLBP (green) produce (D) migratory cells that frequently co-express Nestin and BLBP, which can asymmetrically divide to produce S100β + cells (inset), which then become segregated to the colony periphery. In adult BLBP-cre/Rosa 26 mice (F–I) BLBP (green) and β galactosidase (red: LacZ) are co-expressed (arrowheads) in OECs surrounding axon bundles. BLBP neg β galactosidase + OECs (arrows) are also detected on the periphery of axon bundles and in the LP. (H) BLBP + (green)/β galactosidase + (red) OEC processes are also detected in the nerve fibre layer of the OB, some of which express detectable levels of (I) nuclear Cre recombinase (red). Scale bars represent: in A-100 μm (A, B); C-50 μm; D, E-100 μm; in H-50 μm (F–I)
We have detected BLBP-expressing cells lying directly under the invaginating olfactory pit as early as E10.5 (Richter and Roskams 2007). Although BLBP is co-expressed with nestin in the embryonic CNS (Anthony et al. 2004), these gene products are not co-expressed within the developing OE, and BLBP is only found in cells aligning Doublecortin-expressing immature ORN axons (Fig. 5B, C), a pattern confirmed by transgenic BLBP-GFP reporter mice (Gong et al. 2003). In assaying embryonic OE-derived colonies, Nestin and BLBP expression is usually exclusive but with time in vitro, co-expression can be detected in cells of a more differentiated “glial” phenotype (Fig. 5D). In early colonies, mitotic BLBP-expressing glioblasts derived from semi-adherent colonies clearly give rise to S100β-expressing glia, suggesting that a BLBP+/Lineage negative glial-restricted precursor may be the hierarchical progenitor to OECs (Fig. 5E). Additional insight into the contribution of BLBP + precursors to the OEC lineage is seen in the olfactory nerve of transgenic BLBP-cre/ROSA mice. In these crosses, β galactosidase is induced after cre-mediated excision of a stop codon, regulated by the induction of the BLBP promoter (Fig. 5F–I) (Anthony et al. 2004). The exclusive contribution of BLBP to the OEC lineage is demonstrated by the co-expression of β galactosidase with BLBP in cells immediately surrounding mesaxons (smaller groups of axons within a large axon bundle) in the LP of transgenic BLBP-cre/ROSA mice (Fig. 5F). Interestingly, only the OECs that are in direct contact with axons co-express β-Galactosidase and BLBP. OECs in the outer layer of LP axon bundles (which maintain glial-glial contact), or coursing through the lamina propria, express β-Galactosidase and are clearly derived from a BLBP-expressing glial precursor, but the maintenance of BLBP expression appears to require axonal contact (Fig. 5G). Co-expression of BLBP and reporter predominates in the Olfactory bulb nerve fibre layer (NFL), where Cre expression can also be detected in cells aligning axon bundles (Fig. 5H, I). Given that BLBP is regulated by Notch signaling in radial glia (Anthony et al. 2005), and OECs appear to express Notch 1 and 3 at different stages of their development (Carson et al. 2006), it seems likely that Notch signaling, combined with an axonal-derived signal (Wewetzer and Brandes 2006) may be responsible, at least in part, for the maintenance of a more “developmental” phenotype within BLBP-expressing, adult-residing OECs.
The OE and LP thus contain a complex, inter-dependent system of progenitors and cells at varying stages of glial and neuronal maturity, whose activity first patterns, then maintains, the OE. Here, we have reviewed how advanced gene expression analyses and antigen detection methods, coupled with the use of transgenic reporter mice, has revealed the existence of previously unappreciated distinct precursors that contribute to regionally restricted ORN genesis (Nestin Tg + ORN precursors), adult OE repopulation (Cytokeratin5 Cre- expressing HBCs) and OECs (BLBP + embryonic glial restricted precursors). In addition, we reveal the presence of a Nestin-expressing RGLP and the existence of in vitro colony-forming OE cells that are unique to the embryonic OE, whose full potential has yet to be tested (summarized in Fig. 6). Clearly, the embryonic and adult OE have distinct differences in their progenitor repertoire, and clues to the identity and regulation of adult OE-residing progenitors will certainly be found in understanding more about their hierarchical embryonic parents. With the continued identification of a greater repertoire of candidate stem cell genes in cells of the OE, the definitive identification of more multipotent embryonic and adult neural stem cells using a combination of transgenic, lesion and in vitro approaches, will allow us to unlock the mechanisms used by the olfactory epithelium to adapt to a changing environment, maintain plasticity and chemosensory function.
