Abstract
Plasma membrane (PM) vesicles were isolated from developing embryos of wheat (Triticum aestivum L.) with different drought-tolerance under drought stress by the gradient centrifugation method. The activity of the PM H+-ATPase (EC 3.6.1.35) and contents of polyamine conjugated (covalently and noncovalently) to the PM vesicles were investigated. Results showed that after drought treatment for 3 d, embryo relative water content (ERWC), embryo relative dry weight increase rate (ERDWIR) of drought-sensitive Yumai No. 48 cultivar decreased more significantly than those of drought-tolerant Luomai No. 22 cultivar, while PM H+-ATPase activity, noncovalently conjugated (NCC) spermidine (Spd) and NCC spermine (Spm), the covalently conjugated (CC) putrescine (Put) and CC Spd of PM from Luomai No. 22 cultivar increased more obviously than those from Yumai No. 48 cultivar. As judged by increases in ERWC and ERDWIR, treatment with exogenous Spd alleviated markedly drought injuries to Yumai No. 48, coupled with significant increases in NCC Spd and NCC Spm levels and H+-ATPase activity in the embryo PM vesicle. Under drought stress, the treatment of drought-tolerant Luomai No. 22 cultivar with methylglyoxyl-bis (guanylhydrazone) (MGBG), an inhibitor of S-adenosylmethionine decarboxylase (SAMDC), and phenanthrolin (o−Phen), an inhibitor of transglutaminase (TGase) respectively, caused a decrease of the NCC Spd, NCC Spm, CC Put and CC Spd. Those decreases were associated with decreased PM-H+-ATPase activity and the tolerance of developing wheat embryos to osmotic stress, as judged by decreases in ERWC and ERDWIR. These results suggest that tolerance of the developing wheat embryos to drought stress is associated with the embryo PM H+-ATPase and the levels of NCC Spd, NCC Spm, CC Put and CC Spd in embryo PM vesicles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polyamines (PAs) are biologically aliphatic amines. In plants, PAs include mainly putrescine (Put), spermidine (Spd) and spermine (Spm). The synthesis of Spd and Spm is catalyzed by Spd synthase and Spm synthase via incorporation of aminopropyl to Put and Spd, respectively. S-adenosylmethionine decarboxylase (SAMDC) catalyzes the transformation of S-adenosylmethionine to decarboxylated S-adenosylmethionine, a donor of the aminopropyl to Put and Spd. The Spd and Spm biosynthesis is mainly regulated by SAMDC (Tiburcio et al. 1993). Methylglyoxal-bis (guanylhydrazone) (MGBG) is the potent inhibitor of SAMDC (Slocum 1991). PAs are involved in many aspects of growing and development (Adiga and Prasad 1985; Martin-Tanguy 2001; Iqbal and Ashraf 2005; Cao et al. 2010; Niemenak et al. 2012; Cacho et al. 2013). Furthermore, many studies show that PAs are closely associated with abiotic stress such as osmotic, drought, salt and heavy metal stress, etc. (Tang and Newton 2005; Liu et al. 2006; Goyal and Asthir 2010; Qiao et al. 2012; Do et al. 2013; Grzesiak et al. 2013). Due to their positive charges at physiological pH, PAs are able to bind to macromolecules such as DNA, RNA, and proteins by hydrogen and ionic bonding (Sood and Nagar 2003). By being conjugated to proteins in the Plasma membrane (PM), they could be transformed into noncovalently conjugated PAs (NCC PAs), which are considered to play an important role in stabilizing the function of protein and biomembrane for stress adaptation (Galston and Kaur-Sawhney 1995). Besides the two form PAs mentioned above, by action of Transglutaminase (TGase, E.C. 2.3.2.13), which covalently links free PAs to endoglutamines of proteins, PAs could be transformed into covalently conjugated PAs (CC PAs). Phenanthrolin (o−Phen) is a potent inhibitor of TGase. CC PAs play important roles in the process of post-translational modifications of proteins (Del Duca et al. 1995) and facilitate wheat seedling tolerance to osmotic stress (Liu et al. 2004).
The plant PM H+-ATPase (E.C. 3.6.1.35), P-type ATPase, is a proton pump with ATP hydrolyzing, which plays a key role in plant growth and development and controls lots of cellular processes, such as secondary active transport, which is correlated with cell pH, turgor, and stress adaption (Surowy and Boyer 1991; Sailerova and Zwiazek 1993; Michelet et al. 1994; Michelet and Boutry 1995). Structural analysis indicates that the C-terminus of the P-type H+-ATPase is an auto-inhibitory domain, which is removed with the enzyme to be activated (Palmgren et al. 1991; Johansson et al. 1993). The P-type H+-ATPase is regulated at various levels such as the pre-transcriptional, transcriptional, and post-transcriptional levels (Sussman and Harper 1989; Sussman 1994) and modulated by calcium ions, abscisic acid, auxin, cGMP, light, phospholipids, kinases, and various environmental factors, such as drought, cold, salt, etc. (Serrano 1989; Kinoshita et al. 1995; Suwastika and Gehring 1999; Peng et al. 2003). Therefore, PM H+-ATPase is thought to be one of the important enzymes which are involved in plant response to environmental stresses. Under drought stress, the reports on changes in PM- H+-ATPase activity in plants are inconsistent. Some research indicates that PM H+-ATPase activity is enhanced (Michelet et al. 1994), while other reports demonstrate that its activity is inhibited (Qiu and Zhang 2000).
Drought is one of the main environmental stresses which influence crop growth and development and wheat seed is frequently subjected to drought stress during development. However, to our knowledge, the relationship between PM H+-ATPase activity and levels of noncovalently conjugated (NCC) and CC PAs in PM, purified from developing wheat embryos under drought stress, remains to be elucidated. So, in the experiments presented here, two wheat (Triticum aestivum L.) cultivars (drought-sensitive Yumai No. 48 cv. and drought-tolerant Luomai No. 22 cv.) were used as materials, and we detected PM- H+-ATPase activity and the two conjugated PAs (NCC and CC PAs) in PM vesicles purified from developing wheat embryos subjected to drought stress. The main aim of this work is to elucidate the significances of NCC PAs and CC PAs in PM of wheat embryos under drought stress. A possible relationship between the activity of PM H+-ATPase and the function of the two forms of conjugated PAs is discussed.
