Abstract
The yellow stripe-like (YSL) family of transporters mediates the uptake, translocation, and distribution of various mineral elements in vivo by transferring metal ions chelated with phytosiderophore or nicotianamine (NA). However, little is known about the roles of the YSL genes against cadmium in planta. In this study, we first cloned and characterized a vital member of the YSL gene family, MsYSL1, from the bioenergy plant Miscanthus sacchariflorus. MsYSL1 localized in the plasma membrane and was widely expressed throughout the whole seedling with the highest expression level in the stem. In addition, its expression in the root was stimulated by excess manganese (Mn), cadmium (Cd), and lead, and a shortage of iron (Fe), zinc (Zn), and copper. Functional complementation in yeast indicated that MsYSL1 showed transport activity for Fe(II)–NA and Zn–NA, but not for Cd–NA. Although they exhibited no significant differences versus the wild type under normal cultivation conditions, MsYSL1-overexpressing Arabidopsis lines displayed a higher resistance to Cd accompanied by longer root lengths, lower Cd, Zn, and Mn levels in roots, and higher Cd, Fe, and Mn translocation ratios under Cd stress. Moreover, genes related to NA synthesis, metal translocation, long-distance transport, and Cd exclusion were highly induced in transgenic lines under Cd stress. Thus, MsYSL1 may be an essential transporter for diverse metal–NAs to participate in the Cd detoxification by mediating the reallocation of other metal ions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yellow stripe-like (YSL) proteins belong to an oligopeptide superfamily and participate in the uptake and retranslocation of various metal ions in plants (Curie et al. 2009). YSL transporters usually transport metals as metal complexes formed with phytosiderophores (PSs) or nicotianamines (NAs), which are two vital metal ion ligands that are widely distributed in plants. NA is generated from S-adenosylmethionine through the catalysis of nicotianamine synthetase (NAS) in plants (Higuchi et al. 1994). PS normally consists of a variety of acids, including mugineic acids (MAs) and deoxymugineic acids (DMAs). MA is derived from NA after a deamination step and a reduction process conducted by NA aminotransferase and DMA synthetase, respectively, especially in graminaceous plants (Takahashi et al. 1999; Bashir et al. 2006). YSL transporters are divided into two groups according to the transport substrates. The metal–PS transporter is mainly responsible for the uptake of metal ions in the grass family, while the metal–NA transporter is closely related to long-distance translocation and redistribution in monocots and dicots. In recent decades, researches focused primarily on family members of Zea mays (ZmYS1), Oryza sativa (OsYSL2, 6, 9, 15, 16, and 18), Hordeum vulgare (HvYS1, HvYS2, and HvYSL5), and Arabidopsis thaliana (AtYSL1, 2, 3, 4, and 6). ZmYS1 was the first YSL member discovered and identified as a typical transporter for the uptake of iron(Fe)(III)–PS from rhizosphere soil (Curie et al. 2001). Further research revealed that ZmYS1 transports several substrates, including Fe(III)–DMA, Fe(III)–MA, Fe(II)–NA, zinc (Zn)–DMA, copper (Cu)–MA, nickel (Ni)–MA, Ni–NA, and cadmium (Cd)–DMA (Schaaf et al. 2004). A homologous gene in rice (YSL15) exhibits substrate specificity for Fe(III)–DMA and Fe(II)–NA, and is expressed not only in root epidermis but also in phloem and reproductive organs (Lee et al. 2009), while HvYS1 in barley only transports Fe(III)–DMA and regulates Fe uptake in roots (Murata et al. 2006). Other members are mainly involved in internal metal translocation and redistribution in plants. YSL members in Arabidopsis chiefly mediate Fe(II)–NA and Fe(III)–citrate in vivo because PS is not synthesized or secreted. AtYSL2 is especially expressed in vascular tissues and is capable of transporting Fe(II)–NA and Cu–NA in yeast (DiDonato et al. 2004; Schaaf et al. 2005). AtYSL1 and AtYSL3 are identified as transporters of Fe(II)–NA that mediate Fe, Zn, and Cu redistribution from shoots and leaves to seeds in Arabidopsis, and AtYSL3 also has the capacity to transfer Fe(II)–DMA in yeast (Waters et al. 2006; Chu et al. 2010). Additionally, AtYSL4 and AtYSL6 are reported to localize in the vacuole membrane and chloroplast envelope in response to the detoxification of excessive metals in plant cells although there is no transport capacity for Fe(II)–NA in the yeast (Conte et al. 2013; Divol et al. 2013). OsYSL2 was the first characterized transporter of the 18 members in the rice genome. It delivers Fe(II)–NA and manganese (Mn)–NA from roots to shoots and seeds through the phloem (Koike et al. 2004; Ishimaru et al. 2010). OsYSL6 is responsible for transporting Mn–NA and preventing Mn toxicity (Sasaki et al. 2011). Recent research demonstrated that OsYSL9 functions in transferring the Fe(III)–DMA and Fe(II)–NA from endosperm to embryo in developing grains (Senoura et al. 2017). OsYSL16 not only transports Fe(III)–DMA in the vasculature, but also plays an essential role in node phloem for Cu–NA delivery (Kakei et al. 2012; Zheng et al. 2012). A high mRNA abundance of OsYSL18 with transport activity for Fe(III)–DMA is detected in reproductive tissues (especially in pollen and pollen tubes) and laminar joints in phloem, indicating its function in Fe supply for reproduction (Aoyama et al. 2009). In barley, HvYS2 expresses in the endodermis and possesses a wide range of transport substrates, including Fe(III)/Zn/Mn/Cu/Cobalt (Co)/Ni–DMA and Fe(II)–NA (Araki et al. 2011). HvYSL5 is a vesicle-located protein involved in the transient storage of Fe, and its mRNA expression follows the rhythm of MA secretion. However, the phenotypes of yeast mutants harboring HvYSL5 expression vector cannot be complemented with Fe(III)–DMA or Fe(II)–NA (Zheng et al. 2011). Some YSL genes from cash crops have been cloned in recent years. For example, eight YSL transporters were found in pear (Pyrus bretschneideri), and PbrYSL4 has a high transcript level in all tissues, particularly during pollen tube growth (Yang et al. 2016).
