Abstract
Dengue fever is a vector-borne disease prevalent in tropical and subtropical regions. It is an important public health problem with a considerable and often under-valued disease burden in terms of frequency, cost and quality-of-life. Recent literature reviews have documented the development of mathematical models of dengue fever both to identify important characteristics for future model development as well as to assess the impact of dengue control interventions. Such reviews highlight the importance of short-term cross-protection; antibody-dependent enhancement; and seasonality (in terms of both favourable and unfavourable conditions for mosquitoes). The compartmental model extends work by Bartley (2002) and combines the following factors: seasonality, age-structure, consecutive infection by all four serotypes, cross-protection and immune enhancement, as well as combined vector-host transmission. The model is used to represent dengue transmission dynamics using parameters appropriate for Thailand and to assess the potential impact of combined vector-control and vaccination strategies including routine and catch-up vaccination strategies on disease dynamics. When seasonality and temporary cross-protection between serotypes are included, the model is able to approximate the observed incidence of dengue fever in Thailand. We find vaccination to be the most effective single intervention, albeit with imperfect efficacy (30.2 %) and limited duration of protection. However, in combination, control interventions and vaccination exhibit a marked impact on dengue fever transmission. This study shows that an imperfect vaccine can be a useful weapon in reducing disease spread within the community, although it will be most effective when promoted as one of several strategies for combating dengue fever transmission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dengue fever is associated with severe urban epidemics and has become a major public health problem, with considerable economic, political, and social impacts. The WHO currently ranks dengue fever as the most important mosquito-borne viral disease in the world [1]. Dengue fever occurs in more than 100 countries in the tropical and subtropical regions of Asia-Pacific, the Americas, the Middle East, and Africa with an estimated 3 billion people at-risk [1]. Persons living in areas where dengue fever is endemic can often be infected with three and quite often four dengue serotypes in their lifetime [2]. The reason for this is that whilst the circulation of multiple serotypes was geographically relatively restricted in 1970 for example, it is now apparent that most regions (e.g. Central and South America, central Africa etc.) are prone to the circulation of multiple dengue serotypes [3].
Dengue is a mosquito-borne disease, caused by serologically related but antigenically distinct viruses grouped into four serotypes (DENV-1 to DENV-4). Recovery from infection confers permanent immunity to that serotype, but only short-term cross-immunity to other serotypes [4–6]. All serotypes can cause severe and fatal disease with clinical cases being classified into two groups: dengue fever (DF) and dengue hemorrhagic fever (DHF). Symptoms produced by dengue infection last approximately 3 to 10 days, with an average duration of 6 days following the onset of symptoms [7]. The illness persists for several days after the viraemic period (i.e. virus circulating in the blood) has ended [7]. The symptoms of dengue hemorrhagic fever are more severe than dengue fever symptoms and can lead to death. Dengue hemorrhagic fever may in turn subsequently develop into an acute form of the disease known as dengue shock syndrome (DSS). Risk factors for the incidence of the more serious forms of the disease (Dengue hemorrhagic fever or Dengue shock syndrome) tend to be associated with people who have had past infections with one or more dengue serotypes [8–10]. The theory behind this relates to immune or antibody-dependent enhancement (ADE), i.e. an immune response to one serotype which enhances (rather than negates) future infections and can increase the likelihood of severe disease [8–10].
Dengue embraces a wide clinical spectrum from asymptomatic infections to severe manifestations resulting in large numbers of both unreported and asymptomatic infections. It is estimated that approximately 50–100 million individuals are infected every year [7] with 500,000 cases of dengue hemorrhagic fever and 22,000 deaths [11]. Recent work suggests that the number of ‘true infections’ is considerably greater than the dengue burden estimate of WHO by at least a factor of three [12]. Using advanced mapping techniques, the authors estimate that there are approximately 390 million dengue infections per year with a credible interval of 284–528 million. Furthermore, it is estimated that approximately 96 million of these infections (credible interval 67–136 million) are evident (i.e. any level of disease severity) [12]. The reasons for the growth in dengue fever and dengue hemorrhagic fever as a leading public health challenge tend to be multi-factorial. This includes relatively ineffectual mosquito control, rapid population growth and increase in overseas air travel, an increase in non-biodegradable packaging as well as deteriorations in public health infrastructure [3, 13].
The incidence of dengue fever is shown to exhibit a clear dependence on seasonal variation [14–17]. As can generally be observed, the number of cases is correlated with seasonal patterns with the peak of cases in June and July when environmental conditions are more conducive to mosquito development, i.e. humidity and precipitation are much higher compared with periods of low temperature [14–16].
The most common vector responsible for epidemic dengue is the infected female of the Aedes aegypti mosquito [18]. These predominantly daytime-biting insects live in the vicinity of human habitats and usually lay eggs and produce larvae in artificial containers. In the absence of a vaccine with proven efficacy against all four serotypes or of any drugs for its treatment [19, 20], the control of dengue is currently limited to decreasing Aedes aegypti population densities or preventing their contact with human hosts [21]. Major vector control strategies include environmental management and source reduction (i.e. locating and removing mosquito breeding sites, improved sanitation etc.), use of larvicides (i.e. targeting the larvae forms of mosquitoes by spreading chemical larvicide in breeding sites) and insecticide spray targeting adult mosquitoes (adulticide) [20]. Additional prevention methods include the biological control of vectors and the use of repellents that reduce the contact between infected humans and susceptible mosquitoes in the form of sprays for personal protection, impregnated clothing and curtains, screens on windows and mosquito nets [20].
Mathematical models of dengue fever have been developed to gain insights into disease transmission [5, 22–29], predict outbreaks as well as simulate the impact of interventions for disease control [17, 30–41]. Historically, studies tend to be divided into those that consider mechanical and chemical interventions on the one hand [30, 31, 33–37, 39, 41], and those that consider vaccination on the other [32, 38, 40]. On the whole, few have begun to consider the combined effects of a range of different interventions including vaccination [42]. A summary of the approaches used in dengue fever modelling from 1964 to 2006 is presented by Nishiura (2006) [43]. The majority of studies cited use differential equation compartmental models in their analyses with a small number of studies reporting some form of statistical model. A critical appraisal of dengue fever models was also conducted by Johansson (2011) [44], who noted the importance of short-term cross-protection as well as the fact that force of infection may be significantly underestimated given the absence of cross-protection in many dengue fever models [44]. Analogously, Andraud (2012) [45] carried out a review seeking to identify important characteristics for future model development. The authors advocated the use of combined vector-host transmission models as being the most relevant for health policy in terms of providing projections of combined vaccination and vector control interventions.