Materials and methods
Material and treatments
Wheat (Triticum aestivum L. cv. Luomai No. 22, drought-tolerant; cv. Yumai No. 48, drought-sensitive) seed surface was sterilized in 0.1 % HgCl2 (w/v) for 5 min before they were rinsed with water, and then germinated in pots (30 seeds/pot) (bottom diameter:rim diameter: height: 35: 40: 50 cm) containing nutrient-rich and water–normal topsoil. After the wheat seedlings were vernalized, the pots with seedlings were placed in an environment greenhouse with a temperature of 25/15 °C (day/night), air humidity of 70 %, and 16 h photoperiod at quantum flux density of 600 μmol m−2 s−1 from lamps. On the 10th day after fertilization, the growing wheat plants were treated as followings: (1) Wheat roots were treated under −1.0 MPa soil drought stress; (2) Wheat roots were treated under −1.0 MPa soil drought stress and the wheat flag leaves and ears were sprayed with Spd (1 mM); (3) Wheat roots were treated under −1.0 MPa soil drought stress and the wheat flag leaves and ears were sprayed with MGBG (1 mM); (4) Wheat roots were treated under −1.0 MPa soil drought stress and the wheat flag leaves and ears were sprayed with o−Phen (1 mM). Spd, MGBG, and o−Phen were purchased from Sigma Chemical Co. The materials, of which wheat roots were treated with water–normal soil (−0.15 MPa) and the wheat flag leaves and ears were sprayed with distilled water, were as control group materials. Soil water potential was detected by TEN60 Water Potential Instrument. Wheat flag leaves and ears were sprayed with the reagents mentioned above by 20 ml/pot at 6:00 and 18:00 h per day. After treatment for 3 d, the developing seed embryos were sampled and tested.
Determination of embryo relative water content (ERWC)
Embryo relative water content (ERWC) was calculated according to the following formula: ERWC (%) = (Wf−Wd)/(Wt−Wd) × 100 (Wf, Wd, and Wt represents the embryo fresh weight, dry weight and saturation weight, respectively).
Determination of embryo relative dry weight increase rate (ERDWIR)
Growth rate of embryo dry weight was calculated by the following formula: GR = (Wa−Wb)/Wb (Wa and Wb represents the dry weight of the embryos after 3 and 0 d of treatments, respectively). And then, to counteract the diversity of different cultivar, embryo relative dry weight increase rate (ERDWIR) of every cultivar with different treatment was calculated by the following formula: ERDWIR (%) = (GR of treatment/GR of control) × 100 (Where treatment and control were the same cultivar).
Preparation of embryo PM vesicles and H+-ATPase activity determination
Embryo PM vesicles were purified by sucrose gradient centrifugation methods according to the process described by Qiu and Su (1998) with slight modifications as follows. 2 g fresh embryos were cut into pieces and homogenized in 10 ml of ice-cold extracting solution containing 100 mM KCl, 200 mM sorbitol, 4 mM EGTA, 1 mM PMSF, 2 mM DTT, 15 % (v/v) glycerol, 1.5 % (w/v) PVP, 0.2 % (w/v) BSA, 200 mM K2S2O5 and 60 mM Tris-Hepes (pH 7.8). The homogenate was filtered through 4 layers of gauze and centrifugated at 12,000×g for 20 min. The supernatant was centrifugated for 35 min at 80,000×g to get microsomal membrane precipitate, which was slightly suspended in a buffer containing 0.2 M sucrose, 15 mM KCl, 2 mM DTT, 2 mM EGTA, 0.2 % (w/v) BSA and 2.5 mM Hepes titrated to pH 7.5 with Tris.
The suspending microsomal membrane was layered in dis-continuous sucrose gradient solutions, which consisted of 34 and 41 % (w/w) sucrose layers, containing 2 mM DTT and 2.5 mM Hepes titrated to pH 7.5 with Tris. Then the mixture was centrifugated at 120,000×g for 100 min. The fraction was collected at the interface of 34–41 % sucrose solution and diluted fourfold with the suspension buffer mentioned above. Then the suspension was centrifugated at 75,000×g for 35 min. The PM pellet was then collected. The purity of the PM vesicles was estimated following the method described by Widell and Larsson (1990). The PM, tonoplast and mitochondria H+-ATPase are characterized by vanadate, nitrate and azide inhibition, respectively. In the present research, the H+-ATPase activity of prepared PM vesicle was inhibited by vanadate more than 80 %, and inhibited by nitrate and azide <2 and 1.5 %, respectively, which demonstrated that the purity of isolated PM vesicle was very high.
H+-ATPase activity was assayed by measuring inorganic phosphate from ATP following the method described by Qiu and Su (1998) and Ohinishi et al. (1975) with minor modification. The reaction solution contained 4 mM ATP-Na2, 2.5 mM MgSO4, 0.2 mM Na2MoO4, 1.5 mM NaN3, 60 mM KNO3, 30 mM Hepes titrated to pH 6.5 with Tris and 8 μg proteins membrane vesicles. After 35 min incubation at 37 °C, 15 % (w/v) TCA quenched the reaction.
Determination of PM proteins
To isolate the protein, the prepared PM vesicle solution was added to Triton X-100 1 % (v/v). The solution was then supersonic-treated three times for 20 s with an ultrasonic disintegrator (model 200-w), kept in an ice bath for 35 min, and centrifugated at 22,000×g for 25 min at 4 °C (Zhao et al. 2000). The content of the soluble membrane protein in the supernatant was determined with the method described by Bradford (1976), BSA being standard.