Although most YSL transporter members are induced by Fe deficiency or overload, some respond to other excess nutrient elements or even heavy metals. To explain the high accumulation rate of heavy metals in hyperaccumulators, certain YSL homologous genes were isolated. TcYSL3, TcYSL5, and TcYSL7 were characterized in Thlaspi caerulescens, and further research on TcYSL3 implied a role in Ni–NA translocation (Gendre et al. 2007). In total, 27 members were isolated in Brassica juncea. Some were induced by heavy metals (Das et al. 2011), and the overexpression of BjYSL7 in tobacco enhanced the tolerance for Cd and Ni (Wang et al. 2013). A plasma-located transporter, SnYSL3, is capable of delivering multiple metals chelated with NA (Cd–NA was the first reported) in Solanum nigrum, and the expression level is upregulated by Cd stress (Feng et al. 2016).
Miscanthus is an excellent candidate for phytoremediation based on its strong ecological adaptability and heavy metal tolerance (Chung and Kim 2012). Miscanthus copes with heavy metals by promoting root metabolism (Kayama 2001) and improving photosynthesis or the antioxidant enzyme system (Ezaki et al. 2008; Zhang et al. 2015). However, the molecular mechanisms of heavy metal resistance remain unclear in Miscanthus plants. In our previous study, we found that three Miscanthus species showed different tolerance levels to Cd, resulting in different growth and physiological responses (Guo et al. 2016a). Miscanthus sacchariflorus accumulates less Cd in both roots and leaves by restricting Cd uptake from roots and displays a high Cd tolerance, while Miscanthus floridulus not only absorbs more Cd from roots but also transfers more Cd from roots to shoots (Guo et al. 2016a). According to the transcriptomic analysis of M. sacchariflorus under Cd stress using high-throughput RNA-sequencing technology (Guo et al. 2016b), we identified one YSL gene, MsYSL1, which was significantly upregulated by Cd in M. sacchariflorus. To explore the molecular mechanism of MsYSL1 in M. sacchariflorus, the full-length coding sequence was cloned, characterized, and overexpressed in the yeast and Arabidopsis. The transgenic lines were used to evaluate the functions of MsYSL1 against Cd stress. In addition, the expression levels of genes related to NA synthesis, metal translocation, and long-distance transport, and Cd exclusion were measured under Cd stress in Arabidopsis. MsYSL1 as an essential transporter for diverse metal–NAs participates in Cd detoxification by mediating the reallocation of other metal ions. This study was aimed to increase knowledge regarding the molecular functions of YSLs in plants.
Materials and methods
Plant materials and growth conditions
Mature seeds of M. sacchariflorus were collected in the same place as described in our previous study in 2015 and saved at 4 °C until use (Guo et al. 2016a). The seeds were soaked in deionized water in Petri dishes to germinate at 37 °C for 2 days in the darkness. After germination, the seedlings were transferred to several plastic boxes full of vermiculite in a nutrient solution (pH 5.5) recommended by the International Rice Research Institute to ensure vertical growth (Yoshida et al. 1976). The solution contained the following: 1.43 mM NH4NO3, 0.32 mM NaH2PO4, 0.51 mM K2SO4, 1 mM CaCl2, 1.6 mM MgSO4, 9.5 µM MnCl2, 19 µM H3BO3, 0.152 µM ZnSO4, 0.155 µM CuSO4, 0.075 µM (NH4)6Mo7O24, and 125 µM FeNa2·EDTA. Seven-day-old seedlings were washed with deionized water for hydroponic culture in new boxes, and the nutrient solution (pH 5.5) was changed weekly. Plants were grown in a greenhouse programmed for a 16 h light (white fluorescent light intensity of 1200 µmol photons m−2·s−1)/8 h dark cycle with a daytime temperature of 30 °C and a night temperature of 22 ± 2 °C. The relative humidity was maintained at 60%.
Arabidopsis thaliana (Ecotype: Columbia-0) seeds were sown in peat substrate (Pindstrup, Denmark) (for transformation) and 1/5 Hoagland’s medium (for screening and growth test). The 1/5 Hoagland’s medium (pH 5.5) contained 1 mM KNO3, 1 mM Ca(NO3)2, 0.4 mM MgSO4, 0.2 mM (NH4)H2PO4, 3 µM HBO3, 0.5 µM MnCl2, 0.2 µM CuSO4, 0.4 µM ZnSO4, 1 µM (NH4)6Mo7O24, and 20 µM FeNa2·EDTA. A growth chamber was used for Arabidopsis cultivation and maintained a light/dark cycle of 10/14 h at 24/20 °C and 70% relative humidity.
Nicotiana benthamiana seedlings for MsYSL1–green fluorescent protein (GFP) transient expression were cultivated in vermiculite containing 1/5 Hoagland’s solution (pH 5.5) in a growth chamber under the same conditions as for Arabidopsis.
Gene cloning
The MsYSL1 gene-coding sequence was cloned from M. sacchariflorus cDNA according to the transcriptomic information from our previously published RNA-seq results (Guo et al. 2016b) with specific primers MsYSL1-F and MsYSL1-R (Supplementary Table 1). Total RNA was extracted using a MiniBEST Plant RNA Extraction Kit (Takara, Shiga, Japan) and was then used for cDNA synthesis with a PrimeScript™ 1st-Strand cDNA Synthesis Kit (Takara).