The broad aim of this study is to assess the effectiveness of historical forms of vector control in relation to other forms of disease management including a partially effective vaccine. A dynamic compartmental transmission model is developed simulating the impact of different control strategies, in order to reflect the consequences of these interventions on the epidemiology of dengue in Thailand and determine the optimal combination of approaches to disease control based on the subsequent reduction in incidence. The main contributions of this paper to the dengue modelling literature are the inclusion of the impact of combined vector-control and vaccination strategies on the transmission of dengue fever, age-structure of the model population, seasonality, consecutive infection with all four serotypes as well as considerations of cross-protection and immune-enhancement. In the next section, the model is described, followed by a presentation of results, a brief discussion and ending with conclusions and next steps.
2 Methods
2.1 Mathematical model
The model extends work by Bartley (2002) [24] and includes the following elements: consecutive infections with all four serotypes, age-structure of the population, seasonality, cross-protection and immune enhancement and the impact of combined vector-control and vaccination strategies on the transmission of dengue fever.
Bartley (2002) [24] developed a multi-serotype deterministic compartmental model (SEIR: SEI) incorporating vector-host transmission, seasonality and secondary infection. The influence of seasonality worked through vector parameters including recruitment, mortality, biting rates and duration of extrinsic incubation period (EIP) which were estimated from entomological studies in Bangkok. Antibody-dependent enhancement was explored in the model by the inclusion of a scaling factor in which ADE may lead to increased infectiousness of the individual (by a factor of φhe) infected for a second time. Sensitivity analyses indicated that duration of infectiousness in the host, vector latent period as well as biting and vector mortality rates were key model parameters. Although the model did not calibrate well with observed data (based on goodness-of-fit tests), the authors concluded that strong correlations provided enough evidence for the necessary inclusion of the main determinants of seasonality. Subsequent work carried out by Wearing and Rohani (2006) [5] and building on the work of Bartley (2002) [24], reinforced the importance of seasonality as well as temporary cross-immunity to explain intra-annual and inter-epidemic dynamics observed in dengue endemic areas.
The epidemiological literature and previous modelling studies are used to inform parameter values in the model comparing the effects of different interventions. In this regard, we draw heavily on inputs from published models of dengue fever developed by Bartley (2002) and Burrattini (2008) [24, 30] where the model calibrates well with actual data from Singapore. It is assumed that Singapore is not qualitatively different from Thailand in terms of the manifestation of dengue fever. The sensitivity of the results to changes in parameter values and assumptions are subsequently examined in scenario and sensitivity analyses.
2.2 Dengue surveillance data
Data from National Epidemiological Surveillance in Thailand [46] indicate that there were approximately 90,000 reported cases of dengue fever/dengue hemorrhagic fever in Thailand in 2008 including 51,355, 1626 and 36,645 dengue hemorrhagic fever, dengue shock syndrome and dengue fever infections respectively. There were also 102 deaths reported in 2008 with the great majority (70 %) due to dengue shock syndrome with the remainder attributable to dengue hemorrhagic fever. The highest number of cases were in the 10–14 years age group (n = 24,480) closely followed by the 15–24 years age group (n = 23,966).
2.3 Magnitude of potential under-reporting of dengue fever infections
Wichmann (2011) [47] states that total and inpatient dengue cases in Thailand may have been under-reported by as much as 8.7 and 2.6 times respectively in the period 2003–2007. Moreover, they estimate that greater than 340,000 (median) symptomatic dengue infections occurred annually in these years in children less than 15 years of age, the extent of which is not assessed or reflected in national surveillance figures. Their assessment was based on the numbers of nationally reported inpatient dengue cases as well as average multiplication factors which were generated by comparing Thai provincial reporting data with data from prospective cohort studies in the same province [47].
Any potential under-identification of dengue infections is further corroborated by Undurraga (2013) [48]. The authors estimate average annual dengue fever episodes and under-reporting rates for 12 countries in Southeast Asia (2001–2010) stratified by hospital and ambulatory treatment. Their results suggest average reporting rates of 13.2 % of total symptomatic dengue episodes in the region, implying an expansion factor of 7.6 for converting reported cases into estimated actual cases.
The issue of under-reporting of dengue cases, akin to missing data, has implications for the development of mathematical models seeking to estimate the burden of disease. Our model seeks to calculate the ‘true’ epidemiological burden of dengue fever in Thailand by incorporating an adjustment for estimated under-reporting. Model estimates are therefore calibrated with figures reported by National Epidemiological Surveillance in Thailand in 2008 [46] multiplied by an expansion factor of 7.6 [48]. We use the more recent and lower estimate of Undurraga (2013) [48] to adjust for under-reporting to be conservative in our calculations although both the estimates of Wichamann (2011) and Undurraga (2013) [47, 48] are relatively comparable and consistent with each other.
2.4 Dynamic transmission model
We develop a compartmental transmission model based on SEIR-type of models (Susceptible, Exposed, Infected, and Removed). The epidemiological dynamic transmission model represents the host population as residing in compartments (e.g., susceptibility or disease states) and moving between compartments over time. The movement of the population between compartments is stated mathematically and the system is described by a set of differential equations that represent the flow in and out of each compartment with respect to time. Solving the differential equations allows prediction of the distribution of the population across compartments at given time points, changes over time (e.g., incidence of disease), as well as identification of the equilibrium state. Each flow rate between compartments is dependent upon the value of the input parameters of the transition equations. Parameter values along with data sources are listed in Appendix. Rates are estimated as the inverse of the average time spent in the compartment. Initial conditions were derived by running the model to equilibrium steady-state without any control interventions. The transmission model is used to estimate epidemiological outcomes including the incidence of dengue fever.