Determination of conjugated PA
Conjugated PAs were quantified following the method described by Sharma and Rajam (1995) with minor modification. PCA was dropwise added to the membrane protein extract mentioned above to 5 % terminal concentration, and then the solution was centrifugated at 30,000×g for 45 min. The precipitate was re-suspended in 5 % PCA, mixed with equal volume of 12 N HCl in ampoule, hydrolyzed at 110 °C for 24 h after the ampoule was sealed, and dried at 70 °C after filtration. The pellet was re-dissolved in 2 ml 5 % PCA. The solution contained the CC PAs.
After 2 ml 5 % PCA was added to the purified membrane vesicles, it was centrifugated at 28,000×g for 35 min. The supernatant contained the NCC PAs. The CC and NCC PAs in two solutions above were derivatized with benzoyl chloride respectively following the method described by Di Tomaso et al. (1989) and quantified by HPLC (Waters 2695 with automatic sample injector), with 254 nm as PA detecting wavelength, C-18 reverse-phase column as separation column and 1,6-hexanediamine as an internal standard.
Cultivation and treatment of young wheat embryos, in vitro
To verify the aforementioned experiments, in vivo, the following experiments, in vitro, were carried out. On the 10th day after fertilization, young wheat embryos were taken out from developing seed, and inoculated onto sterile MS solid medium. After cultivation for 3 d, the embryos were treated with 20 % PEG-6000 (−0.5 MPa), 20 % PEG (−0.5 MPa) + Spd (0.5 mM), 20 % PEG (−0.5 MPa) + MGBG (0.5 mM) and 20 % PEG (−0.5 MPa) + o−Phen (0.5 mM) for 2 d, respectively and control embryos were kept on sterile MS solid medium without PEG, Spd, MGBG or o−Phen. Then, the treated and control embryos were sampled for assays of H+-ATPase activity and conjugated PAs in PM following the methods mentioned above.
Statistical analysis
The experiment was repeated three times and 3 samples were taken every experiment. Data were analysed using software of SPSS 10.0 and Microsoft Excel. Values reported in the paper are means ± stand error (SE) of three independent tests. Significant differences among the samples were determined by Duncan’s multiple range tests at a significance level of 0.05.
Results
Effects of drought stress, exogenous Spd, MGBG and o−Phen on ERWC and ERDWIR of developing wheat embryos
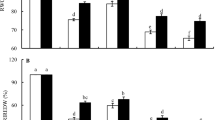
Resistance to drought stress has been associated with the higher ERWC and ERDWIR of stressed plants (Schonfeld et al. 1988). Drought stress for 3 d caused decreases of ERWC and ERDWIR of two wheat cultivars, Yumai No. 48 and Luomai No. 22, and the changes in the former cultivar were more apparent than those in the latter (Fig. 1). This result proved that Luomai No. 22 was drought-tolerant and Yumai No. 48 was drought-sensitive. Treatment of Yumai No. 48 with exogenous Spd alleviated the drought injury, as judged by increases in ERWC and ERDWIR, while exogenous Spd affected the resistance of Luomai No. 22 slightly. Drought injury to Luomai No. 22 was greater compared to that of Yumai No. 48 after treatment with MGBG, as judged by the larger decreased ranges of ERWC and ERDWIR (Fig. 1). O−Phen treatment enhanced the drought-induced decreases of ERWC and EDWIR of both cultivars, and the effect on Yumai No. 48 was slighter than that on Luomai No. 22 (Fig. 1).
Effects of drought stress, exogenous Spd, MGBG and o−Phen on ERWC (a) and ERDWIR (b) of developing wheat embryos. On the 10th day after fertilization, the wheat roots were treated under −1.0 MPa soil drought stress and the wheat roots with water–normal soil were as control group. The wheat flag leaves and ears were sprayed with Spd (1 mM), MGBG (1 mM) and o−Phen (1 mM), respectively. The control group was sprayed with distilled water. After treatment for 3 d, the developing seed embryos were sampled and tested. Each value in the figure represents the mean of three experiments ± SE (standard error), error bars indicate SE (n = 9), and different letters (a–f) above the column are significantly different at P < 0.05 (Duncan’s multiple range tests). DR, drought stress treatment; DR + Spd, treatment with drought stress and Spd (by spraying 1 mM) simultaneously; DR + MGBG, treatment with drought stress and MGBG (by spraying 1 mM) simultaneously; DR + o−Phen, treatment with drought stress and o−Phen (by spraying 1 mM) simultaneously
To elucidate the mechanism of presentation above, the following tests were presented.
Effects of drought stress, exogenous Spd, MGBG on NCC PA contents in PM vesicles from developing wheat embryos
Drought stress brought about increases in contents of NCC Put, NCC Spd and NCC Spm in PM vesicles purified from both wheat cultivar embryos (Fig. 2a, b, c), and the contents of NCC Spd and NCC Spm of Luomai No. 22 increased more markedly than those of Yumai No. 48 (Fig. 2b, c). Exogenous Spd treatment caused a significant increase in the contents of NCC Spd and NCC Spm in PM vesicles from Yumai No. 48 embryos under drought stress and the effects of Spd on Luomai No. 22 were slight (Fig. 2b, c). MGBG treatment caused a significant reduction in the contents of NCC Spd and NCC Spm in PM vesicles from the developing embryos of Luomai No. 22 under drought stress and the effect of MGBG on Yumai No. 48 was slight (Fig. 2b, c).