Proposed new classes of progenitor in the embryonic and adult OE. (A) Embryonic OE contains an OE-spanning, Nestin-expressing, Prominin 1-expressing Radial Glia-Like Progenitor (RGLP), a CNS Nestin transgene-expressing (Nes Tg+) zonally restricted Neural Restricted Precursor (NRP) for ORNs, and a BLBP-expressing, (Notch1-expressing) Glial Restricted Precursor (GRP), that are rarely found in adult OE. (B) Postnatal OE, in turn, is largely devoid of RGLPs, and its most multipotent cell is currently found in a subpopulation of HBCs. Mitotic, BLBP-negative (BLBP-Cre-reporter-positive) GRP/immature OECs are found outside ORN axon bundles within the LP. INP-immediate neuronal precursor; ORN/IRN-olfactory/immature receptor neuron; OEC-olfactory ensheathing cell
References
Altman J (1969) Postnatal neurogenesis and the problem of neural plasticity. In: Himwich W (ed) Developmental neurobiology. Charles C. Thomas, Springfield, III., pp 197–257
Anthony TE, Klein C, Fishell G, Heintz N (2004) Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41:881–890
Anthony TE, Mason HA, Gridley T, Fishell G, Heintz N (2005) Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes Dev 19:1028–1033
Asson-Batres MA, Smith WB (2006) Localization of retinaldehyde dehydrogenases and retinoid binding proteins to sustentacular cells, glia, Bowman's gland cells, and stroma: potential sites of retinoic acid synthesis in the postnatal rat olfactory organ. J Comp Neurol 496:149–171
Attisano L, Wrana JL (2002) Signal transduction by the TGF-beta superfamily. Science 296:1646–1647
Au E, Roskams AJ (2003) Olfactory ensheathing cells of the lamina propria in vivo and in vitro. Glia 41:224–236
Balmer CW, LaMantia AS, Bhasin N, Rhodes K, Heemskerk J (2005) Noses and neurons: induction, morphogenesis, and neuronal differentiation in the peripheral olfactory pathway Mesenchymal/epithelial induction mediates olfactory pathway formation. Dev Dyn 234:464–481
Bauer S, Rasika S, Han J, Mauduit C, Raccurt M, Morel G, Jourdan F, Benahmed M, Moyse E, Patterson PH (2003) Leukemia inhibitory factor is a key signal for injury-induced neurogenesis in the adult mouse olfactory epithelium. J Neurosci 23:1792–1803
Beites CL, Kawauchi S, Crocker CE, Calof AL (2005) Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res 306:309–316
Bergman U, Ostergren A, Gustafson AL, Brittebo B (2002) Differential effects of olfactory toxicants on olfactory regeneration. Arch Toxicol 76:104–112
Bergstrom U, Giovanetti A, Piras E, Brittebo EB (2003) Methimazole-induced damage in the olfactory mucosa: effects on ultrastructure and glutathione levels. Toxicol Pathol 31:379–387
Booth C, Potten CS (2000) Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest 105:1493–1499
Brazel CY, Rao MS (2004) Aging and neuronal replacement. Ageing Res Rev 3:465–483
Brittebo EB (1995) Metabolism-dependent toxicity of methimazole in the olfactory nasal mucosa. Pharmacol Toxicol 76:76–79
Caggiano M, Kauer JS, Hunter DD (1994) Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron 13:339–352
Calof AL, Chikaraishi DM (1989) Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron 3:115–127
Calof AL, Mumm JS, Rim PC, Shou J (1998a) The neuronal stem cell of the olfactory epithelium. J Neurobiol 36:190–205
Calof AL, Rim PC, Askins KJ, Mumm JS, Gordon MK, Iannuzzelli P, Shou J (1998b) Factors regulating neurogenesis and programmed cell death in mouse olfactory epithelium. Ann N Y Acad Sci 855:226–229
Camara CG, Harding JW (1984) Thymidine incorporation in the olfactory epithelium of mice: early exponential response induced by olfactory neurectomy. Brain Res 308:63–68
Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, Ffrench-Constant C (2004) Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development 131:3433–3444
Carr VM, Farbman AI (1992) Ablation of the olfactory bulb up-regulates the rate of neurogenesis and induces precocious cell death in olfactory epithelium. Exp Neurol 115:55–59