Model compartments comprise those for both human and vector populations. The human population (Nh) is divided into susceptible to dengue infection, Sh; exposed but not yet infectious (i.e. incubating the virus), Eh; infected and infectious humans, Ih; temporary cross-protection, Cp; temporary cross-enhancement (CE) and immune (R) compartments. Temporary cross-protection to recurrent infections (CP) lasts for approximately 6 months in the base case whilst cross-enhancement (CE) (i.e. enhancement of viral infectiousness caused by antibodies that do not neutralise [8–10]) lasts for approximately 3 months. The final recovery state R imparts permanent immunity to that serotype, but only temporary immunity to other serotypes.
The model assumes that the four dengue serotypes have comparable infectiousness and prevalence as a simple proxy for complex dengue virus circulation dynamics. This is consistent with other modelling studies in this field [5, 22–24, 31, 37]. For example, hosts can experience a primary infection with one serotype followed by the possibility of subsequent infections with other serotypes. Accordingly, exposed, infectious and immune states are further stratified by the number of infections suffered (i.e. primary, secondary, tertiary etc.) in the form Eh, Eh2, Eh3 and Eh4.
In contrast, it is assumed that mosquitoes will be infected by one serotype only and that they will remain infectious until death. The life cycle of the mosquito is represented in the model by two developmental phases. The aquatic phase comprising egg, larva and pupa stages is denoted by Av. The adult stage is divided into three compartments: number of susceptible mosquitoes, Sv; number of exposed but not yet infectious mosquitoes (i.e. incubating the virus), Ev and infected and infectious mosquitoes, Iv. The total mosquito population is Nv (i.e. Nv = Sv + Ev + Iv).
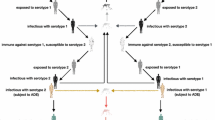
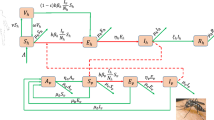
The force of infection λ [1…4] is equal to bβh (Iv/Nh) where b is the average number of bites per mosquito per day, βh is the age-specific transmission probability, and Iv as well as Nh are defined as above (i.e. number of infected and infectious mosquitoes and the total human population respectively). The probability of acquiring the dengue virus is likewise differentiated by infection which is fixed at 1.0, 0.75, 0.50, and 0.25 for each respective dengue infection. Accordingly, primary, secondary, tertiary and quaternary infections occur at rates of λ1, λ2, λ3 and λ4 respectively where λ2 for example, is equal to bβh (0.75Iv/Nh) and λ3 is equal to bβh (0.50Iv/Nh). These rates are less than λ1 [bβh (Iv/Nh)] because fewer mosquitoes are assumed to be infected and infectious (Iv) with the serotype to which humans with temporary cross-protection remain susceptible [37]. Similarly, the force of infection or per-capita incidence rate amongst mosquitoes is bβv (Ih1 + Ih2 + Ih3 + Ih4 + ((Ihe2φhe) + (Ihe3φhe) + (Ihe4φhe))/Nh) where b is as above; βv is the probability of transmission from human to vector and (Ih1 + Ih2 + Ih3 + Ih4 + ((Ihe2φhe) + (Ihe3φhe) + (Ihe4φhe))/Nh) is the proportion of infectious individuals where Nh is the total human population. The disease dependent death rate α is similarly stratified by infection, in that secondary infections have the potential to be more severe [8–10, 49]. The flow diagram of the infection process is presented in Fig. 1. Additional model assumptions relate to the following:
Flow diagram of the infection process. Due to space constraints, the following expression (Ih1 + Ih2 + Ih3 + Ih4 + ((Ihe2φhe) + (Ihe3φhe) + (Ihe4φhe))) is signified by I*. Underlying background mortality (μh) is applied to all compartments but not shown on the figure. Only dengue-induced mortality (μD) is displayed
-
The population is homogeneous, which means that every individual in a compartment is homogenously mixed with the other individuals;
-
Mosquito bites are homogeneously distributed amongst all human hosts; this means that each mosquito bite has an equal probability of being taken from any particular human host.
-
The total size of the mosquito population is allowed to vary over time.
-
There is no natural protection, i.e. humans and mosquitoes are assumed to be born susceptible and losses of immunity are not considered, nor are maternally derived antibodies.
-
The mosquito has no resistant phase due to its relatively short life expectancy.
2.5 Age stratification
The model population includes the entire population for the country (i.e. Thailand) where the model is applied and reflects current demographic characteristics such as age, based on recent census data and population projections from national statistics for 2008. For simplicity, the model assumes that population size is constant. Hence, births are equal to deaths and possible migration of infected individuals into the human population is not considered. Individuals survive until 70 years of age (life expectancy) and then die (known as Type I survivorship) [50].
The model is age-stratified with the total population divided into six age cohorts: 0–11 months; 1–4 years; 5–9 years; 10–14 years; 15–24 years and 25 years and over. At each time lag, individuals age and therefore move to the next age class. We assumed uniform aging over time. Thus, each differential equation includes the addition of a 1/L (where L denotes the width of the age class) proportion of individuals from the previous age class, and the withdrawal of the same proportion of individuals in the age class considered.
2.6 Seasonality
Seasonality terms adapted from Coutinho (2005, 2006) [51, 52] and Burrattini (2008) [30] are incorporated into the aquatic maturation rate and transition rate to adult mosquitoes using the following expression:
The assumption is that the vector population fluctuates seasonally with rainfall and other climactic factors affecting the availability of breeding sites and therefore recruitment into the vector population. This is a sinusoidal function with a period of 365 days and where π is equal to 3.1416. The parameters c and d are climactic factors adjusting winters and summers. Accordingly, the length and severity of winters can be simulated with the variation in c and d; if c < d, the winter is relatively severe and of a longer duration. Conversely, if c > d the winter is comparatively mild and short. The Heaviside θ-function [θ(c − d(sin(2πft + σ)))] prevents the expression from becoming negative; it is equal to zero when the argument is negative (i.e. <0) and one when the argument is ≥0. The parameter f(365−1) represents the frequency with which high and low transmission seasons vary and equates to one reproductive cycle per year. The phase parameter σ is used to synchronise the adult mosquito population at a minimum when the aquatic maturation/progression rate is similarly at a minimum [30, 51, 52].
2.7 Control Interventions
Building on previous scholarship in this area [17, 30, 31, 33, 34, 37, 39, 49], we assess the following control interventions individually and in combination:
-
i.
No control
-
ii.
Larvicides
-
iii.
Adulticides
-
iv.