Effects of drought stress, exogenous Spd, MGBG on NCC Put (a), NCC Spd (b) and NCC Spm (c) contents in PM vesicles from developing wheat embryos. On the 10th day after fertilization, the wheat roots were treated under −1.0 MPa soil drought stress and the wheat roots with water–normal soil were as control group. The wheat flag leaves and ears were sprayed with Spd (1 mM) and MGBG (1 mM), respectively. The control group was sprayed with distilled water. After treatment for 3 d, the developing seed embryos were sampled and tested. Each value in the figure represents the mean of three experiments ± SE (standard error), error bars indicate SE (n = 9), and different letters (a–d) above the column are significantly different at P < 0.05 (Duncan’s multiple range tests). DR, drought stress treatment; DR + Spd, treatment with drought stress and Spd (by spraying 1 mM) simultaneously; DR + MGBG, treatment with drought stress and MGBG (by spraying 1 mM) simultaneously
Effects of PEG, exogenous Spd, MGBG on NCC PA contents in PM vesicles from wheat embryos developing on MS solid medium, in vitro
To elucidate further the changes in NCC PAs from PM vesicles, we used wheat embryos developing on the MS solid medium as experiment material. Following PEG treatment for 2 d, NCC Spd (Fig. 3b) and NCC Spm (Fig. 3c) in PM vesicles from embryos of Luomai No. 22 cv. increased more significantly than those of Yumai No. 48 cv. Exogenous Spd enhanced the osmotic-induced increase in NCC Spd (Fig. 3b) and NCC Spm (Fig. 3c) contents of Yumai No. 48 cv., and MGBG inhibited the osmotic-induced increase in NCC Spd (Fig. 3b) and NCC Spm (Fig. 3c) contents of Luomai No. 22 cv. (Fig. 3). The results, in vitro, were consistent with those, in vivo.
Effects of PEG, exogenous Spd, MGBG on NCC Put (a), NCC Spd (b) and NCC Spm (c) contents in PM vesicles from wheat embryos developing on MS solid medium, in vitro. On the 10th day after fertilization, young wheat embryos were taken out from developing seed and inoculated onto MS solid medium. After cultivation for 3 d, the embryos were treated with PEG (−0.5 MPa), PEG (−0.5 MPa) + Spd (0.5 mM), and PEG (−0.5 MPa) + MGBG (0.5 mM) for 2 d, respectively and control embryos were kept on MS solid medium without PEG, Spd or MGBG. Then, the treated and control embryos were sampled for assays of NCC PAs in PM. Each value in the figure represents the mean of three experiments ± SE (standard error), error bars indicate SE (n = 9), and different letters (a–d) above the column are significantly different at P < 0.05 (Duncan’s multiple range tests). PEG, treatment with PEG; PEG + Spd, treatment with PEG and Spd simultaneously; PEG + MGBG, treatment with PEG and MGBG simultaneously
Effects of drought stress and o−Phen on CC PAs contents in PM vesicles from developing wheat embryos
The contents of CC Put and CC Spd could be detected in PM vesicles purified from embryos of both cultivars under drought stress (Fig. 4), while the content of CC Spm was too low to be detected. Drought stress treatment markedly elevated the contents of CC Put (Fig. 4a) and CC Spd (Fig. 4b) of Luomai No. 22, but had a little effect on the levels of CC Put (Fig. 4a) and CC Spd of Yumai No. 48 (Fig. 4b). The treatment with o−Phen inhibited the drought stress-induced increases in CC Put (Fig. 4a) and CC Spd (Fig. 4b) contents in PM from the embryos of both cultivars and the effect of o−Phen on drought-treated Luomai No. 22 was greater than that observed on drought-treated Yumai No. 48 (Fig. 4).
Effects of drought stress and o−Phen on CC Put and CC Spd contents in PM vesicles from developing wheat embryo. On the 10th day after fertilization, the wheat roots were treated under −1.0 MPa soil drought stress and the wheat roots with water–normal soil were as control group. The wheat flag leaves and ears were sprayed with o−Phen (1 mM). The control was sprayed with distilled water. After treatment for 3 d, the developing seed embryos were sampled and tested. Each value in the figure represents the mean of three experiments ±SE (standard error), error bars indicate SE (n = 9), and different letters (a–d) above the column are significantly different at P < 0.05 (Duncan’s multiple range tests). DR, drought stress treatment; DR + o−Phen, treatment with drought stress and o−Phen (by spraying 1 mM) simultaneously
Effects of PEG and o−Phen on CC PA contents in PM vesicles from wheat embryos developing on MS solid medium, in vitro
To verify further the changes in CC PAs from PM vesicles, we used wheat embryos developing on MS solid medium as experiment material. Following PEG treatment for 2 d, CC Put (Fig. 5a) and CC Spd (Fig. 5b) in PM vesicles from the embryos of Luomai No. 22 cv. increased more significantly than those of Yumai No. 48 cv. O−Phen inhibited markedly the osmotic-induced increase in CC Put and CC Spd contents of Luomai No. 22 cv. (Fig. 5). These results in vitro were consistent with those, in vivo, as noted above.
Effects of PEG and o−Phen on CC Put and CC Spd contents in PM vesicles from wheat embryos developing on MS solid medium, in vitro. On the 10th day after fertilization, young wheat embryos were taken out from developing seed and inoculated onto MS solid medium. After cultivation for 3 d, the embryos were treated with PEG (−0.5 MPa) and PEG (−0.5 MPa) + o−Phen (0.5 mM) for 2 d, respectively, and control embryos were kept on MS solid medium without PEG or o−Phen. Then, the treated and control embryos were sampled for assays of CC PAs in PM. Each value in the figure represents the mean of three experiments ± SE (standard error), error bars indicate SE (n = 9), and different letters (a–c) above the column are significantly different at P < 0.05 (Duncan’s multiple range tests). PEG, treatment with PEG; PEG + o−Phen, treatment with PEG and o−Phen simultaneously
Effects of drought stress, exogenous Spd, MGBG and o−Phen on PM H+-ATPase activity of developing wheat embryos
PM-H+-ATPase activity in embryos of Luomai No. 22 increased more significantly than that of Yumai No. 48 (Fig. 6) in response to drought stress. Exogenous Spd treatment enhanced the increase of PM-H+-ATPase activity in embryos of Yumai No. 48 subjected to drought stress, and MGBG and o−Phen treatments inhibited the drought stress-induced increases of PM-H+-ATPase activity in Luomai No. 22 embryos to a markedly greater extent than in Yumai No. 48 (Fig. 6).
