Abstract
Moringa oleifera is a multipurpose plant which is now being promoted as a fodder crop. The present study was conducted to induce the tolerance in moringa plants to emerge and grow under saline conditions. For this, moringa seeds were primed with aerated water (hydropriming) and moringa leaf extract (MLE) for 12 and 24 h and studied for its emergence, potential growth behaviour, mineral composition, chlorophyll contents and antioxidant activities in comparison with unprimed seeds to investigate the physiological changes in moringa plants under saline conditions. The seeds were sown in plastic pots filled with acid washed sand at four salinity levels (3, 6, 10, 14 dS m−1) in a completely randomized design with three replications. It was found that salinity >6 dS m−1 reduced the emergence, growth and vigour of moringa plants but hydropriming (12 h) enhanced moringa emergence at 10 dS m−1 followed by MLE priming (12 h). Maximum aboveground biomass and photosynthetic pigments were recorded when the seeds were hydroprimed (12 h) but maximum root length and number of roots were found in MLE primed (12 h) moringa plants. Significant decrease in K+:Na+ ratio with increasing salinity levels resulted in low K+ and Mg2+ uptake and Na+ toxicity in moringa leaves which resulted in reduced chlorophyll contents at 14 dS m−1 but a significant increase in chlorophyll a and b contents and total phenolics were found in hydroprimed seeds (12 h) while the antioxidant activities of superoxide dismutase, peroxidase and catalas were improved by MLE priming (12 h). This study concludes that moringa emergence and growth performance can be improved by hydropriming under saline conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Salinity affects seed emergence, plant growth behaviour and its development through disturbing the physiological and cellular processes due to decrease in soil water potential resulting less absorption of water by seeds (Munns 2002; Jafar et al. 2012). The responsible factor for these problems is the accumulation of sodium (Na+) and chloride (Cl−) in soils that results in declined growth and less productivity of plants. This accumulation induces osmotic stress which hinders water absorption and uptake of essential minerals by plants and also provokes ion toxicity which ultimately affects the plant growth and yield (Munns and Tester 2008). The adverse effects of salinity like low yield of crops have been reported in many crops like wheat (Afzal et al. 2008), maize (El-Tayeb 2005) and cotton (Sattar et al. 2010). Moringa oleifera (here after only moringa) is not an exception. No doubt, it can tolerate moderate saline conditions (up to 8 dS m−1) but at this level, mild reduction in plant growth, chlorophyll contents and mineral composition have been recorded (Nouman et al. 2012a). Beside this, the importance of moringa as a field or fodder crop, vegetable, crop growth enhancer, biopesticides, alley crop, medicines, water purifier, biogas etc. cannot be neglected (Foidl et al. 2001). Its leaves are rich in digestible proteins, calcium, potassium, magnesium, iron, and vitamins (Makkar and Becker 1996; Nouman et al. 2014). Moreover, these are also rich sources of cytokinin, ascorbic acid, vitamins, phenolics, flavonoids and antioxidants which make it usable for physicians and nutritionists to combat malnutrition problems especially making up vitamin A deficiency (Makkar and Becker 1996; Nambiar 2006). The presence of enzymatic and non-enzymatic antioxidants has been reported in moringa leaves make it a good source of strengthening the immune system (Nouman et al. 2013). Keeping in view the importance of moringa, there is a need to induce tolerance in moringa plants to tolerate saline conditions with less decrease in its growth, biomass and mineral composition.
Recently, different strategies are employed to induce tolerance in different plants under saline conditions. Among these, seed priming is considered as an important strategy to mitigate the adverse effects of salinity (Jisha et al. 2013). Afzal et al. (2008, 2012) reported hydropriming, CaCl2, KCl, NaCl and Kinetin as effective priming agents which can alleviate the adverse effects of salinity in various crops. Being a rich source of calcium, potassium, ascorbic acid and zeatin (cytokinin), moringa leaf extract (MLE) is also being used as natural plant growth enhancer which improves the germination of various range grasses (Nouman et al. 2012b), wheat (Yasmeen et al. 2012) and Maize plants (Afzal et al. 2012). Previously, hydropriming and MLE priming have been used improving the stand establishment and nutritional quality of moringa plants under normal and stress conditions (Santos et al. 2011; Nouman et al. 2012c) but no study has been yet reported describing the improvement in growth, mineral composition and antioxidant system of moringa plants under salinity by inducing seed priming techniques. Keeping in view these findings, the present investigation was carried out to explore the increase in plant biomass, photosynthetic pigments, mineral contents and improvement in antioxidant system of moringa plants under saline conditions.