Carson C, Murdoch B, Roskams AJ (2006) Notch 2 and Notch 1/3 segregate to neuronal and glial lineages of the developing olfactory epithelium. Dev Dyn 235:1678–1688
Carter LA, MacDonald JL, Roskams AJ (2004) Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci 24:5670–5683
Cau E, Casarosa S, Guillemot F (2002) Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development 129:1871–1880
Cau E, Gradwohl G, Fode C, Guillemot F (1997) Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development 124:1611–1621
Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F, Fode C (2000) Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development 127:2323–2332
Cayouette M, Raff M (2003) The orientation of cell division influences cell-fate choice in the developing mammalian retina. Development 130:2329–2339
Cayouette M, Barres BA, Raff M (2003) Importance of intrinsic mechanisms in cell fate decisions in the developing rat retina. Neuron 40:897–904
Chen X, Fang H, Schwob JE (2004) Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol 469:457–474
Chuah MI, Teague R (1999) Basic fibroblast growth factor in the primary olfactory pathway: mitogenic effect on ensheathing cells. Neuroscience 88:1043–1050
Corbin JG, Nery S, Fishell G (2001) Telencephalic cells take a tangent: non-radial migration in the mammalian forebrain. Nat Neurosci 4(Suppl):1177–1182
Corti S, Nizzardo M, Nardini M, Donadoni C et al (2007) Isolation and characterization of murine neural stem/progenitor cells based on Prominin-1 expression. Exp Neurol 205:547–562
Costanzo RM, Graziadei PP (1983) A quantitative analysis of changes in the olfactory epithelium following bulbectomy in hamster. J Comp Neurol 215:370–381
Cowan CM, Roskams AJ (2004) Caspase-3 and caspase-9 mediate developmental apoptosis in the mouse olfactory system. J Comp Neurol 474:136–148
Cowan CM, Thai J, Krajewski S, Reed JC, Nicholson DW, Kaufmann SH, Roskams AJ (2001) Caspases 3 and 9 send a pro-apoptotic signal from synapse to cell body in olfactory receptor neurons. J Neurosci 21:7099–7109
Cummings DM, Brunjes PC (1997) The effects of variable periods of functional deprivation on olfactory bulb development in rats. Exp Neurol 148:360–366
Cuschieri A, Bannister LH (1975) The development of the olfactory mucosa in the mouse: light microscopy. J Anat 119:277–286
Davis JA, Reed RR (1996) Role of Olf-1 and Pax-6 transcription factors in neurodevelopment. J Neurosci 16:5082–5094
DeHamer MK, Guevara JL, Hannon K, Olwin BB, Calof AL (1994) Genesis of olfactory receptor neurons in vitro: regulation of progenitor cell divisions by fibroblast growth factors. Neuron 13:1083–1097
De_Carlos JA, Lopez_Mascaraque L, Valverde F (1995) The telencephalic vesicles are innervated by olfactory placode-derived cells: a possible mechanism to induce neocortical development. Neuroscience 68:1167–1178
Doyle KL, Khan M, Cunningham AM (2001) Expression of the intermediate filament protein nestin by sustentacular cells in mature olfactory neuroepithelium. J Comp Neurol 437:186–195
Ezeh PI, Farbman AI (1998) Differential activation of ErbB receptors in the rat olfactory mucosa by transforming growth factor-alpha and epidermal growth factor in vivo. J Neurobiol 37:199–210
Farbman AI (1990) Olfactory neurogenesis: genetic or environmental controls? Trends Neurosci 13:362–365
Farbman AI (1992) Cell Biology of Olfaction. Cambridge University Press, Cambridge
Farbman AI, Margolis FL (1980) Olfactory marker protein during ontogeny: immunohistochemical localization. Dev Biol 74:205–215
Farbman AI, Buchholz JA (1996) Transforming growth factor-alpha and other growth factors stimulate cell division in olfactory epithelium in vitro. J Neurobiol 30:267–280
Farbman AI, Brunjes PC, Rentfro L, Michas J, Ritz S (1988) The effect of unilateral naris occlusion on cell dynamics in the developing rat olfactory epithelium. J Neurosci 8:3290–3295
Feng L, Hatten ME, Heintz N (1994) Brain lipid-binding protein (BLBP): a novel signaling system in the developing mammalian CNS. Neuron 12:895–908