Effects of drought stress, exogenous Spd, MGBG and o−Phen on PM H+-ATPase activity of developing wheat embryos. On the 10th day after fertilization, the wheat roots were treated under −1.0 MPa soil drought stress and the wheat roots with water–normal soil were as control group. The wheat flag leaves and ears were sprayed with Spd (1 mM), MGBG (1 mM) and o−Phen (1 mM), respectively. The control group was sprayed with distilled water. After treatment for 3 d, the developing seed embryos were sampled and tested. Each value in the figure represents the mean of three experiments ±SE (standard error), error bars indicate SE (n = 9), and different letters (a–d) above the column are significantly different at P < 0.05 (Duncan’s multiple range tests). DR, drought stress treatment; DR + Spd, treatment with drought stress and Spd (by spraying 1 mM) simultaneously; DR + MGBG, treatment with drought stress and MGBG (by spraying 1 mM) simultaneously; DR + o−Phen, treatment with drought stress and o−Phen (by spraying 1 mM) simultaneously
Effects of PEG, exogenous Spd, MGBG and o−Phen on PM H+-ATPase activity from wheat embryos developing on MS solid medium, in vitro
To verify further the change in PM-H+-ATPase activity from embryos, we used wheat embryos developing on MS solid medium as experiment material. Following PEG treatment for 2 d, PM-H+-ATPase activity in embryos of Luomai No. 22 increased more markedly than that of Yumai No. 48 (Fig. 7). Under PEG stress, the treatment with exogenous Spd increased PM-H+-ATPase activity in the embryos of Yumai No. 48 more than in the embryos of Luomai No. 22, while MGBG and o−Phen inhibited markly the osmotic-induced increases of PM H+-ATPase activity in Luomai No. 22 (Fig. 7). These results, in vitro, were in accord with those, in vivo, as mentioned above.
Effects of PEG, exogenous Spd, MGBG and o−Phen on PM H+-ATPase activity from wheat embryos developing on MS solid medium, in vitro. On the 10th day after fertilization, young wheat embryos were taken out from developing seed and inoculated onto MS solid medium. After cultivation for 3 d, the embryos were treated with PEG (−0.5 MPa), PEG (−0.5 MPa) + Spd (0.5 mM), PEG (−0.5 MPa) + MGBG (0.5 mM), and PEG (−0.5 MPa) + o−Phen (0.5 mM) for 2 d, respectively and control embryos were kept on MS solid medium without PEG, Spd, MGBG or o−Phen. Then, the treated and control embryos were sampled for assays of H+-ATPase activity in PM. Each value in the figure represents the mean of three experiments ± SE (standard error), error bars indicate SE (n = 9), and different letters (a–c) above the column are significantly different at P < 0.05 (Duncan’s multiple range tests). PEG, treatment with PEG; PEG + Spd, treatment with PEG and Spd simultaneously; PEG + MGBG, treatment with PEG and MGBG simultaneously; PEG + o−Phen, treatment with PEG and o−Phen simultaneously
Discussion
Relationship between drought stress and the contents of two forms of conjugated PAs in PM from developing wheat embryos under drought stress
It has been well-documented that PAs are associated with plant tolerance to various abiotic stress (Liu et al. 2004; Goyal and Asthir 2010; Qiao et al. 2012; Do et al. 2013; Grzesiak et al. 2013; Li et al. 2013). Furthermore, PAs are essential for normal seed development and active polyamine metabolism occurs in this period (Astarita et al. 2003; Uranoa et al. 2005; Cao et al. 2010). Because of their poly-cationic nature at physiological pH, PAs bind strongly to negative charges in cellular components such as nucleic acids, proteins, and phospholipids (Gupta et al. 2013) to form NCC PAs. In the present research, it was suggested that NCC Spd and NCC Spm might involve in the tolerance of wheat embryos to drought stress (Fig. 2). The outcome of additional experiments with exogenous Spd and MGBG treatments supported this suggestion (Fig. 2). The results of experiments, in vitro, also corroborated the suggestion (Fig. 3). Our finding is consistent with previously published findings mentioned below. With more positive charges, Spd and Spm bind noncovalently to protein and phospholipids more easily than Put (Dutra et al. 2013). Despite the fact that the precise role of Put in environmental stress tolerance is still controversial, there are many reports emphasizing the protective role for Spd and Spm against drought stress in plants (Yamaguchi et al. 2007; Kubis 2008). Farooq et al. (2009) have argued that among the PAs, Spm is the most effective in improving drought tolerance. However, more in depth study is required to come to a comprehensive conclusion regarding regulation of PA metabolism in drought stress.
In plants, other than free and NCC PAs, PAs could covalently link to endoglutamines of proteins by action of TGase to be transformed into CC PAs, which stabilize the configuration and function of proteins (Del Duca et al. 1995). In this work, we observed that drought stress brought about more significant increases in CC Put and CC Spd levels in PM of drought-tolerant wheat embryos (Fig. 4), which is indicative of possible involvement of CC Put and CC Spd in the tolerance of wheat embryos to drought stress. The outcome of the additional experiment with o−Phen treatment supported this finding (Fig. 4) and the results of experiments, in vitro, also corroborated it (Fig. 5). The finding of Del Duca et al. (1995) is consistent with ours. To our knowledge, the exact mechanism by which CC PAs mediate plant growth and development under drought stress remains be ascertained. Therefore, in the future, with the availability of cutting-edge imaging and genomic techniques, the study of the arena would be interesting and significant.
Relationship between the activity of H+-ATPase and the contents of two forms of conjugated PAs in PM from developing wheat embryos under drought stress
To further elucidate the function of the two conjugated PAs, the activity of H+-ATPase in PM was detected. In the present research, drought treatment brought about a more significant increase of H+-ATPase activity in PM from drought-tolerant Luomai No. 22 embryos than that from drought-sensitive Yumai No. 48 (Fig. 6). The result suggested a possible involvement of PM H+-ATPase in drought stress tolerance of wheat embryos. The suggestion is consistent with the result of previous work (Surowy and Boyer 1991; Michelet et al. 1994).