Reverse transcription and quantitative real-time PCR (qRT-PCR) analysis
To investigate the expression patterns in M. sacchariflorus, 6-week-old seedlings were used to detect MsYSL1 expression levels in roots, stems, and leaves. These seedlings were grown in the different nutrient solutions containing 400 µM Fe, 200 µM Zn, 100 µM Mn, 200 µM Cu, 100 µM Cd, or 100 µM lead for 6 h. Solutions deficient in Fe, Zn, Mn, or Cu were also utilized in a 5-day experiment. For related gene expression analyses in transgenic Arabidopsis, 6-week-old seedlings were exposed to 50 µM CdSO4 for 24 h. After treatments, Miscanthus (roots, stems and leaves) and Arabidopsis (roots) were harvested. The harvest tissues were quickly frozen in liquid nitrogen and stored at − 80 °C for subsequent experiments. For the qRT-PCR analysis, three biological replications were performed.
Total RNA was extracted using a MiniBEST Plant RNA Extraction Kit (Takara), and cDNA was synthesized from 1 µg of RNA using a PrimeScript™ RT reagent Kit with gDNA Eraser (Takara). The qRT-PCR analysis was conducted on a LightCycler® 480II machine (Roche, Penzberg, Bavaria, Germany) with SYBR® Green I Master PCR mix (Roche) in triplicate. The expression data were normalized using Actin in M. sacchariflorus and Actin2/UBQ10 in Arabidopsis. Primers for qRT-PCR were listed in Supplementary Table 1. Data were analyzed by LightCycler® 480 software v1.5.0 and LinRegPCR v2016.1 software according to a previous report (Aglawe et al. 2012).
Plasmid construction
To construct the overexpression vector, the MsYSL1 coding sequence was amplified without the stop codon using the gene specific primers MsYSL1OE-F and MsYSL1OE-R into the BamHI/XbaI sites of pCAMBIA1300–GFP (CaMV35s promoter). The vector was also used for the MsYSL1–GFP fusion protein that was transiently expressed in N. benthamiana.
For the yeast complementation assay, the MsYSL1 expression cassette was cloned using primers MsYSL1YE-F and MsYSL1YE-R and introduced into the yeast expression vector pDR196 digested by EcoRI/XhoI. The pDR196–OsYSL15 and pDR196–OsYSL2 expression vectors were constructed by inserting OsYSL15 and OsYSL2 coding sequences, respectively, using primers with the same enzyme cutting sites as MsYSL1 (OsY15-YE-F/OsY15-YE-R and OsY2-YE-F/OsY2-YE-R, respectively). All primers applied for construction of vectors were given in Supplementary Table 1.
Subcellular localization
The CaMV35s::MsYSL1–GFP vector and red fluorescent protein (RFP) marker pm-rbCD3-1008 that localized to the plasma membrane (Nelson et al. 2007) were introduced into Agrobacterium tumefaciens GV3101 and transiently expressed in the leaves of N. benthamiana as previously described (Xu et al. 2014). After treatment, the leaves were cut into small pieces, and mesophyll cells were observed through a confocal laser scanning microscope (LSM 710, Carl Zeiss, Jena, Germany) with lasers at 488 and 584 nm.
Yeast complementation assay
The yeast complementation assay was adapted to the following Saccharomyces cerevisiae mutant strains: Fe uptake-defective mutant DEY1453 (MATa can1 his3 leu2 trp1 ura3 fet3-2::HIS3 fet4-1::LEU2), Zn uptake-defective mutant ZHY3 (MATa ade6 can1 his3 leu2 trp1 ura3 zrt1::LEU2; zrt2::HIS3), and the wild type BY4741 (MATa, his3∆1, leu2∆0, met15∆0, ura3∆0). The polyethylene glycol/lithium acetate method was used for the yeast transformation (Gietz and Schiestl 1995), and a synthetic dropout medium (SD-Ura, pH 5.8), containing 0.67% yeast nitrogen base (Sigma-Aldrich, St. Louis, MO, USA), 0.2% appropriate amino acid lacking uracil (DO Supplement-Ura, Takara) and 2% glucose, was used to screen transformants harboring expression vectors.
The empty pDR196 vector, pDR196–MsYSL1, pDR196–OsYSL15, and pDR196–OsYSL2 were independently transformed into yeast strains DEY1453, ZHY3, and BY4741. Complementation of the yeast mutant phenotype was tested on the solid SD-Ura medium containing 0.67% yeast nitrogen base (Sigma-Aldrich), 0.2% appropriate amino acid lacking uracil (DO Supplement-Ura, Takara), 2% glucose, and 2% agar at pH 7. NA (TRC, Toronto, Ontario, Canada) was purchased from Toronto Research Chemicals. Before solidification, the fresh metal–NA complex was generated as previously reported (Schaaf et al. 2004), and added to the medium. Positive yeast transformants were developed in liquid SD-Ura medium at 30 °C for ~ 16–18 h until the optical density (OD) was over 1.5 at 600 nm. Then, a specified volume of yeast suspension was transferred into fresh SD-Ura liquid medium to grow for ~ 6–8 h from 0.2 to 1.0 at OD600. The fresh yeast suspension was centrifuged at 3000×g for 10 min and suspended in autoclave-sterilized water three times, resulting in final serial dilutions (0.1, 0.01, 0.001 and 0.0001 at OD600) of the yeast culture in water. The diluted solutions were spotted on plates with 20 µM Fe–NA, 10 µM Zn–NA, and 20 µM Cd–NA medium for the Fe, Zn, and Cd uptake assays, respectively. After spotting, plates were inverted and incubated at 30 °C for 4 days.
MsYSL1 overexpressing in Arabidopsis
The floral dip method incorporating A. tumefaciens GV3101 with the CaMV35s::MsYSL1–GFP vector was utilized for overexpressing MsYSL1 in Arabidopsis (Clough and Bent 1998). Positive transgenic seedlings were identified at the genome level by PCR and at the transcript level by semi-quantitative PCR using the respective primers listed in Supplementary Table 1.