Fishell G, Kriegstein AR (2003) Neurons from radial glia: the consequences of asymmetric inheritance. Curr Opin Neurobiol 13:34–41
Fishell G, Kriegstein A (2005) Cortical development: new concepts. Neuron 46:361–362
Furuta A, Rothstein JD, Martin LJ (1997) Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci 17:8363–8375
Getchell TV, Narla RK, Little S, Hyde JF, Getchell ML (2000) Horizontal basal cell proliferation in the olfactory epithelium of transforming growth factor-alpha transgenic mice. Cell Tissue Res 299:185–192
Getchell TV, Shah DS, Partin JV, Subhedar NK, Getchell ML (2002) Leukemia inhibitory factor mRNA expression is upregulated in macrophages and olfactory receptor neurons after target ablation. J Neurosci Res 67:246–254
Gleeson JG, Lin PT, Flanagan LA, Walsh CA (1999) Doublecortin is a microtubule associated protein and is expressed widely by migrating neurons. Neuron 23:257–271
Goldstein BJ, Schwob JE (1996) Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J Neurosci 16:4005–4016
Goldstein BJ, Wolozin BL, Schwob JE (1997) FGF2 suppresses neuronogenesis of a cell line derived from rat olfactory epithelium. J Neurobiol 33:411–428
Goldstein BJ, Fang H, Youngentob SL, Schwob JE (1998) Transplantation of multipotent progenitors from the adult olfactory epithelium. Neuroreport 9:1611–1617
Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, Heintz N (2003) A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature 425:917–925
Gordon MK, Mumm JS, Davis RA, Holcomb JD, Calof AL (1995) Dynamics of MASH1 expression in vitro and in vivo suggest a non-stem cell site of MASH1 action in the olfactory receptor neuron lineage. Mol Cell Neurosci 6:363–379
Gotz M, Huttner WB, Haubensak W, Attardo A, Denk W (2005) The cell biology of neurogenesis Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Nat Rev Mol Cell Biol 6:777–788
Graziadei PP, Graziadei GA (1979) Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol 8:1–18
Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL (1993) Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75:463–476
Halpern M, Martinez-Marcos A (2003) Structure and function of the vomeronasal system: an update. Prog Neurobiol 70:245–318
Harding JW, Getchell TV, Margolis FL (1978) Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res 140:271–285
Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R (1996) Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature 380:171–175
Hockfield S, McKay RD (1985) Identification of major cell classes in the developing mammalian nervous system. J Neurosci 5:3310–3328
Holbrook EH, Szumowski KE, Schwob JE (1995) An immunochemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol 363:129–146
Holcomb JD, Mumm JS, Calof AL (1995) Apoptosis in the neuronal lineage of the mouse olfactory epithelium: regulation in vivo and in vitro. Dev Biol 172:307–323
Hsu P, Yu F, Feron F, Pickles JO, Sneesby K, Mackay-Sim A (2001) Basic fibroblast growth factor and fibroblast growth factor receptors in adult olfactory epithelium. Brain Res 896:188–197
Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE (1998) Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol 400:469–486
Hurley RW, McCarthy JB, Verfaillie CM (1995) Direct adhesion to bone marrow stroma via fibronectin receptors inhibits hematopoietic progenitor proliferation. J Clin Invest 96:511–519
Hurley RW, McCarthy JB, Wayner EA, Verfaillie CM (1997) Monoclonal antibody crosslinking of the alpha 4 or beta 1 integrin inhibits committed clonogenic hematopoietic progenitor proliferation. Exp Hematol 25:321–328
Illing N, Boolay S, Siwoski JS, Casper D, Lucero MT, Roskams AJ (2002) Conditionally immortalized clonal cell lines from the mouse olfactory placode differentiate into olfactory receptor neurons. Mol Cell Neurosci 20:225–243
Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR (2002) A stem cell molecular signature. Science 298:601–604
Iwema CL, Fang H, Kurtz DB, Youngentob SL, Schwob JE (2004) Odorant receptor expression patterns are restored in lesion-recovered rat olfactory epithelium. J Neurosci 24:356–369