Environmental management embracing source reduction, i.e. elimination of breeding sites and ‘clean-up’ campaigns, improvements in sanitation as well as health educational measures using the Government of Singapore’s 2005 “10-Minute Mozzie Wipe-out” initiative as one such example
-
v.
Vaccination
We simulate the impact of chemical adulticide and larvicide interventions on the incidence of disease by increasing mortality rates for both adult mosquito and aquatic life forms. Using the square pulse function in Berkeley Madonna [53], adulticide and larvicide are administered in condensed intermittent bursts (analogous to a ‘clean-up’ campaign of 1 day per week for 5 weeks) at the beginning of the dengue season. This is done for 1 year only as well as each year for 5 years at the same time each year, i.e. at the beginning of the dengue season. Conversely, the aquatic carrying capacity (K) is decreased to simulate environmental management and associated activities as defined above. Reflecting the on-going nature of this package of interventions, the aquatic carrying capacity is reduced by 40 %, 50 %, 60 % and 70 % for the duration of the dengue season, approximately day 100–170 in the calendar year equating to higher temperatures and rainfall. This is done for 1 year only and the effects are evaluated over 5 and 10 years.
The balance between vaccination coverage, vaccine efficacy, and the waning of vaccine-induced protection determine the relative impact of vaccination on the epidemiology of dengue fever. In the model, infants aged 0–11 months are not vaccinated; rather vaccination takes place at 1 year of age with 70 % coverage. There is also catch-up vaccination for those more than 1 year and less than 5 years of age with 30 % coverage. Given the uncertainty associated with the uptake of an imperfect vaccine, we have adopted conservation assumptions related to coverage and explored alternatives in scenario analyses. Efficacy is assumed to be 30.2 % [19]. Vaccine-acquired protection is assumed to wane over time to take into consideration imperfect vaccine-induced immunity. For the purposes of the current model under consideration, it is assumed that vaccination consists of one dose only.
3 Results
3.1 Comparison of predicted with observed rates of infection adjusting for under-reporting
Without vector control, the model predicts approximately 675,000 dengue infections per year at steady state in Thailand, all age groups combined. This compares to the number of reported dengue fever/dengue hemorrhagic fever infections in Thailand in 2008 [46] adjusted for under-reporting [48], all age groups combined (n = 681,158). Figure 2 presents the results of the baseline epidemiological model without any control interventions compared to observed dengue infections using the best fitting combination of parameter values. It can be seen that model output compares well with observed data with the exception of the 25 years and over age group which underestimates the data slightly. Given that the main burden of disease is located in the younger age groups, primarily in the teenage and young adult age groups, this is not considered to be a major source of bias. The best fit was obtained for a model with cross-protection only and without the inclusion of cross-enhancement. Although various levels of cross-enhancement ranging from a 2-fold to 5-fold increase in infectiousness were tested and compared, none provided a better fit than the base model with cross-protection only.
3.2 Evaluation of single interventions
To evaluate the impact of the different control strategies, we compared the base case steady state without interventions, as shown in Fig. 2, to the average annual number of cases during the years that follow the introduction of controls. Vaccination being a continuous intervention, its effects are accumulated over the years that follow introduction. Conversely, environmental management, larvicide or adulticide are one-off or relatively short-term interventions, therefore their effects are evident much sooner.
Beginning first with vaccination, Table 1 presents the baseline estimates by age at infection and the impact of vaccination over 5, 10 and 20 year periods. Base case characteristics comprise the following: vaccination of 1 year olds with 70 % coverage, catch-up vaccination for those more than 1 year and less than 5 years of age with 30 % coverage, efficacy of 30.2 % and vaccine waning over 10 years. Some vaccines may take longer than others, potentially years, to realise the full benefit of the vaccine and one may observe this outcome to some extent in the simulated results. For example, in the first 5 years after vaccination, the impact of vaccination across age groups is relatively marginal and amounts to a 9–16 % decrease in incidence of dengue infections. The most pronounced effect is in the 1–4 years age group reflecting the fact that catch-up vaccination took place in this age-group initially. In contrast, the reduction in incidence 10 years post-vaccination jumps to approximately 35–42 %. In the same way, reductions in incidence 20 years post-vaccination range from between 58 and 63 % across age groups illustrating that the full benefits of vaccination are often derived in the longer term.
We also carried out sensitivity and scenario analyses to explore different model assumptions. For example, we examined different waning periods including 5 and 20 years, different coverage levels, using both 50 % and 90 %; an alternative estimate of efficacy using the upper limit of the 95 % confidence interval (56.6 %) from the recently published dengue vaccine trial [19] and finally, different assumptions around the level of coverage attached to catch-up vaccination; 50 % and 70 %. Assumptions were tested univariately, i.e. one value at a time rather than being examined multivariately.
Table 2 presents the results of these scenario analyses. Once again, one may observe that the short-term impacts of vaccination, in the realm of 5 years post-vaccination, are relatively marginal, with the exception being when efficacy is increased to 56.6 %. One then observes marked gains in the reduction of disease. A similar pattern as above is witnessed in the 10 year and 20 year post-vaccination scenarios. Namely, the full benefits of vaccination become much more evident in the long term particularly when the estimate of efficacy is meaningfully increased.
Table 3 presents the results of analyses examining the impact of chemical and environmental management strategies on the incidence of dengue. Depending on the time horizons of the treatment intervention, results are reported either in the years when the control strategy is active or the 5 years following the end of the intervention or a combination of both. For example, when larvicide and adulticide are administered 1 day per week for 5 weeks at the start of the dengue season, we report results in the active year and the subsequent 4 years. When larvicide and adulticide treatments are administered at the start of the dengue season 1 day per week for 5 weeks for 5 consecutive years, we report the reductions in incidence during these years of active treatment but also the reductions in incidence in the 5 years following the end of treatment. Results indicate that in the most conservative circumstances, i.e. insecticide spraying for 1 day per week for 5 weeks for 1 year only and a reduction in egg-carrying capacity of 40 %, adulticide and environmental management are the most effective interventions. In contrast, larvicide, again in the most conservative circumstances, performs relatively poorly when compared to the latter. As the duration of each intervention increases, every year for 5 years in the case of adulticide or an increase in effectiveness in the case of environment management, a corresponding reduction in the disease burden is observed.