The regulatory mechanism of H+-ATPase activity needs to be further studied. There have been extensive researches concerning the capacity of PAs to enhance the membrane-associated enzyme activities (Srivastava and Rajbabu 1983; Reggiani et al. 1992; Lester 2000; Janicka-Russak et al. 2010), and Zepeda-Jazo et al. (2011) reported that PAs improve Ca2+ and K+ transport functions. The regulatory mechanism by which PAs regulate H+-ATPase activity remains to be answered. In our research, we found that drought treatment brought about significant increases of not only the contents of NCC Spd, NCC Spm (Fig. 2), CC Put and CC Spd (Fig. 4), but also H+-ATPase activity (Fig. 6) in PM of the drought-tolerant Luomai No. 22 embryos. These results are indicative of possible involvement of NCC Spd, NCC Spm, CC Put and CC Spd in the association with H+-ATPase activity in PM. This notion was supported by the results of additional experiments with the treatments of exogenous Spd and inhibitors, MGBG and o−Phen (Figs. 2, 4, 6). The results of the experiments, in vitro (Figs. 3, 5, 7), were consistent with the results noted above. Statistical analysis indicated that the sum of NCC Spd and NCC Spm was markedly positively correlated with the H+-ATPase activities in PM (r0.05 = 0.95, r0.01 = 0.83, n = 8), and statistical analysis indicated that there was a markedly positive correlation between H+-ATPase activity and the sum of contents of CC Put and CC Spd in PM (r0.05 = 0.82, r0.01 = 0.81, n = 6).
The reason why NCC Spd and NCC Spm, but not NCC Put could enhance the H+-ATPase activity in PM is to be attributed to their cationic nature at physiological pH, as suggested by Sood and Nagar (2003). With more positive charges, NCC Spd and NCC Spm could modulate H+-ATPase activity by noncovalently binding to protein more easily to affect its configuration and function than NCC Put. The C-terminal end of H+-ATPase in PM plays a role as an auto-inhibitory regulatory domain (Palmgren et al. 1991; Johansson et al. 1993; Svennelid et al. 1999; Jelich et al. 2001) and it is hypothesized that factors which increase H+-ATPase activity are likely to have the domain as their ultimate target. For example, H+-ATPase in PM is activated due to the binding of 14-3-3 protein to its C-terminal amino acids (Svennelid et al. 1999; Jelich et al. 2001). Furthermore, it is suggested that PAs, especially Spd and Spm, could activate 14-3-3 protein by noncovalently binding acidic residues in the loop 8 of 14-3-3 protein to neutralize the negative charge (Athwal and Huber 2002). Our finding is consistent with the researches of Garufi et al. (2007) and Shen and Huber (2006), which revealed that among the different PAs, Spm brings about more stimulation of the H+-ATPase activity and this effect is due to an increase in 14-3-3 levels associated with the enzyme. This implies that Spd and Spm with more positive charges bind noncovalently to 14-3-3 protein more easily. However, the report of Dutra et al. (2013) indicated that PAs reduces the activities of proton pumps, such as H+-ATPase in embryogenic suspension cultures of Araucaria angustifolia. The diversity might be attributed to different plant species, organ, tissue, and the development stage. Although studies on the mode of action of PAs on the proton pumps remain to be in-depth in plants, we can suggest that PAs could modulate the activity of proton pumps due to its poly-cationic nature, involving a phosphorylation cascade and 14-3-3 dependent modulation, which is also complicated. For example, Camoni et al. (2012) reported that binding of phosphatidic acid to 14-3-3 proteins hampers their ability to activate the plant PM H+-ATPase. Besides the reason discussed above, Spd and Spm might affect the PM’s physical state by noncovalently conjugating to membrane phospholipids with negative charges. The change in the PM physical state is associated with H+-ATPase activity in PM (Zhang et al. 2002).
As to CC PAs, it is reported (Del Duca et al. 1995) that PAs could have an important function in chloroplasts by being covalently conjugated to endo-glutamyl residues of the chlorophyll a/b antenna complex, CP26, CP24, CP26 and the large subunit of Rubisco. The conversion of free PAs to CC PAs, forming protein-Glu-PAs and protein–Glu-PAs-protein, could stabilize the configuration and function of PM proteins (including H+-ATPase, of course) by preventing these proteins from denaturing under drought stress and thus promote the activity of these enzymes. Obviously, the effect of conjugated PAs on H+-ATPase activity in PM is complex, interesting, and deserves further investigation. Further studies underling the proton pumps modulation by PAs would shade new light on the mechanism of action in plants under various stresses.
Summarily, to our knowledge, the present research is the first to show that the elevated NCC Spd, NCC Spm, together with CC Put and CC Spd induced by drought stress could promote H+-ATPase activity in PM, and via doing so, might enhance the tolerance of developing wheat embryos to drought stress. It is very well-known that PAs are present in all the plant cell compartments, which is a proof of its indispensable nature and role in diverse cellular processes (Kumer et al. 1997; Kaur-Sawhney et al. 2003; Legocka and Sobieszczuk-Nowicka, 2012). Further research is needed to ascertain the sub-cellular distribution of all forms of PAs, configuration and function of biomacromolecule in cell treated with drought stress, exogenous PAs, and the application of PA biosynthesis inhibitor.