Jang W, Youngentob SL, Schwob JE (2003) Globose basal cells are required for reconstitution of olfactory epithelium after methyl bromide lesion. J Comp Neurol 460:123–140
Joyner AL, Zervas M (2006) Genetic inducible fate mapping in mouse: establishing genetic lineages and defining genetic neuroanatomy in the nervous system. Dev Dyn 235:2376–2385
Kawauchi S, Shou J, Santos R, Hebert JM, McConnell SK, Mason I, Calof AL (2005) Fgf8 expression defines a morphogenetic center required for olfactory neurogenesis and nasal cavity development in the mouse. Development 132:5211–5223. Epub 2005 Nov 5212
Kim J, Wu HH, Lander AD, Lyons KM, Matzuk MM, Calof AL (2005) GDF11 controls the timing of progenitor cell competence in developing retina. Science 308:1927–1930
Krishna NS, Little SS, Getchell TV (1996) Epidermal growth factor receptor mRNA and protein are expressed in progenitor cells of the olfactory epithelium. J Comp Neurol 373:297–307
LaMantia AS, Bhasin N, Rhodes K, Heemskerk J (2000) Mesenchymal/epithelial induction mediates olfactory pathway formation. Neuron 28:411–425
Lemischka I (2001) Stem cell dogmas in the genomics era. Rev Clin Exp Hematol 5:15–25
Leung CT, Coulombe PA, Reed RR (2007) Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci 10(6):720–726
Levesque JP, Simmons PJ (1999) Cytoskeleton and integrin-mediated adhesion signaling in human CD34 + hemopoietic progenitor cells. Exp Hematol 27:579–586
Lledo PM, Carleton A, Vincent JD (2002) [Odors and olfaction]. J Soc Biol 196:59–65
Luskin MB (1993) Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron 11:173–189
Mackay-Sim A, Kittel P (1991) Cell dynamics in the adult mouse olfactory epithelium: a quantitative autoradiographic study. J Neurosci 11:979–984
Mahanthappa NK, Schwarting GA (1993) Peptide growth factor control of olfactory neurogenesis and neuron survival in vitro: roles of EGF and TGF-beta s. Neuron 10:293–305
Manglapus GL, Youngentob SL, Schwob JE (2004) Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol 479:216–233
Marzesco AM, Janich P, Wilsch-Brauninger M, Dubreuil V, Langenfeld K, Corbeil D, Huttner WB (2005) Release of extracellular membrane particles carrying the stem cell marker prominin-1 (CD133) from neural progenitors and other epithelial cells. J Cell Sci 118:2849–2858
McKay R (2000) Stem cells and the cellular organization of the brain. J Neurosci Res 59:298–300
Mehler MF, Mabie PC, Zhu G, Gokhan S, Kessler JA (2000) Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci 22:74–85
Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G (2004) Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol 469:311–324
Morrison SJ (2001) Neuronal potential and lineage determination by neural stem cells. Curr Opin Cell Biol 13:666–672
Morshead CM, Benveniste P, Iscove NN, van der Kooy D (2002) Hematopoietic competence is a rare property of neural stem cells that may depend on genetic and epigenetic alterations. Nat Med 8:268–273
Moulton DG (1974) Dynamics of cell populations in the olfactory epithelium. Ann N Y Acad Sci 237:52–61
Mumm JS, Shou J, Calof AL (1996) Colony-forming progenitors from mouse olfactory epithelium: evidence for feedback regulation of neuron production. Proc Natl Acad Sci USA 93:11167–11172
Murdoch B, Roskams A (2007) A Novel Embryonic Nestin-Expressing Radial Glia-Like Progenitor Gives Rise To Spatially Restricted Olfactory And Vomeronasal Neurons. J Neurosci under review
Murray RC, Navi D, Fesenko J, Lander AD, Calof AL (2003) Widespread defects in the primary olfactory pathway caused by loss of Mash1 function. J Neurosci 23:1769–1780
Murrell W, Feron F, Wetzig A, Cameron N, Splatt K, Bellette B, Bianco J, Perry C, Lee G, Mackay-Sim A (2005) Multipotent stem cells from adult olfactory mucosa. Dev Dyn 233:496–515
Nan B, Getchell ML, Partin JV, Getchell TV (2001) Leukemia inhibitory factor, interleukin-6, and their receptors are expressed transiently in the olfactory mucosa after target ablation. Journal of Comparative Neurology 435:60–77