Transgenic Arabidopsis phenotype and metal content analysis
MsYSL1-overexpressing Arabidopsis T3 seeds were surface-sterilized and sown on plates containing 1/5 Hoagland’s solid medium (pH 5.5) in the presence of 50 µM CdSO4 or not for 8 days to screen the Cd tolerance by observing the growth. Six-week-old hydroponic seedlings were cultivated for another 5-days period with 10 µM CdSO4 and then washed with 20 mM Na2·EDTA and deionized water three times. After wiping off the water, fresh samples were weighed and digested in 5 mL HNO3 and 1 mL H2O2 at 160 °C for metal concentration measurement by inductively coupled plasma mass spectrometry (DRC-e, Perkin-Elmer, Norwalk, CT, USA). The ratio of the metal amount in shoots to the amount in the whole plant was considered the translocation ratio. Three biological replicates were applied in these experiments.
Statistical analysis
Experimental data from at least three individual replicates were shown as the means ± SD. Two-tailed t-tests were performed, and significance was determined as *P < 0.05 or **P < 0.01.
Results
Phylogenetic analysis of MsYSL1
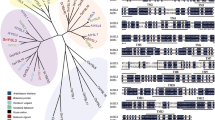
The MsYSL1 gene, which encodes a polypeptide of 678 amino acids, was obtained from RNA-seq results of M. sacchariflorus. A phylogenetic analysis revealed that MsYSL1 belonged to the Group I subfamily that includes some typical Fe uptake transporters, such as ZmYS1/HvYS1/OsYSL15 from Gramineae and AtYSL1-3 from Arabidopsis (Fig. 1). The MsYSL1 protein shared a 94% identity with ZmYS1 and contained 13 predicted transmembrane domains (Fig. S1). The prediction of transmembrane helices also showed that MsYSL1 possessed an N terminus in the cytosol and C terminus outside the membrane (Fig. S2).
Expression patterns and subcellular localization of MsYSL1
To understand whether the expression of MsYSL1 was induced by metals, the transcript levels of the MsYSL1 gene were detected in the main tissues of M. sacchariflorus, suffering from a deficiency or excess of metals, by qRT-PCR with MsActin as an internal reference gene for normalization. Under normal conditions, MsYSL1 had a highest expression level in stems compared with that in roots and leaves (Fig. 2a). In roots, the expression of MsYSL1 was induced by excess metal treatments (especially by lead, Mn, and Cd) and deficiencies in Fe, Zn, and Cu (Fig. 2b, e). In stems, a deficiency of Fe and an excess of Cu led to an increase of MsYSL1 at the transcript level (Fig. 2c, f). Either a deficiency or an excess of Fe significantly upregulated the expression of MsYSL1 in leaves (Fig. 2d, g).
Expression patterns of MsYSL1 in M. sacchariflorus. a Tissue-dependent expression of MsYSL1. b–d MsYSL1 expression levels in roots, stems and leaves under excessive metal conditions for 6 h. e–g MsYSL1 expression levels in roots, stems and leaves under various metal deficiency treatments for 5 days. The expression levels were determined by qRT-PCR. MsActin was applied as an internal reference and experimental data were indicated as the means ± SD (n = 3)
To determine the function, the subcellular localization of MsYSL1 was investigated. The coding sequence, without the stop codon, was introduced into a GFP-fusion vector with a CaMV35S promoter, and then the fusion vector and an RFP marker (pm-rbCD3-1008) were transiently expressed in tobacco leaves (Fig. S3a, b, c). The GFP signal was clearly merged with the pm-rbCD3-1008 marker protein in the plasma and nuclear membranes (Fig. S3d), confirming that MsYSL1 is a plasma membrane protein in M. sacchariflorus. Thus, MsYSL1 could be a transporter located in the plasma membrane.
Yeast transport activity assay of MsYSL1
To investigate its transport activity and substrate specificity, the MsYSL1 expression vector was introduced into yeast mutants defective in Fe or Zn uptake. The corresponding yeast mutants transformed with pDR196 and pDR196–OsYSL2/15 were used as negative and positive controls, respectively. When exposed to 20 µM FeSO4, all of the Fe uptake-defective mutant DEY1453 yeast lines maintained similar low growth rates. When subjected to 50 µM Fe(III)–citrate, all of the lines grew well and showed no differences. However, when exposed to 20 µM Fe–NA, the lines with OsYSL2 and MsYSL1 expression vectors grew better compared with the line containing the empty vector (Fig. 3a). In addition, Zn uptake-defective mutant ZHY3, harboring the MsYSL1 expression vector, showed better growth versus its counterpart harboring the empty vector in the presence of 10 µM ZnSO4–NA (Fig. 3b). However, there was no difference in growth under the 10 µM ZnSO4 and 100 µM ZnSO4 treatments, suggesting that MsYSL1 only transported chelated ZnSO4–NA. For the wild type yeast BY4741, the lines containing either the pDR196 or MsYSL1 expression vector showed similar growth in the presence of 20 µM CdSO4–NA and 20 µM CdSO4 (Fig. 3c), indicating that MsYSL1 was not able to transfer Cd–NA or Cd ions.