3.3 Evaluation of multiple interventions
When we examine the impact of combination interventions on the incidence of dengue fever, results are reported for 5, 10 and 20 years post-intervention to ensure that the full benefits of vaccination are captured. Table 4 presents the results of these analyses. For adulticide, we assess the contribution of insecticide spraying in the form of 1 day per week for 5 weeks over 5 consecutive years. For environmental management, a reduction in egg-carrying capacity of 40 % is used. For vaccination, we adopt the base case characteristics. When vaccination is used in combination with environmental management, model projections suggest annual reductions in incidence of 45 %, 57 % and 62 % for 5, 10 and 20 years post-vaccination respectively. Similarly, when vaccination is used in conjunction with adulticide, model projections indicate annual reductions in incidence of 53 %, 75 % and 81 % for 5, 10 and 20 years post-vaccination respectively. Finally, when all three interventions are used in combination, model projections show annual reductions in the dengue disease burden of 62 %, 81 % and 86 % for 5, 10 and 20 years post-vaccination.
4 Discussion
This paper describes the results of using mathematical modelling to compare a range of dengue control strategies and their impact on the epidemiology of dengue fever in Thailand. The interventions under consideration include chemical (i.e. larvicides, adulticides), environmental management and vaccination. The base age-structured epidemiological model (i.e. without any control interventions) is shown to calibrate well with reported dengue fever/dengue hemorrhagic fever cases in Thailand in different age-groups from the year 2008 [43] adjusted for under-reporting [45]. This suggests that the inputs and initial values used to populate the mathematical model are consistent with the decision problem.
As highlighted by Undurraga (2012) [48], estimating the degree of under-reporting in dengue cases with sufficient accuracy is very challenging. The authors stress that generating more rigorous estimates is conditional on having greater insights into the epidemiology of dengue fever. It is suggested that greater accuracy could be achieved with long-term nationally representative cohort studies albeit with considerably more investment in both time and resources. In the absence of the above, researchers must necessarily rely on regional/local cohort studies, capture-recapture studies, Delphi panels and similar such designs with the attendant uncertainty in estimates that this entails [48]. In the context of the present study, one may hypothesise that using a single standardised value (7.6) to adjust for under-reporting across heterogeneous age-groups exposes a potential weakness in EF calculations. For example, the majority of study inputs underpinning EF calculations are based on children and young adults rather than being taken from a range of age groups in that the majority of cases occur in these age groups. Consequently, there is considerable uncertainty surrounding the appropriate adjustment for under-reporting in older age groups. If the 95 % confidence intervals accompanying the point estimate of 7.6 (95 % CI: 7–8.6) are used in the adjustment calculations, the discrepancy between model estimates and observed data may be less than 1,000 or as large as 30,000 cases. Hence, variability in estimates is driven by the appropriate choice of EFs in older age groups rather than model structure.
Seasonality in the form of a sinusoidal variation fitted to the aquatic maturation rate was incorporated to provide a degree of ecological and biological veracity. Similarly, temporary cross-protection was included for the same reasons as well as being consistent with recommended good practice in the modelling of dengue fever [44, 45]. Finally, sensitivity and scenario analyses were conducted to assess the impact on model fit and results when additional parameters were added or underlying assumptions changed. The results of our simulations indicate that singular interventions can make useful inroads into dengue fever transmission, particularly adulticide in the short term and vaccination in the medium to long term. These interventions subsequently come into their own when used in combination with a 75–85 % reduction in the incidence of dengue fever infections when vaccination is combined with either environmental management or adulticide or when all three interventions are combined.
Chemical and environmental management interventions have formed the basis of efforts to control dengue fever over the last 50 years in spite of acknowledged limitations in terms of effectiveness, mode of delivery, cost, and duration of sustainability [54, 55] but may still have an important role to play in the short to medium term. Each form of control has their merits as well as drawbacks [55, 56]. For example, ‘environmental management’ and all that this encompasses, whether in the form of government driven (top-down) campaigns or community-based (bottom-up) initiatives are predicated to a great extent on the level of local community compliance as well as health educational and inter-agency collaborative enforcement of these schemes. Expert commentators point out that effectiveness could be substantially improved if, for example, efforts were redirected towards eliminating the most ‘productive’ breeding sites rather than all potential sites using surveys to measure ‘pupal productivity’ and ‘key container’ or ‘key premise’ indices to facilitate identification [57–60]. Likewise with adulticides or larvicides, poor compliance as well as a growing lack of acceptance for the widespread use of chemicals is an important factor limiting the effectiveness of these interventions. For example, residents in areas where insecticide spraying is taking place may keep their doors and windows shut hampering the effective dissemination of the agent to access indoor populations of mosquitoes. This is compounded by pragmatic considerations surrounding correct dosing, functionality of sprayers as well as concerns around sustainability and growing mosquito resistance to insecticides [55]. Studies in Asia and the Americas have shown that resistance is becoming an issue of escalating importance [61, 62]. Consequently, many commentators state that insecticide fogging or spraying should only be used in clearly delineated geographical areas and for a limited time only [20].
As highlighted previously, few mathematical modelling studies have explored the combined effects of different interventions including vaccination and their impact on the epidemiology of dengue transmission. A number of reasons necessitate a wider consideration. Firstly, recently published efficacy results of a dengue vaccine were relatively low and differed by serotype [19]. Secondly, even if the reported efficacy had been very high, i.e. in the range 80–90 %, there is still a case for some form of mixed strategy incorporating vaccination as well as environmental management and appropriate chemical control. For example, the vaccine is still not 100 % effective and there may be occurrences of primary and secondary vaccine failure in the periods both pre- and post-vaccination. Moreover, if we consider the analogous vector-borne disease yellow fever, the vaccine is recognised as being safe and very effective in preventing yellow fever in different age groups with durable protection. Nonetheless, the number of yellow fever cases continues to expand in spite of this. Estimates from WHO indicate that there are approximately 200, 000 cases and 30, 000 deaths linked to yellow fever annually worldwide [63]. Reasons put forward include increasing deforestation, urbanisation, and climate change as well as waning population immunity leading to greater mosquito/virus contact [63]. Mosquito control is advocated using breeding site destruction and larvicides as well as insecticide spraying to kill adult mosquitoes during outbreaks [63]. This would suggest that there is a still place for other forms of vector control in addition to vaccination for the control of yellow fever and by extension dengue fever and mathematical modelling studies can aid in these policy debates.