Newman MP, Feron F, Mackay-Sim A (2000) Growth factor regulation of neurogenesis in adult olfactory epithelium. Neuroscience 99:343–350
Novak A, Guo C, Yang W, Nagy A, Lobe CG (2000) Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28:147–155
Othman M, Lu C, Klueber K, Winstead W, Roisen F (2005) Clonal analysis of adult human olfactory neurosphere forming cells. Biotech Histochem 80:189–200
Othman MM, Klueber KM, Roisen FJ (2003) Identification and culture of olfactory neural progenitors from GFP mice. Biotech Histochem 78:57–70
Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS (1995) The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci USA 92:9647–9651
Peretto P, Cummings D, Modena C, Behrens M, Venkatraman G, Fasolo A, Margolis FL (2002) BMP mRNA and protein expression in the developing mouse olfactory system. J Comp Neurol 451:267–278
Piras E, Franzen A, Fernandez EL, Bergstrom U, Raffalli-Mathieu F, Lang M, Brittebo EB (2003) Cell-specific expression of CYP2A5 in the mouse respiratory tract: effects of olfactory toxicants. J Histochem Cytochem 51:1545–1555
Pixley SK (1996) Characterization of olfactory receptor neurons and other cell types in dissociated rat olfactory cell cultures. Int J Dev Neurosci 14:823–839
Potocnik AJ, Brakebusch C, Fassler R (2000) Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 12:653–663
Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA (2002) “Stemness”: transcriptional profiling of embryonic and adult stem cells. Science 298:597–600
Ramer LM, Au E, Richter MW, Liu J, Tetzlaff W, Roskams AJ (2004) Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol 473:1–15
Ramon-Cueto A, Nieto-Sampedro M (1992) Glial cells from adult rat olfactory bulb: immunocytochemical properties of pure cultures of ensheathing cells. Neuroscience 47:213–220
Rao M (2004) Stem and precursor cells in the nervous system. J Neurotrauma 21:415–427
Regad T, Roth M, Bredenkamp N, Illing N, Papalopulu N (2007) The neural progenitor-specifying activity of FoxG1 is antagonistically regulated by CKI and FGF. Nat Cell Biol 9:531–540
Reynolds BA, Weiss S (1992) Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science 255:1707–1710
Reynolds BA, Weiss S (1996) Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol 175:1–13
Richter M, Roskams A (2007) The cell biology of olfactory ensheathing glia. Glia submitted
Roisen FJ, Klueber KM, Lu CL, Hatcher LM, Dozier A, Shields CB, Maguire S (2001) Adult human olfactory stem cells. Brain Res 890:11–22
Roskams AJ, Bredt DS, Dawson TM, Ronnett GV (1994) Nitric oxide mediates the formation of synaptic connections in developing and regenerating olfactory receptor neurons. Neuron 13:289–299
Roskams AJ, Bethel MA, Hurt KJ, Ronnett GV (1996) Sequential expression of Trks A, B, and C in the regenerating olfactory neuroepithelium. J Neurosci 16:1294–1307
Roskams AJ, Cai X, Ronnett GV (1998) Expression of neuron-specific beta-III tubulin during olfactory neurogenesis in the embryonic and adult rat. Neuroscience 83:191–200
Satoh M, Yoshida T (1997) Promotion of neurogenesis in mouse olfactory neuronal progenitor cells by leukemia inhibitory factor in vitro. Neurosci Lett 225:165–168
Satoh M, Yoshida T (2000) Expression of neural properties in olfactory cytokeratin-positive basal cell line. Brain Res Dev Brain Res 121:219–222
Schlessinger J (2000) Cell signaling by receptor tyrosine kinases. Cell 103:211–225
Schwanzel-Fukuda M (1999) Origin and migration of luteinizing hormone-releasing hormone neurons in mammals. Microsc Res Tech 44:2–10
Schwob JE (2002) Neural regeneration and the peripheral olfactory system. Anat Rec 269:33–49
Schwob JE (2005) Restoring olfaction: a view from the olfactory epithelium. Chem Senses 30 (Suppl 1):i131–i132
Schwob JE, Szumowski KE, Stasky AA (1992) Olfactory sensory neurons are trophically dependent on the olfactory bulb for their prolonged survival. J Neurosci 12:3896–3919
Schwob JE, Youngentob SL, Meiri KF (1994) On the formation of neuromata in the primary olfactory projection. J Comp Neurol 340:361–380