Functional complementation of mutant yeasts by MsYSL1. a DEY1453 yeast expressing empty vector pDR196, vectors with OsYSL2/OsYSL15 (positive control) or MsYSL1 was grown on SD-Ura (pH 7) containing 20 µM FeSO4, 20 µM FeSO4–NA or 50 µM Fe(III)–citrate. b ZHY3 yeast harboring empty vector pDR196 and vector with MsYSL1 was grown on SD-Ura (pH 7) containing 10 µM ZnSO4, 10 µM ZnSO4–NA or 100 µM ZnSO4. c BY4741 yeast with empty vector pDR196 and vector with MsYSL1 was spotted on SD-Ura (pH 7) containing 0 µM CdSO4, 10 µM CdSO4–NA or 20 µM CdSO4. A serial of culture dilutions (OD600 = 0.1, 0.01, 0.001, 0.0001) were spotted on media and been incubated at 30 °C for 4 days
MsYSL1 improved the tolerance of transgenic Arabidopsis to Cd
To identify the physiological and biochemical functions of MsYSL1 in plants, MsYSL1 was overexpressed using pCAMBIA1300–GFP as the overexpression vector under the CaMV35s promoter in Arabidopsis (Fig. S4a). A PCR analysis was conducted to ensure that the genetic transformation was successful after obtaining transgenic lines (Fig. S4b). Then, semi-quantitative PCR was used to verify the expression levels in the lines (Fig. S4c). Finally, three lines (Y15, Y21, and Y27) were chosen for further experiments. There was no difference in growth between the wild type and transgenic plants (Fig. 4a, c) under normal cultivation, while the root lengths of the transgenic lines were approximately two-fold longer than those of the wild type after exposure to 50 µM CdSO4 (Fig. 4b, d). Thus, MsYSL1 played a role in the resistance of Arabidopsis to Cd toxicity.
Phenotype of MsYSL1-overexpressing lines under Cd stress. a Response of transgenic lines and wild-type line under normal solution. b Response of transgenic lines and wild-type line under Cd treatment. c Root length under normal condition. d Root length under Cd stress. Seedlings were cultivated on plates containing treatment media for 8 days after sowing. Normal solution was 1/5 Hoagland’s solution (pH 5.5) and Cd treatment solution was 1/5 Hoagland’s solution with 50 µM CdSO4 (pH 5.5). Data were means ± SD (n = 3)
MsYSL1 was involved in metal ion homeostasis
To understand why MsYSL1 improved the resistance of Arabidopsis to Cd toxicity, the concentrations of various metal ions were analyzed and their translocation ratios were calculated. No differences, either in concentrations or in translocation ratios, were found under control conditions (Fig. 5a–c). However the concentrations of Cd, Zn, and Mn in the roots of MsYSL1-overexpressing Arabidopsis were lower than those in the roots of wild type (Col-0) under Cd stress, and the concentrations of all the above mentioned metals were not significantly different in the shoots of the lines (Fig. 5d, e). For the translocation ratios, the transgenic lines exhibited obvious transport activities for Cd, Fe, and Mn from roots to shoots (Fig. 5f).
Metal content determination of MsYSL1-overexpressing lines under normal condition and Cd stress. a Metal content of roots under normal condition. b Metal content of shoots under normal condition. c Translocation ratios of multi metal in plants under normal condition. d Metal content of roots under Cd stress. e Metal content of shoots under Cd stress. f Translocation ratios of multiple metals in plants under Cd stress. Hydroponic seedlings were cultivated in 1/5 Hoagland’s solution (pH 5.5) for 6 weeks before a 5-day 10 µM CdSO4 treatment. Metal content determination was calculated by fresh weight (FW). Data were means ± SD (n = 3)
Expression levels of genes involved in Cd uptake and translocation in transgenic Arabidopsis
To understand whether the differences of Cd transport activity between transgenic lines and wild type were related to Cd uptake or translocation, we examined the expression levels of genes involved in metal translocation and heavy metal tolerance (Fig. 6). Three homologous YSL genes (YSL1, YSL2, and YSL4) that participated in metal–NA translocation were investigated. Although the expression of AtYSL1 was not different among the lines, the expression levels of AtYSL2 and AtYSL4 in the transgenic lines were higher than those in wild type line. NAS1 and NAS2, which regulate the synthesis of NA, showed a nearly twofold increase in transcript levels in transgenic lines compared to wild type lines. The expression levels of natural resistance-associated macrophage protein 4 (NRAMP4) and heavy metal transporting ATPase 2/4 (HMA2/HMA4), which are related to redistribution from roots to shoots through the vascular bundle, were almost twofold higher than in control lines. The expression level of AtNRAMP3 was not consistent in the different lines. In addition, two Cd exclusion genes, detoxification 1 (DTX1) and pleiotropic drug resistance 8 (PDR8), were also significantly induced in transgenic plants under Cd stress compared to that in the control.
Expression levels of metal transport and heavy metal tolerance genes in roots of MsYSL1-overexpressing lines under Cd stress. Six-week-old hydroponic Arabidopsis roots were captured after 24 h Cd stress (50 µM CdSO4). Relative mRNA expression level of each gene was standardized by AtActin2/AtUBQ10. Data were analyzed according to LinReg method. Data were means ± SD (n = 3)
Discussion
MsYSL1 functioned as a vital metal–NA transporter in vivo
A phylogenetic tree analysis classified the YSL members into four individual subfamilies, and MsYSL1 was categorized as a Group I member along with many other characterized transporters, including primary uptake transporters (ZmYS1, HvYS1, and OsYSL15) and translocation-related transporters (OsYSL2, HvYS2, and AtYSL1/2/3) from different plant species, indicating that MsYSL1 may have similar functions as the abovementioned transporters. Additionally, the subcellular location of MsYSL1 in tobacco was in the plasma membrane (Fig. S3), which was identical to members in the same subfamily (Curie et al. 2009), and also implied a consistent function. MsYSL1 had a high identity with ZmYS1, which has already been identified as a multi-metal transporter for absorbing metal–PS and –NA complexes (Schaaf et al. 2004). Functional complementation assays indicated that MsYSL1 possessed the capacity to transfer Fe(II)–NA and Zn–NA in yeast cells and rescued the mutant yeast phenotype (Fig. 3). MsYSL1 expression rescued the Fe-deficiency phenotype by regulating Fe(II) absorption in DEY1453 yeast. This was similar to its homologous proteins ZmYS1 from maize, OsYSL15 from rice (Lee et al. 2009), and AtYSL1/2/3 from Arabidopsis (DiDonato et al. 2004; Waters et al. 2006). In addition, MsYSL1 was confirmed to possess the ability to transport Zn–NA in the Zn uptake-defective mutant ZHY3, which could also be rescued by an YSL family member from Solanum nigrum (SnYSL3) (Feng et al. 2016). Thus, the MsYSL1 transporter may play an important role in regulating the uptake and translocation of Fe and Zn in vivo. Although MsYSL1 was induced by Cd stress in M. sacchariflorus, the overexpression of MsYSL1 in wild type yeast did not directly mediate the transport of Cd–NA or Cd2+. This implied that MsYSL1 may indirectly respond to Cd stress by mediating other metal ion homeostasis in M. sacchariflorus.