Given this background, we conducted an assessment of the relative impact of different dengue control interventions incorporating learning from previous studies. The premise being that it is generally not viable to eliminate dengue fever in Thailand using current technologies and their corresponding effectiveness, rather the aim is to control the transmission of dengue fever. The follow-up question then becomes one of identifying the optimal mix of control interventions again using the tools currently available to us today including an imperfect vaccine.
This study is subject to a number of limitations. Firstly, a number of potentially effective interventions are not included in our evaluation. In a recent analysis, Amaku (2013) [39] highlighted the effectiveness of strategies that reduce contact between humans and vectors through the use of, for example, insect repellents or insecticide-treated clothes. Other interventions not considered relate to biological control including predatory copepods and larvivorous (larvae-eating) fish as well as genetically modified mosquitoes. Evidence from Vietnam indicates that the copepod Mesocyclops is very effective at eradicating Aedes aegypti when introduced into water receptacles where mosquitoes breed [64–66]. Potential caveats relate to the practical necessity for continual replacement of these organisms in containers as householders regularly empty and clean the water containers. Moreover, additional concerns relate to cultural sensitivities and objections to putting living things in household water receptacles. For example, it is considered unacceptable in Thailand to wash and bathe in water that contains living creatures including small fish [67]. This may prevent the widespread adoption of these interventions.
An additional limitation relates to the absence of heterogeneity in the model with the exception of age. Spatial/geographical heterogeneity is not considered; dengue fever may vary widely across the country but be more homogeneous within cites but the model does not take this into account. Heterogeneities in host-vector contact are also not considered, for example, in hosts getting bitten or biting by mosquitoes [68]. Woolhouse (1997) [69] identifies the 80/20 “rule” in which 80 % of all transmission is due to 20 % of all individuals. The authors maintain that the rule is applicable to a variety of disease systems. Similarly, de Benedictis (2003) [70] used polymerase chain reaction to identify human DNA from blood meals in Aedes aegypti collected in 22 homes and found that only 3 people accounted for 56 % of the meals, thus showing feeding is non-random, with a bias towards young adults and males. The implications of heterogeneity imply that as with ‘pupal’ surveys and ‘key container’ or ‘key premise’ indices, interventions that can be focused on key groups can potentially be hugely effective. Conversely, strategies that fail to reach their target groups will tend to be less successful than perhaps anticipated in reducing population-level disease burden [59, 69].
Whilst compartmental models have their strengths as evidenced by their relative popularity, they also have important shortcomings related to the lack of spatial capabilities and their fixed deterministic status [71]. In contrast to dynamic models, individual-based or agent-based models take a ‘bottom-up’ rather than ‘top-down’ approach to specify how individuals and even vectors interact with each other according to an explicit set of rules [29, 40, 72]. Moreover, geographic and/or spatially explicit capabilities are integral to these approaches. With increased computing power, these new mathematical methodologies offer great potential in capturing improved realism although an accompanying caveat concerns data availability as the new model formulations tend to be data hungry [71].
This project forms the first step in a body of work examining the impact of different dengue fever intervention strategies on the epidemiology of dengue fever in Thailand. Subsequent steps will in turn examine both the cost-effectiveness of these multiple intervention strategies as well as determine the optimal mix of strategies for the prevention of dengue fever under constraints. Cost-effectiveness does not directly address the problem that decision-makers are increasingly constrained by a fixed-budget and may not be able to fund new more expensive interventions, even if they have been shown to represent good value for money. In this regard, quantitative methods applied to the optimal allocation of fixed resources in order to obtain maximum of benefits may be of assistance [73].
References
WHO (2011) Working to overcome the global impact of neglected tropical diseases Update 2011. WHO/HTM/NTD/2011.3 (World Health Organization)
Gubler DJ (1998) Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11(3):480–496
Gubler DJ (2002) Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol 10(2):100–103
WHO (2009) Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. WHO/HTM/NTD/DEN/2009.1 (World Health Organization)
Wearing HJ, Rohani P (2006) Ecological and immunological determinants of dengue epidemics. Proc Natl Acad Sci U S A 103(31):11802–11807
Halstead SB (2004) Antibody-dependent enhancement of infection: a mechanism for indirect virus entry into cells. Cellular Receptor for Animal Viruses, Cold Spring Harbor Laboratory Press 28:493–516. 0-87969-429-7/94 (Chapter 25)
WHO (2007) Report of the Scientific Working Group meeting on dengue. (World Health Organization). Available: http://apps.who.int/tdr/svc/publications/tdr-research-publications/swgreport-dengue. Accessed March 20, 2013.