Abbreviations
- BSA:
-
Bovine serum albumin
- CC:
-
Covalently conjugated
- DR:
-
Drought
- DTT:
-
Dithiothreitol
- EGTA:
-
Ethylene glycol tetraacetic acid
- ERDWIR:
-
Embryo relative dry weight increase rate
- ERWC:
-
Embryo relative water content
- Hepes:
-
2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid
- HPLC:
-
High performance liquid chromatography
- MGBG:
-
Methylglyoxyl-bis (guanylhydrazone)
- NCC:
-
Noncovalently conjugated
- O−Phen:
-
Phenanthrolin
- PA:
-
Polyamine
- PCA:
-
Perchloric acid
- PEG:
-
Polyethylene glycol–6000
- PM:
-
Plasma membrane
- PMSF:
-
Phenylmethanesulfonyl fluoride
- Put:
-
Putrescine
- PVP:
-
Polyvinyl pyrrolidone
- SAMDC:
-
S-adenosylmethionine decarboxylase
- Spd:
-
Spermidine
- Spm:
-
Spermine
- TCA:
-
Trichloroacetic acid
- TGase:
-
Transglutaminase
References
Adiga PR, Prasad GL (1985) Biosynthesis and regulation of polyamines in higher plants. Plant Growth Regul 3:205–226
Astarita LV, Handro W, Floh ES (2003) Changes in polyamines content associated with zygotic embryogenesis in the Brazilian pine, Araucaria angustifolia (Bert.) O. Ktze. Rev Bras Bot 26(2):163–168
Athwal GS, Huber SC (2002) Divalent cations and polyamines bind to loop-8 of 14-3-3 proteins, modulating their interaction with phosphorylated nitrate reductase. Plant J 29:119–129
Bradford MM (1976) A rapid and sensitive methods for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binging. Anal Biochem 72:248–254
Cacho M, Torres DA, Elena-Rosselló JA (2013) Role of polyamines in regulating silymarin production in Silybum marianum (L.) Gaertn (Asteraceae) cell cultures under conditions of calcium deficiency. J Plant Physiol 170(15):1344–1348
Camoni L, Lucente CD, Pallucca R, Visconti S, Aducci P (2012) Binding of phosphatidic acid to 14-3-3 proteins hampers their ability to activate the plant plasma membrane H+-ATPase. IUBMB Life 64:710–716
Cao DD, Hu J, Zhu SJ, Hu WM, Knapp A (2010) Realtionship between changes in endogenous polyamines and seed quality during development of sh 2 sweet corn (Zea mays L.) seed. Sci Horti 123:301–307
Del Duca S, Beninati S, Serafini-Fracassini D (1995) Polyamines in chloroplasts: identification of their glutamyl and acetyl derivatives. Biochem J 305:233–237
Di Tomaso JM, Shaff JE, Kochian LV (1989) Putrescine-induced wounding and its effects on membrane integrity and ion transport processes in roots of intact corn seedlings. Plant Physiol 90:988–995
Do PT, Degenkolbe T, Erban A, Heyer AG, Kopka J, Kohl KL, Hincha DK, Zuther E (2013) Dissecting rice polyamine metabolism under controlled long-term drought stress. PLoS ONE 8(4):e60325. doi:10.1371/journal.pone.0060325
Dutra NT, Silveira V, Azevedo IG, Gomes-Neto LR, Facanha AR, Steiner N, Guerra MP, Floh EIS, Santa-Catarina C (2013) Polyamines affect the cellular growth and structure of pro-embryogenic masses in Araucaria angustifolia embryogenic cultures through the modulation of proton pump activities and endogenous levels of polyamines. Physiol Plant 148:121–132
Farooq M, Wahid A, Lee DJ (2009) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945
Galston AW, Kaur-Sawhney R (1995) Polyamines as endogenous growth regulators. In: Davies PJ (ed) Plant Hormones: Physiology. Dordrecht, Kluwer Academic Publishers, Biochemistry and Molecular Biology, pp 158–178
Garufi A, Visconti S, Camoni L, Aducci P (2007) Polyamines as physiological regulators of 14-3-3 interaction with the plant plasma membrane H+-ATPase. Plant Cell Physiol 48:434–440
Goyal M, Asthir B (2010) Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul 60:13–25
Grzesiak M, Filek M, Barbasz A, Kreczmer B, Hartikainen H (2013) Relationships between polyamines, ethylene, osmoprotectants and antioxidant enzymes activities in wheat seedlings after short-term PEG- and NaCl-induced stresses. Plant Growth Regul 69:177–189
Gupta K, Dey A, Gupta B (2013) Plant polyamines in abiotic stress responses. Acta Physiol Plant 35:2015–2036
Iqbal M, Ashraf M (2005) Changes in growth, photosynthetic capacity and ionic relations in spring wheat (Triticum aestivum L.) due to pre-sowing seed treatment with polyamines. Plant Growth Regul 46:19–30
Janicka-Russak M, Kabala K, Mlodzinska E, Klobus G (2010) The role of polyamines in the regulation of the plasma membrane and the tonoplast proton pumps under salt stress. J Plant Physiol 167:261–269
Jelich OC, Weiler EW, Oeching C (2001) Binding of regulatory 14-3-3 proteins to the C-terminus of the plant plasma membrane H+-ATPase involves part of its autoinhibitory region. J Biol Chem 276:39852–39857
Johansson F, Sommarin M, Larsson C (1993) Fusicoccin activates the plasma membrane H+-ATPase by a mechanism involving the C-terminal inhibitory domain. Plant Cell 5:321–327
Kaur-Sawhney R, Tiburcio AF, Atabella T, Galston AW (2003) Polyamines in plants: an overview. J Cell Mol Biol 2:1–12
Kinoshita T, Nishimura M, Shimazaki K (1995) Cytosolic concentration of C 2+a regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell 7:1333–1342
Kubis J (2008) Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165:397–406
Kumer A, Altabella T, Taylor MA, Tiburcio AF (1997) Recent advances in polyamine research. Trends Plant Sci 2:124–130
Legocka J, Sobieszczuk-Nowicka E (2012) Sorbitol and NaCl stresses affect free, microsome-associated and thylakoid-associated polyamine content in Zea mays and Phaseolus vulgaris. Acta Physiol Plant 34:1145–1151
Lester GE (2000) Polyamines and their cellular anti-senescence properties in honeydew muskmelon fruit. Plant Sci 160:105–112
Li XN, Jiang HD, Liu FL, Cai J, Dai TB, Cao WX, Jiang D (2013) Induction of chilling tolerance in wheat during germination by pre-soaking seed with nitric oxide and gibberellin. Plant Growth Regul 71:31–40
Liu HP, Dong BH, Zhang YY, Liu ZP, Liu YL (2004) Relationship between osmotic stress and the levels of free, conjugated and bound Polyamines in leaves of wheat seedlings. Plant Sci 166:1261–1267