Overexpression of MsYSL1 enhanced Cd tolerance in Arabidopsis
Cd is a non-essential and toxic element to plants, and restricts their normal metabolism, growth, and development (Singh et al. 2016), thus reducing the yield (Cao et al. 2015). Cd is absorbed through some transporters, like divalent cations (Fe, Zn and Mn) or ion channels (Ca and Mg), indicating that a competitive interaction between essential mineral nutrients and Cd occurs in vivo (Lin and Aarts 2012). To further confirm the role of MsYSL1 in responding to Cd stress, MsYSL1 was overexpressed in Arabidopsis, and the transgenic lines were exposed to a Cd solution. The overexpression of MsYSL1 in Arabidopsis significantly increased its Cd tolerance, resulting in longer root lengths and lower Cd concentrations in roots compared with wild type plants (Figs. 4, 5d). According to the functional complementation experiment of yeast, MsYSL1 did not transport Cd2+ or Cd–NA into yeast cells (Fig. 3c). Therefore, we hypothesized that MsYSL1 mediated Cd tolerance mainly by regulating the redistribution of other metals in transgenic Arabidopsis and M. sacchariflorus. OsYSL2 is involved in the reallocation of Fe and Mn from roots to shoots through vascular bundles (Koike et al. 2004). This was in agreement with the effects of Cd stress on the accumulation and translocation ratios of other metals, especially Fe and Mn, in the roots of transgenic Arabidopsis (Fig. 5d, f). In addition, the highest expression level of MsYSL1 was found in stems under normal conditions, while its expression was inhibited under Cd stress, further indicating that MsYSL1 may play an important role in regulating the translocation of metal ions from the root to the shoot in plants.
Cd tolerance was maintained by upregulating metal translocation genes in the MsYSL1-overexpressing line
Cd tolerance can be maintained by regulating metal uptake and translocation transporters to mediate metal ion reallocation (Wang et al. 2013; Sasaki et al. 2014; Lin et al. 2016). SnYSL3, which delivers a broad range of metal–NA complexes, increased Cd tolerance in transgenic Arabidopsis by decreasing Fe and Mn concentrations in the roots, and increasing the root-to-shoot translocation ratios of Fe and Mn (Feng et al. 2016). BjYSL7 encodes a plasma-localized metal–NA transporter, and the overexpression of BjYSL7 in tobacco enhanced Cd and Ni tolerance by increasing their translocation from roots to shoots (Wang et al. 2013). In this study, the overexpression of MsYSL1 in Arabidopsis partly induced the expression of three AtYSL genes and two AtNAS genes under Cd stress(Fig. 6), indicating that MsYSL1 participated in the translocation of Fe, Zn, and Mn from roots to shoots in transgenic Arabidopsis, thus increasing Cd tolerance (Figs. 3a, b, 5). This was in agreement with that the high expression levels of the NAS genes contributed to metal redistribution from roots to shoots (Kim et al. 2005; Klatte et al. 2009), and enhanced Cd resistance (Wu et al. 2012). In addition, in transgenic Arabidopsis, the expression profiles of genes involved in Cd uploading in the vasculature, such as AtHMA2/HMA4 and AtNRAMP4 (Eren and Arguello 2004; Verret et al. 2004; Pottier et al. 2015), were upregulated (Fig. 6). Although the expression level of AtHMA2 in the Y27 line was lower than that of the wild type, and the mRNA levels of AtNRAMP3 were not consistent in the transgenic lines, this might be the result of high AtYSL2 and AtNRAMP4 expression levels. The overexpression of AtHMA4/AtNRAMP4 decreased the Zn and Cd accumulation in roots (Verret et al. 2004; Pottier et al. 2015). This may also explain why the overexpression of MsYSL1 in Arabidopsis increased Cd, Fe, and Mn translocation ratios under Cd stress (Fig. 5f). In addition, two Cd efflux genes, AtDTX1 and AtPDR8 (Li et al. 2002; Kim et al. 2007), were significantly induced by Cd in transgenic Arabidopsis (Fig. 6), indicating that the high Cd tolerance and low Cd accumulation in roots may be partly due to the increase in Cd efflux under Cd stress in transgenic Arabidopsis. However, the specific mechanism of the MsYSL1 overexpression-induced increase in the two Cd efflux gene transcript levels under Cd stress in Arabidopsis is not clear and needs to be further investigated in future studies.
Conclusions
In this study, an YSL1 gene from M. sacchariflorus was cloned and characterized. The heterologous expression of MsYSL1 in yeast indicated that MsYSL1 was a transporter that participated in delivering metal–NA complexes in vivo. The overexpression of MsYSL1 in Arabidopsis enhanced Cd tolerance by decreasing Cd accumulation in roots and increasing Cd translocation from roots to shoots. In addition, the genes related to NA synthesis, metal translocation and long-distance transport, and Cd exclusion were highly induced in transgenic lines under Cd stress, further indicating that MsYSL1 may play a crucial role in Cd detoxification and internal metal reallocation in M. sacchariflorus.