Halstead SB (2007) Dengue Lancet 370(9599):1644–1652
Aguiar M, Stollenwerk N, Kooi BW (2009) Torus bifurcations, isolas and chaotic attractors in a simple dengue model with ADE and temporary cross immunity (Chapter 3). Int J Comput Math 86:1867–1877
Aguiar M et al (2011) The role of seasonality and import in a minimalistic multi-strain dengue model capturing differences between primary and secondary infections: complex dynamics and its implications for data analysis (Chapter 4). J Theor Biol 289:181–196
WHO (2012) Dengue and severe dengue - Fact sheet N°117, http://www.who.int/mediacentre/factsheets/fs117/en/ (Accessed 2012-03-27)
Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI (2013) The global distribution and burden of dengue. Nature 496(7446):504–507
Gubler DJ, Wilson ML (2005) The global resurgence of vector-borne diseases: lessons learned from successful and failed adaptation, Integration of public health with adaptation to climate change: lessons learned and new directions. In: Ebi KL, Smith J, Burton I (eds) Taylor and Francis, London, pp 44–59
Asia-Pacific Dengue Program Managers Meeting [proceedings] (2008) World Health Organization Western Pacific Region: National Environment Agency
Kongnuy R, Pongsumpun P (2011) Mathematical Modeling for Dengue Transmission with the Effect of Season. World Academy of Science, Engineering and Technology 51
Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW (2011) Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. PNAS 108(18):7460–7465
Massad E, Coutinho FA (2011) The cost of dengue control. Lancet 377(9778):1630
Guzmán MG, Kouri G (2002) Dengue: an update. Lancet Infect Dis 2(1):33–42
Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J (2012) Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380(9853):1559–1567
WHO (2011) Regional Office for South-East Asia. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever; Revised and expanded edition. (SEARO Technical Publication Series No. 60)
Morrison AC, Zielinski-Gutierrez E, Scott TW, Rosenberg R (2008) Defining challenges and proposing solutions for control of the virus vector Aedes aegypti. PLoS Med 18:5(3)
Derouich M, Boutayeb A, Twizell EH (2003) A model of dengue fever. Biomed Eng Online 2:4
Derouich M, Boutayeb A (2006) Dengue fever: Mathematical modelling and computer simulation. Appl Math Comput 177:528–544
Bartley LM, Donnelly CA, Garnett GP (2002) The seasonal pattern of dengue in endemic areas: mathematical models of mechanisms. Trans R Soc Trop Med Hyg 96(4):387–397
Focks DA, Daniels E, Haile DG, Keesling JE (1995) A simulation model of the epidemiology of urban dengue fever: Literature analysis, model development, preliminary validation and samples of simulation results. Am J Trop Med Hyg 53(5):489–506
Nagao Y, Koelle K (2008) Decreases in dengue transmission may act to increase the incidence of dengue hemorrhagic fever. Proc Natl Acad Sci U S A 105(6):2238–2243
Esteva L, Vargas C (1998) Analysis of a dengue disease transmission model. Math Biosci 150(2):131–151
Chikaki E, Ishikawa H (2009) A dengue transmission model in Thailand considering sequential infections with all four serotypes. J Infect Dev Ctries 3(9):711–722
Otero M, Barmak DH, Dorso CO, Solari HG, Natiello MA (2011) Modeling dengue outbreaks. Math Biosci 232(2):87–95
Newton EAC, Reiter P (1992) A model of the transmission of dengue fever with an evaluation of the impact of ultralow volume (ULV) insecticide applications of dengue epidemics. Am J Trop Med Hyg 47:709–720
Burattini MN, Chen M, Chow A, Coutinho FA, Goh KT, Lopez LF, Ma S, Massad E (2008) Modelling the control strategies against dengue in Singapore. Epidemiol Infect 136(3):309–319
Derouich M (2009) Dengue Fever: A Mathematical Model with Immunization Program in Handbook of Research on Systems Biology Applications in Medicine. Editor Andriani Daskalaki (Max Planck Institute for Molecular Genetics, Germany)
Luz PM, Codeco CT, Medlock J, Struchiner CJ, Valle D et al (2009) Impact of insecticide interventions on the abundance and resistance profile of Aedes aegypti. Epidemiol Infect 137(8):1203–1215
Yang HM, Ferreira CP (2008) Assessing the effects of vector control on dengue transmission. Appl Math Comput 198:401–413
Rodrigues HS, Monteiro MTT, Torres DFM, Zinober A (2010a) Control of Dengue disease: a case study in Cape Verde. Proc. 10th International Conference on Mathematical Methods in Science and Engineering, Almerıa, pp 26–30
Rodrigues HS, Monteiro MTT, Torres DFM (2010b) Insecticide control in a Dengue epidemics model. In: Simos T (ed) Numerical Analysis and Applied Mathematics. AIP Conf. Proc, 1281 pp 979–982
Oki M, Sunahara T, Hashizume M, Yamamoto T (2011) Optimal timing of insecticide fogging to minimize dengue cases: modeling dengue transmission among various seasonalities and transmission intensities. PLoS Negl Trop Dis 5(10):e1367
Coudeville L, Garnett GP (2012) Transmission dynamics of the four dengue serotypes in southern Vietnam and the potential impact of vaccination. PLoS One 7(12):e51244
Amaku M, Coutinho FAM, Raimundo SM, Lopez LF, Burrattini MN, Massad E. A Comparative Analysis of the Relative Efficacy of Vector-Control Strategies against Dengue Fever. Manuscript under review. http://arxiv.org/abs/1302.1166.
Chao DL, Halstead SB, Halloran ME, Longini IM Jr (2012) Controlling dengue with vaccines in Thailand. PLoS Negl Trop Dis 6(10):e1876
Barmak DH, Dorso CO, Otero M, Solari HG (2013) Modelling interventions during a dengue outbreak. Epidemiol Infect 26:1–17
Boccia TM, Burattini MN, Coutinho FA, Massad E (2013) Will people change their vector-control practices in the presence of an imperfect dengue vaccine? Epidemiol Infect 5:1–9
Nishiura H (2006) Mathematical and statistical analyses of the spread of dengue. Dengue Bull 30:51–67, Table 1, p53
Johansson MA, Hombach J, Cummings DAT (2011) Models of the impact of dengue vaccines: A review of current research and potential approaches. Vaccine 29(35):5860–5868
Andraud M, Hens N, Marais C, Beutels P (2012) Dynamic epidemiological models for dengue transmission: a systematic review of structural approaches. PLoS One 7(11):e49085
Bureau of Epidemiology (2008) Ministry of Public Health, Thailand
Wichmann O, Yoon IK, Vong S, Limkittikul K, Gibbons RV, Mammen MP, Ly S, Buchy P, Sirivichayakul C, Buathong R, Huy R, Letson GW, Sabchareon A (2011) Dengue in Thailand and Cambodia: an assessment of the degree of underrecognized disease burden based on reported cases. PLoS Negl Trop Dis 5(3):e996
Undurraga EA, Halasa YA, Shepard DS (2013) Use of expansion factors to estimate the burden of dengue in Southeast Asia: a systematic analysis. PLoS Negl Trop Dis 7(2):e2056
Luz PM, Vanni T, Medlock J, Paltiel AD, Galvani AP (2011) Dengue vector control strategies in an urban setting: an economic modelling assessment. Lancet 377(9778):1673–1680
Anderson RM, May RM (1991) Infectious Diseases of Humans, Dynamics and Control. Oxford University Press, Oxford
Coutinho FAB, Burattini MN, Lopez LF, Massad E (2005) An approximate threshold condition for non-autonomous system: An application to a vector-borne infection. Math Comput Simul 70:149–158
Coutinho FAB, Burattini MN, Lopez LF, Massad E (2006) Threshold conditions for a nonautonomous epidemic system describing the population dynamics of dengue. Bull Math Biol 68(8):2263–2282
Berkeley Madonna 8.02 (2009)
Gubler DJ, Kuno G (eds) (2001) Dengue and dengue hemorrhagic fever. CABI Publishing, Wallingford, UK 425–62
Chang MS et al (2011) Challenges and future perspective for dengue vector control in the Western Pacific Region. W Pac Surveill Response J 2(2):9–16
McCall PJ, Kittayapong P (2006) Control of dengue vectors: tools and strategies. Working paper for the Scientific Working Group on Dengue Research, convened by the Special Programme for Research and Training in Tropical Diseases, Geneva, 1–5
Nathan MB, Focks DA, Kroeger A (2006) Pupal/demographic surveys to inform dengue-vector control. Ann Trop Med Parasitol 100(Suppl 1):S1–S3
Focks D, Alexander N. Multicountry study of Aedes aegypti pupal productivity survey methodology: findings and recommendations. TDR/IRM/Den /06.1 Copyright © World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases, 2006.