Materials and methods
Plant material
Moringa leaves were harvested from mature trees afternoon from the nursery of the Department of Forestry, University of Agriculture Faisalabad, Pakistan and were washed thoroughly with water. The leaves were analyzed for their enzymatic (SOD, POD, CAT) and non-enzymatic antioxidants (ascorbic acid and total phenolics) and mineral contents (Tables 4, 5). These analyses were conducted on overnight frozen leaves which extract was further used in priming.
MLE extraction
The overnight frozen moringa leaves were pressed in a locally fabricated machine to extract its juice. The extract was filtered by passing through cheesecloth and 30 times diluted with distilled water (Nouman et al. 2012b, Yasmeen et al. 2012).
Seed material
Moringa seeds were collected from the Department of Forestry, University of Agriculture Faisalabad-Pakistan and their initial germination percentage (67.33 %) was recorded by placing moringa seeds between Whatman filter paper No. 1 in petri dishes at 32 ± 2 °C.
Seed priming treatments
Shelled moringa seeds were soaked in aerated solution of MLE i.e., MLE priming (Nouman et al. 2012b) and aerated water i.e., hydropriming for 12 and 24 h. Unprimed moringa seeds were used as control. After priming, seeds were rinsed with distilled water and dried to their original weight under shade at 28 ± 2 °C temperature (Basra et al. 2002).
Seed emergence and plant vigour evaluation
The experiment was carried out under wire-house conditions at temperature 32 ± 2 °C and 16 h daylight and 8 h night conditions. Primed and unprimed seeds were sown in acid washed sand filled pots (15 × 30 cm) (20 seeds per pot). The experiment was conducted in completely randomized design (CRD) with two factor factorial arrangement (salinity and seed priming) with three replications in nursery of the Department of Forestry, University of Agriculture Faisalabad, Pakistan. Salinity levels (3, 6, 10, 14 dS m−1) were induced at the start of the experiment with NaCl. The water (pH 6.9) was applied to pots when required. After complete emergence, Hoagland solution was applied to provide the nutrients for plant growth. Emerging seeds were counted daily according to AOSA (1990). Final emergence percentage (FEP) values ranged between (<30 and >70 %), so, angular transformation of the FEP was calculated using the formula arcsine√FEP (Gomez and Gomez 1983). Time taken to 50 % emergence (E50) was calculated according to Farooq et al. (2005) and mean emergence time (MET) was calculated by using the equation of Ellis and Roberts (1981) while emergence index (EI) was calculated following the formula given by AOSA (1983). After final emergence, six seedlings per replication/pot were maintained for investigating plant vigour and biochemical analyses. After 3 weeks of emergence, the plants were harvested for analyzing growth, physiological and mineral parameters. Three plants were randomly selected for growth parameters and mineral analyses while the remaining plants were used for biochemical assays.
Growth parameters
Growth parameters like shoot length, root length, number of leaves and roots were recorded by using standard procedures after three weeks of emergence.
Photosynthetic pigments
The protocol devised by Nagata and Yamashta (1992) was used for the quantification of chlorophyll a, b, and β-carotene contents in moringa leaves. One gram moringa fresh leaves were ground in 10 mL of acetone (80 %) and filtered through Whatman filter paper No. 1 and stored in falcon tubes. The absorbance was recorded at 663, 645, 505 and 453 nm by using UV spectrophotometer (UV-4000, ORI Germany). The contents were quantified by using the absorbance readings according to protocol.