Abbreviations
- YSL:
-
Yellow stripe-like
- PSs:
-
Phytosiderophores
- NAs:
-
Nicotianamines
- NAS:
-
Nicotianamine synthetase
- MAs:
-
Mugineic acids
- DMAs:
-
Deoxymugineic acids
References
Aglawe SB, Fakrudin B, Patole CB, Bhairappanavar SB, Koti RV, Krishnaraj PU (2012) Quantitative RT-PCR analysis of 20 transcription factor genes of MADS, ARF, HAP2, MBF and HB families in moisture stressed shoot and root tissues of Sorghum. Physiol Mol Biol Plants 18:287–300
Aoyama T, Kobayashi T, Takahashi M, Nagasaka S, Usuda K, Kakei Y, Ishimaru Y, Nakanishi H, Mori S, Nishizawa NK (2009) OsYSL18 is a rice iron(III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol Biol 70:681–692
Araki R, Murata J, Murata Y (2011) A novel barley yellow stripe 1-like transporter (HvYSL2) localized to the root endodermis transports metal-phytosiderophore complexes. Plant Cell Physiol 52:1931–1940
Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006) Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem 281:32395–32402
Cao FB, Cai Y, Liu L, Zhang M, He XY, Zhang GP, Wu FB (2015) Differences in photosynthesis, yield and grain cadmium accumulation as affected by exogenous cadmium and glutathione in the two rice genotypes. Plant Growth Regul 75:715–723
Chu HH, Chiecko J, Punshon T, Lanzirotti A, Lahner B, Salt DE, Walker EL (2010) Successful reproduction requires the function of Arabidopsis YELLOW STRIPE-LIKE1 and YELLOW STRIPE-LIKE3 metal-nicotianamine transporters in both vegetative and reproductive structures. Plant Physiol 154:197–210
Chung JH, Kim DS (2012) Miscanthus, as a potential bioenergy crop in east Asia. J Crop Sci Biotechnol 15:65–77
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Conte SS, Chu HH, Chan-Rodriguez D, Punshon T, Vasques KA, Salt DE, Walker EL (2013) Arabidopsis thaliana yellow stripe1-like4 and yellow stripe1-like6 localize to internal cellular membranes and are involved in metal ion homeostasis. Front Plant Sci 4:283
Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL (2001) Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature 409:346–349
Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103:1–11
Das S, Sen M, Saha C, Chakraborty D, Das A, Banerjee M, Seal A (2011) Isolation and expression analysis of partial sequences of heavy metal transporters from Brassica juncea by coupling high throughput cloning with a molecular fingerprinting technique. Planta 234:139–156
DiDonato RJ Jr, Roberts LA, Sanderson T, Eisley RB, Walker EL (2004) Arabidopsis Yellow Stripe-Like2 (YSL2): a metal-regulated gene encoding a plasma membrane transporter of nicotianamine-metal complexes. Plant J 39:403–414
Divol F, Couch D, Conéjéro G, Roschzttardtz H, Mari S, Curie C (2013) The Arabidopsis yellow stripe like4 and 6 transporters control iron release from the chloroplast. Plant Cell 25:1040–1055
Eren E, Arguello JM (2004) Arabidopsis HMA2, a divalent heavy metal-transporting P(1B)-type ATPase, is involved in cytoplasmic Zn2+ homeostasis. Plant Physiol 136:3712–3723
Ezaki B, Nagao E, Yamamoto Y, Nakashima S, Enomoto T (2008) Wild plants, Andropogon virginicus L. and Miscanthus sinensis Anders, are tolerant to multiple stresses including aluminum, heavy metals and oxidative stresses. Plant Cell Rep 27:951–961
Feng SS, Tan JJ, Zhang YX, Liang S, Xiang SQ, Wang H, Chai TY (2016) Isolation and characterization of a novel cadmium-regulated yellow stripe-like transporter (SnYSL3) in Solanum nigrum. Plant Cell Rep 36:1–16
Gendre D, Czernic P, Conéjéro G, Pianelli K, Briat JF, Lebrun M, Mari S (2007) TcYSL3, a member of the YSL gene family from the hyper-accumulator Thlaspi caerulescens, encodes a nicotianamine-Ni/Fe transporter. Plant J 49:1–15
Gietz RD, Schiestl RH (1995) Transforming yeast with DNA. Methods Mol Cell Biol 5:255–269
Guo HP, Hong CT, Chen XM, Xu YX, Liu Y, Jiang DA, Zheng BS (2016a) Different growth and physiological responses to cadmium of the three Miscanthus species. PLoS ONE 11:e0153475
Guo HP, Hong CT, Xiao MZ, Chen XM, Chen HM, Zheng BS, Jiang DA (2016b) Real-time kinetics of cadmium transport and transcriptomic analysis in low cadmium accumulator Miscanthus sacchariflorus. Planta 244:1–14
Higuchi K, Kanazawa K, Nishizawa NK, Chino M, Mori S (1994) Purification and characterization of nicotianamine synthase from Fe-deficient barley roots. Plant Soil 165:173–179
Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, Nishizawa NK (2010) Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J 62:379–390
Kakei Y, Ishimaru Y, Kobayashi T, Yamakawa T, Nakanishi H, Nishizawa NK (2012) OsYSL16 plays a role in the allocation of iron. Plant Mol Biol 79:583–594
Kayama M (2001) Comparison of the aluminum tolerance of Miscanthus sinensis Anderss. and Miscanthus sacchariflorus Bentham in hydroculture. Int J Plant Sci 162:1025–1031
Kim S, Takahashi M, Higuchi K, Tsunoda K, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK (2005) Increased nicotianamine biosynthesis confers enhanced tolerance of high levels of metals, in particular nickel, to plants. Plant Cell Physiol 46:1809–1818
Kim DY, Bovet L, Maeshima M, Martinoia E, Lee Y (2007) The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J 50:207–218
Klatte M, Schuler M, Wirtz M, Fink-Straube C, Hell R, Bauer P (2009) The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol 150:257–271
Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2004) OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J 39:415–424