Liu J, Yu BJ, Liu YL (2006) Effects of spermidine and spermine levels on salt tolerance associated with tonoplast H+-ATPase and H+-PPase activities in barley roots. Plant Growth Regul 49:119–126
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34:135–148
Michelet B, Boutry M (1995) The plasma membrane H+-ATPase: a highly regulated enzyme with multiple physiological functions. Plant Physiol 108:1–6
Michelet B, Lukaszewiez M, Dupriez V, Boutry M (1994) A plant plasma membrane proton-ATPase gene is regulated by development and environment and shows signs of a translational regulation. Plant Cell 6:1375–1389
Niemenak N, Awah TM, Lieberei R (2012) Establishment of suspension culture in Theobroma cacao and polyamines associated with cacao embryogenesis. Plant Growth Regul 67:1–8
Ohinishi T, Gall RG, Mayer ML (1975) An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. Anal Biochem 689:261–267
Palmgren MG, Sommarin M, Serrano R, Larson C (1991) Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H+-ATPase. J Biol Chem 266:20470–20475
Peng YB, Lu YF, Zhang DP (2003) Abscisic acid activates ATPase in developing apple fruit especially in fruit phloem cells. Plant, Cell Environ 26:1329–1342
Qiao XQ, Shi GX, Jia R, Chen L, Tian XL, Xu J (2012) Physiological and biochemical responses induced by lead stress in Spirodela polyrhiza. Plant Growth Regul 67:217–225
Qiu QS, Su XF (1998) The influence of extracellular-side Ca2+ on the activity of the plasma membrane H+-ATPase from wheat roots. Aust J Plant Physiol 25:923–928
Qiu QS, Zhang N (2000) Water stress inhibits P-nitrophenyl phosphate hydrolysis activity of the plasma membrane H+-ATPase from soybean hypocotyls. Aust J Plant Physiol 27:717–721
Reggiani R, Zaina S, Bertani A (1992) Plasma membrane ATPase in rice coleoptiles: stimulation by putrescine and polyamines. Phytochemistry 31:417–419
Sailerova E, Zwiazek JJ (1993) Effects of triadimefon and osmotic stress on the plasma membrane composition and ATPase activity in the white spruce (Picea Glauca) needles. Physiol Plant 87:475–482
Schonfeld MA, Johnson RC, Carver BF, Mornhinwey DW (1988) Water relation in winter wheat as drought resistance indicators. Crop Sci 28:526–537
Serrano R (1989) Structure and function of plasma membrane H+-ATPase. Annu Rev Plant Physiol Plant Mol Biol 40:61–94
Sharma P, Rajam MV (1995) Spatial and temporal changes in endogenous polyamine levels associated with osmotic embryogenesis from different hypocotyls segments of eggplant (Solanum melongena L.). J Plant Physiol 146:658–664
Shen W, Huber SC (2006) Polycations globally enhance binding of 14-3-3ω to target proteins in spinach leaves. Plant Cell Physiol 47:764–771
Slocum RD (1991) Polyamine biosynthesis in plant. In: Slocum RD, Flores HE (eds) Polyamines in Plants. CRC Press, Florida, pp 23–40
Sood S, Nagar PK (2003) The effect of polyamines on leaf senescence in two diverse rose species. Plant Growth Regul 39:155–160
Srivastava SK, Rajbabu P (1983) Effect of amines and guanidines on ATPase from maize scutellum. Phytochemistry 22:2675–2679
Surowy TK, Boyer JS (1991) Low water potentials affect expression of genes encoding vegetative storage proteins and plasma membrane protein ATPase in soybean. Plant Mol Biol 16:251–262
Sussman MR (1994) Molecular analysis of protein in the plasma membrane. Annu Rev Plant Physiol Plant Mol Biol 45:213–234
Sussman MR, Harper JF (1989) Molecular biology of the plasma membrane of higher plants. Plant Cell 1:953–960
Suwastika IN, Gehring CA (1999) The plasma membrane H+-ATPase from Tradescantia stem and leaf tissue is modulated in vitro by Cgmp. Arch Biochem Biophys 367:137–139
Svennelid F, Olsson A, Piotrowski M, Rosenquist M, Ottman C, Larsson C, Oecking C, Sommarin M (1999) Phosphorylation of Thr-948 at the C-terminus of the plasma membrane H+-ATPase creats a binding site for the regulatory 14-3-3 protein. Plant Cell 11:2379–2391
Tang W, Newton RJ (2005) Polyamines reduce salt-induced oxidative damage by increasing the activities of antioxidant enzymes and decreasing lipid peroxidation in Virginia pine. Plant Growth Regul 46:31–43
Tiburcio AF, Campos JL, Figueras X (1993) Recent advances in the understanding of polyamines functions during plant development. Plant Growth Regul 12:331–340
Uranoa K, Hoboa T, Shinozaki K (2005) Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed development. FEBS Lett 579:1557–1564
Widell S, Larsson C (1990) A critical evaluation of markers used in plasma membrane purification. In: Larsson C, Moller IM (eds) The Plant Plasma Membrane. Springer-Verley, Berlin, pp 16–43
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ, Kusano T (2007) A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem Biophys Res Commun 352:486–490
Zepeda-Jazo I, Velarde-Buendia AM, Enriquez-Figueroa R, Bose J, Shabala S, Muniz-Murguia J, Pottosin II (2011) Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membrane. Plant Physiol 157:2167–2180
Zhang WH, Chen Q, Liu YL (2002) Relationship between H+-ATPase activity and fluidity of tonoplast in barley roots under NaCl stress. Acta Botanic Sinic 44:292–296
Zhao FG, Sun C, Liu YL, Liu ZP (2000) Effect of salinity stress on the levels of covalently and noncovalently conjugated polyamines in plasma membrane and tonoplast isolated from barley seedlings. Acta Botanic Sinic 42:920–926
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No. 31271627), the Special Fund of Industrial (Agriculture) Research for Public Welfare of China (Grant No. 201203077) and the Education Department Natural Science Foundation of Henan Province (Grant No. 2011A180033).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, H., Zhou, X., Yang, Q. et al. Changes in H+-ATPase activity and conjugated polyamine contents in plasma membrane purified from developing wheat embryos under short-time drought stress. Plant Growth Regul 75, 1–10 (2015). https://doi.org/10.1007/s10725-014-9925-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9925-9