Total phenolic contents
The protocol devised by Singleton and Rossi (1965) revised by Waterhouse (2001) was used for calculating total phenolic contents in moringa leaves. The absorbance of gallic acid standards (100, 150, 250, 500 mg L−1) and moringa samples was noted at 760 nm by using UV spectrophotometer (UV-4000, ORI Germany).
Antioxidants’ assay
Firstly, total soluble proteins were determined by using Bradford assay (Bradford 1976). For enzymatic antioxidants determination, fresh moringa leaves were harvested before sunrise and stored at −80 °C to avoid heat and light effect on enzymatic antioxidants. One gram leaf sample was ground in 10 mL of 50 mM cooled phosphate buffer (pH 7.8) and centrifuged (15,000 rpm) at 4 °C. The supernatant was stored in Eppendorf tubes for enzymatic antioxidants’ assay. Superoxide dismutase (SOD) (EC 1.15.1.1) activity was noted at 560 nm by using UV spectrophotometer (UV-4000, O.R.I. Germany) according to procedure described by Giannopolitis and Ries (1977). Peroxidase (POD) (EC 1.11.1.7) and catalase (CAT) (EC 1.11.1.6) activities were determined by following the procedure of Chance and Maehly (1955) at 470 and 240 nm wavelength, respectively.
Mineral analysis
Moringa leaves were oven dried at 60 °C to a constant weight and ground to pass 2 mm sieve. The samples were digested by using HNO3 and HCLO4 (2:1) by following the procedure adapted by Rashid (1986). The presence of sodium (Na+) and potassium (K+) contents were recorded using flame photometer (Jenway PEP-7) (Chapman and Pratt 1961). Magnesium (Mg) contents were determined by atomic absorption spectrophotometer (Model: Z-8200).
Statistical analysis
The significance of variance (p < 0.05) was determined for the data by using MSTAT-C Computer Program (MSTAT Development Team 1989). LSD test at 5 % level of probability was used to test the differences among mean values (Steel et al. 1997).
Results
Salinity affected moringa seeds’ emergence significantly by reducing final emergence percentage from 55.0 to 16.7 %. Moringa seeds which were soaked in water for 12 h showed increasing trend in FEP at all salinity levels but it was more effective till 10 dS m−1, where moringa seeds exhibited 70.0 % FEP while unprimed seeds showed only 43.3 % (Table 1). Hydropriming (24 h) and MLE priming (12 h) were also effective in improving emergence under saline conditions. Maximum emergence index (17.6) was observed in hydroprimed seeds (12 h) at 3 dS m−1 which remained effective till 10 dS m−1. Salinity at 14 dS m−1 drastically reduced EI (2.7) which was not effectively improved by priming techniques. Hydropriming (12 h) was followed by MLE priming (12 h) in improving EI of moringa seeds (Table 1). MET under moderate saline conditions (10 dS m−1) was maximally reduced by hydropriming (12 h) which was statistically at pat with hydropriming (24 h) and MLE priming (12 h) at 3 dS m−1. Similar observations were recorded in case of E50 when seeds were priming with hydropriming for 12 h at 6 dS m−1, hydropriming (12 h) was statistically similar with MLE priming (12 h) (Table 1).
All priming treatments significantly improved seedling vigour of moringa seeds till 10 dS m−1. Maximum shoot length (27.6 cm) was recorded at 3 dS m−1 when moringa seeds were subjected with hydropriming (12 h) (Table 2). This treatment remained effective significantly till 10 dS m−1 but at 14 dS m−1, it could not give good results. Hydropriming (12 h) was mostly effective in improving number of leaves (52.0, 45.3 and 31.0) at 3, 6 and 10 dS m−1, respectively (Table 2). No doubt, the number of roots increased with increasing salinity levels without any priming treatment (9.4–14.6), but primed moringa seeds exhibited significant effect on moringa roots elongation and increment in number of roots. Maximum root length of moringa seedlings (23.4 cm) was recorded under normal saline conditions (10 dS m−1) when the seeds were primed with MLE for 12 h was followed by hydropriming (12 h). No priming treatment could improve number of leaves under high salinity level (14 dS m−1). MLE priming (12 h) was more effective at all salinity levels (Table 2). Maximum roots (29.4 and 24.6) were found at 10 and 14 dS m−1, respectively.