Lee S, Chiecko JC, Kim SA, Walker EL, Lee Y, Guerinot ML, An G (2009) Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol 150:786–800
Li L, He Z, Pandey GK, Tsuchiya T, Luan S (2002) Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J Biol Chem 277:5360–5368
Lin YF, Aarts MGM (2012) The molecular mechanism of zinc and cadmium stress response in plants. Cell Mol Life Sci 69:3187–3206
Lin H, Fang CX, Li YZ, Lin WW, He JY, Lin RY, Lin WX (2016) Cadmium-stress mitigation through gene expression of rice and silicon addition. Plant Growth Regul 81:1–11
Murata Y, Ma JF, Yamaji N, Ueno D, Nomoto K, Iwashita T (2006) A specific transporter for iron(III)-phytosiderophore in barley roots. Plant J 46:563–572
Nelson BK, Cai X, Nebenfuhr A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51:1126–1136
Pottier M, Oomen R, Picco C, Giraudat J, Scholz-Starke J, Richaud P, Carpaneto A, Thomine S (2015) Identification of mutations allowing natural resistance associated macrophage proteins (NRAMP) to discriminate against cadmium. Plant J 83:625–637
Sasaki A, Yamaji N, Xia J, Ma JF (2011) OsYSL6 is involved in the detoxification of excess manganese in rice. Plant Physiol 157:1832–1840
Sasaki A, Yamaji N, Ma JF (2014) Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. J Exp Bot 65:6013–6021
Schaaf G, Ludewig U, Erenoglu BE, Mori S, Kitahara T, Von Wirén N (2004) ZmYS1 functions as a proton-coupled symporter for phytosiderophore- and nicotianamine-chelated metals. J Biol Chem 279:9091–9096
Schaaf G, Schikora A, Häberle J, Vert G, Ludewig U, Briat JF, Curie C, Von Wirén N (2005) A putative function for the Arabidopsis Fe-phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant Cell Physiol 46:762–774
Senoura T, Sakashita E, Kobayashi T, Takahashi M, Aung MS, Masuda H, Nakanishi H, Nishizawa NK (2017) The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol Biol 95:375–387
Singh S, Singh A, Bashri G, Prasad SM (2016) Impact of cd stress on cellular functioning and its amelioration by phytohormones: an overview on regulatory network. Plant Growth Regul 80:253–263
Takahashi M, Yamaguchi H, Nakanishi H, Shioiri T, Nishizawa NK, Mori S (1999) Cloning two genes for nicotianamine aminotransferase, a critical enzyme in iron acquisition (Strategy II) in graminaceous plants. Plant Physiol 121:947–956
Verret F, Gravot A, Auroy P, Leonhardt N, David P, Nussaume L, Vavasseur A, Richaud P (2004) Overexpression of AtHMA4 enhances root-to-shoot translocation of zinc and cadmium and plant metal tolerance. FEBS Lett 576:306–312
Wang JW, Li Y, Zhang YX, Chai TY (2013) Molecular cloning and characterization of a Brassica juncea yellow stripe-like gene, BjYSL7, whose overexpression increases heavy metal tolerance of tobacco. Plant Cell Rep 32:651–662
Waters BM, Chu HH, DiDonato RJ, Roberts LA, Eisley RB, Lahner B, Salt DE, Walker EL (2006) Mutations in Arabidopsis Yellow Stripe-Like1 and Yellow Stripe-Like3 reveal their roles in metal ion homeostasis and loading of metal ions in seeds. Plant Physiol 141:1446–1458
Wu HL, Chen CL, Du J, Liu HF, Cui Y, Zhang Y, He YJ, Wang YQ, Chu CC, Feng ZY, Li JM, Ling HQ (2012) Co-overexpression FIT with AtbHLH38 or AtbHLH39 in Arabidopsis enhanced cadmium tolerance via increased cadmium sequestration in roots and improved iron homeostasis of shoots. Plant Physiol 158:790–800
Xu YX, Zhang SN, Guo HP, Wang SK, Xu LG, Li CY, Qian Q, Chen F, Geisler M, Qi YH, Jiang DA (2014) OsABCB14 functions in auxin transport and iron homeostasis in rice (Oryza sativa L.). Plant J 79:106–117
Yang J, Chen JQ, Chen X, Ma G, Wang P, Fabrice MR, Zhang SL, Wu JY (2016) Phylogenetic and expression analysis of pear yellow stripe-like transporters and functional verification of PbrYSL4 in pear pollen. Plant Mol Biol Rep 34:737–747
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Routine procedures for growing rice plants in culture solution, 3rd edn. International Rice Research Institute, Los Banos
Zhang J, Yang SY, Huang YJ, Zhou SB (2015) The tolerance and accumulation of Miscanthus sacchariflorus (maxim.) Benth., an energy plant species, to cadmium. Int J Phytoremediat 17:538–545
Zheng LQ, Fujii M, Yamaji N, Sasaki A, Yamane M, Sakurai I, Sato K, Ma JF (2011) Isolation and characterization of a barley yellow stripe-like gene, HvYSL5. Plant Cell Physiol 52:765–774
Zheng LQ, Yamaji N, Yokosho K, Ma JF (2012) YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice. Plant Cell 24:3767–3782
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31571577, 31371591) and the National Science and Technology Support Plan of China (Grant No. 2012BAC09B01).
Author information
Authors and Affiliations
Contributions
CHM, GHP and JDA conceived and designed the experiments. CHM and ZC performed the experiments. CHM, HYM and HY analyzed the data. CHM, GHP and JDA wrote the paper. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
These authors have declared that no competing interests exist.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Zhang, C., Guo, H. et al. Overexpression of a Miscanthus sacchariflorus yellow stripe-like transporter MsYSL1 enhances resistance of Arabidopsis to cadmium by mediating metal ion reallocation. Plant Growth Regul 85, 101–111 (2018). https://doi.org/10.1007/s10725-018-0376-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-018-0376-6