Tun-Lin W, Lenhart A, Nam VS, Rebollar-Téllez E, Morrison AC, Barbazan P, Cote M, Midega J, Sanchez F, Manrique-Saide P, Kroeger A, Nathan MB, Meheus F, Petzold M (2009) Reducing costs and operational constraints of dengue vector control by targeting productive breeding places: a multi-country non-inferiority cluster randomized trial. Trop Med Int Health 14(9):1143–1153
WHO HELI (2013) Better environmental management for control of dengue. Health and Environment Linkages Initiative (HELI) - Accessed 15 June/2013: http://www.who.int/heli/risks/vectors/denguecontrol/en/
Marcombe S, Mathieu RB, Pocquet N, Riaz MA, Poupardin R, Sélior S, Darriet F, Reynaud S, Yébakima A, Corbel V, David JP, Chandre F (2012) Insecticide resistance in the dengue vector Aedes aegypti from Martinique: distribution, mechanisms and relations with environmental factors. PLoS One 7(2):e30989
Ranson H, Joseph Burhani J, Nongkran, Lumjuan N, Black WC (2010) Insecticide resistance in dengue vectors. TropIKA.net vol.1 no.1 Jan./Mar. 2010
Yellow fever Fact sheet N°100 (2013). Accessed 30 June 2013: http://www.who.int/mediacentre/factsheets/fs100/en/
Nam VS, Thi Yen N, Minh Duc H, Cong Tu T, Trong Thang V, Le Hoang N, Hoang San L, Le Loan L, Que Huong VT, Kim Khanh LH, Thuy Trang HT, Lam LZ, Kutcher SC, Aaskov JG, Jeffery JA, Ryan PA, Kay BH (2012) Community-based control of Aedes aegypti by using Mesocyclops in southern Vietnam. Am J Trop Med Hyg 86(5):850–859
Vu SN, Nguyen TY, Kay BH, Marten GG, Reid JW (1998) Eradication of Aedes aegypti from a village in Vietnam, using copepods and community participation. Am J Trop Med Hyg 59(4):657–660
Vu SN, Nguyen TY, Tran VP, Truong UN, Le QM, Le VL, Le TN, Bektas A, Briscombe A, Aaskov JG, Ryan PA, Kay BH (2005) Elimination of dengue by community programs using Mesocyclops(Copepoda) against Aedes aegypti in central Vietnam. Am J Trop Med Hyg 72(1):67–73
Cattand P, Desjeux P, Guzmán MG, Jannin J, Kroeger A, Médici A, Musgrove P, Nathan MB, Shaw A, Schofield CJ (2006) Tropical Diseases Lacking Adequate Control Measures: Dengue, Leishmaniasis, and African Trypanosomiasis. Disease Control Priorities in Developing Countries, 2nd edn. Oxford University Press, New York, pp 451–466. doi:10.1596/978-0-821-36179-5/Chpt-23
Lloyd AL, Zhang J, Root AM (2007) Stochasticity and heterogeneity in host-vector models. J R Soc Interface 4(16):851–863
Woolhouse ME, Dye C, Etard JF, Smith T, Charlwood JD, Garnett GP, Hagan P, Hii JL, Ndhlovu PD, Quinnell RJ, Watts CH, Chandiwana SK, Anderson RM (1997) Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc Natl Acad Sci U S A 94(1):338–342
De Benedictis J, Chow-Shaffer E, Costero A, Clark GG, Edman JD, Scott TW (2003) Identification of the people from whom engorged Aedes aegypti took blood meals in Florida, Puerto Rico, using polymerase chain reaction-based DNA profiling. Am J Trop Med Hyg 68(4):437–446
Rahmandad H, Sterman J (2008) Heterogeneity and network structure in the dynamics of diffusion: Comparing agent-based and differential equation models. Manag Sci 54(5):998–1014
Jacintho LFO, Batista AFM, Ruas TL, Marietto MGB, Silva FA (2010) An agent-based model for the spread of the Dengue fever: a swarm platform simulation approach. An agent-based model for the spread of the Dengue fever: a swarm platform simulation approach. SpringSim ‘10: Proceedings of the 2010 Spring Simulation Multiconference
Brandeau ML, Zaric GS, Richter A (2003) Resource-allocation for control of infectious diseases in multiple independent populations: beyond cost-effectiveness analysis. J Health Econ 22:575–598
For 1970–2000, Thailand, source: United Nations
Luz PM, Lima-Camara TN, Bruno RV, Castro MG, Sorgine MH, Lourenço-de-Oliveira R, Peixoto AA (2011) Potential impact of a presumed increase in the biting activity of dengue-virus-infected Aedes aegypti (Diptera: Culicidae) females on virus transmission dynamics. Mem Inst Oswaldo Cruz 106(6):755–758
Acknowledgments
The authors wish to thank Julie Roiz for her expert insights into infectious disease modelling and Eduardo Massad for his patient answering of queries related to his work in dengue modelling.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Knerer, G., Currie, C.S.M. & Brailsford, S.C. Impact of combined vector-control and vaccination strategies on transmission dynamics of dengue fever: a model-based analysis. Health Care Manag Sci 18, 205–217 (2015). https://doi.org/10.1007/s10729-013-9263-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10729-013-9263-x