Schwob JE, Youngentob SL, Mezza RC (1995) Reconstitution of the rat olfactory epithelium after methyl bromide- induced lesion. J Comp Neurol 359:15–37
Shetty RS, Bose SC, Nickell MD, McIntyre JC, Hardin DH, Harris AM, McClintock TS (2005) Transcriptional changes during neuronal death and replacement in the olfactory epithelium. Mol Cell Neurosci 30:90–107
Shihabuddin LS, Palmer TD, Gage FH (1999) The search for neural progenitor cells: prospects for the therapy of neurodegenerative disease. Mol Med Today 5:474–480
Shingo T, Sorokan ST, Shimazaki T, Weiss S (2001) Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci 21:9733–9743
Shou J, Rim PC, Calof AL (1999) BMPs inhibit neurogenesis by a mechanism involving degradation of a transcription factor [see comments]. Nat Neurosci 2:339–345
Shou J, Murray RC, Rim PC, Calof AL (2000) Opposing effects of bone morphogenetic proteins on neuron production and survival in the olfactory receptor neuron lineage. Development 127:5403–5413
Smart IH (1971) Location and orientation of mitotic figures in the developing mouse olfactory epithelium. J Anat 109:243–251
Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1:4
Suzuki A, Zheng Y, Kondo R, Kusakabe M, Takada Y, Fukao K, Nakauchi H, Taniguchi H (2000) Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology 32:1230–1239
Tietjen I, Rihel JM, Cao Y, Koentges G, Zakhary L, Dulac C (2003) Single-cell transcriptional analysis of neuronal progenitors. Neuron 38:161–175
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, Bock R, Klein R, Schutz G (1999) Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet 23:99–103
van der Kooy D, Weiss S (2000) Why stem cells? Science 287:1439–1441
Vanderluit JL, Ferguson KL, Nikoletopoulou V, Parker M, Ruzhynsky V, Alexson T, McNamara SM, Park DS, Rudnicki M, Slack RS (2004) p107 regulates neural precursor cells in the mammalian brain. J Cell Biol 166(6):853–863.Epub 2004 Sep 2007
Voura EB, Billia F, Iscove NN, Hawley RG (1997) Expression mapping of adhesion receptor genes during differentiation of individual hematopoietic precursors. Exp Hematol 25:1172–1179
Watt FM (2001) Stem cell fate and patterning in mammalian epidermis. Curr Opin Genet Dev 11:410–417
Weiler E, Farbman AI (1997) Proliferation in the rat olfactory epithelium: age-dependent changes. J Neurosci 17:3610–3622
Weiss S (1999) Pathways for neural stem cell biology and repair. Nat Biotechnol 17:850–851
Weissman IL, Anderson DJ, Gage F (2001) Stem and progenitor cells: origins, phenotypes, lineage commitments, and transdifferentiations. Annu Rev Cell Dev Biol 17:387–403
Wewetzer K, Brandes G (2006) Axonal signaling and the making of olfactory ensheathing cells: a hypothesis. Neuron Glia Biol 2:217–224
Whitlock KE (2004) A new model for olfactory placode development. Brain Behav Evol 64:126–140
Wray S, Grant P, Gainer H (1989) Evidence that cells expressing luteinizing hormone-releasing hormone mRNA in the mouse are derived from progenitor cells in the olfactory placode. Proc Natl Acad Sci USA 86:8132–8136
Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL (2003) Autoregulation of neurogenesis by GDF11. Neuron 37:197–207
Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM (1991) Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64:841–848
Yoshida Y, Han B, Mendelsohn M, Jessell TM (2006) PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron 52:775–788
Yu TT, McIntyre JC, Bose SC, Hardin D, Owen MC, McClintock TS (2005) Differentially expressed transcripts from phenotypically identified olfactory sensory neurons. J Comp Neurol 483:251–262
Zhang X, Klueber KM, Guo Z, Lu C, Roisen FJ (2004) Adult human olfactory neural progenitors cultured in defined medium. Exp Neurol 186:112–123
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murdoch, B., Roskams, A.J. Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology. J Mol Hist 38, 581–599 (2007). https://doi.org/10.1007/s10735-007-9141-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-007-9141-2