Seed priming significantly (p < 0.05) affected the uptake of sodium (Na+), potassium (K+) and magnesium (Mg2+) contents in moringa leaves. Likewise, K+:Na+ ratio also exhibited significant difference among treatments at different salinity levels. Maximum K+ contents (3,289.5 mg kg−1) were recorded in moringa leaves when primed with MLE for 12 h at 6 dS m−1 followed by hydropriming (12 h) under same saline conditions i.e., 6 dS m−1 (Table 3). No priming treatment could improve or maintain K+ contents in moringa leaves at 14 dS m−1. Maximum Na+ contents (1,011.7 mg kg−1) were found in moringa leaves at 14 dS m−1 when no seed priming treatment was applied to moringa seeds followed by hydro- and MLE priming (12 h). Even at 10 dS m−1, no seed priming treatment could reduce the uptake of Na+ contents (Table 3). The decreasing K+ contents and increasing Na contents with increasing salinity levels result in decreasing K+:Na+ ratio (Table 3). MLE priming (12 h) significantly improved Mg2+ contents (11,730.3 mg kg−1) followed by MLE priming (24 h) (10,856.9 mg kg−1) at 6 dS m−1 (Table 3). The least Mg2+ contents were exhibited at 14 dS m−1 and no seed priming treatment could effectively improve or maintain the uptake (Table 3).
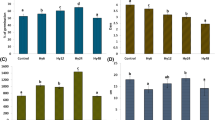
Hydropriming (12 h) also improved chlorophyll a contents. Maximum chlorophyll a contents were recorded in hydroprimed (12 h) seedlings under 10 dS m−1 salinity level (23.0 μg g−1) which was statistically at par with same priming treatment under 6 dS m−1 (22.7 μg g−1) (Fig. 1). No priming treatment could improve chlorophyll a contents in moringa leaves grown at 14 dS m−1 salinity level. Chlorophyll b contents were also significantly affected by salt stress. The most severe damage was observed when moringa seedlings were subjected to 14 dS m−1. Maximum chlorophyll b contents were found in moringa seedlings grown at 10 dS m−1 (Fig. 2). At 3, 6 and 10 dS m−1, hydropriming (12 h) was the best priming treatment in improving TPC while no improvement in TPC was observed in primed seeds at 14 dS m−1 (Fig. 3). Maximum TPC were recorded in hydroprimed (12 h) moringa seedlings (869.4 μg g−1) followed by MLE priming (12 h) under same salinity level (816.8 μg g−1).
Enzymatic antioxidants (CAT, POD and SOD) were significantly affected under saline conditions and seed priming treatments. Maximum CAT activity (124.9 unit mg−1 protein) was recorded in MLE primed (12 h) moringa seedlings under 10 dS m−1 which was followed by hydropriming (12 h) at same salinity level (11.2 unit mg−1 protein). Similar trend was observed at 3 and 6 dS m−1 salinity levels (Fig. 4). Maximum POD and SOD activities were recorded in MLE primed (12 h) moringa seedlings (1,301.5 and 478.1 unit mg−1 protein, respectively) followed by MLE priming (24 h) (1,062.1 and 390.5 unit mg−1 protein, respectively) at 10 dS m−1. MLE priming (12 h) also improved SOD activity (340.2 unit mg−1 protein) followed by hydropriming (12 h) (291.0 unit mg−1 protein) under normal saline conditions (6 dS m−1). No priming treatment could improve antioxidants system at 14 dS m−1 (Figs. 5, 6).
Discussion
Soil salinity adversely affects the seed emergence, plant growth and its establishment by disturbing the physiological and metabolic activities along with cellular processes of plants (Munns 2011; Wakeel 2013). The adverse effects of salinity can be observed from the destruction of photosynthetic system and protein synthesis which ultimately reduce the seed emergence and weaken the plant development. The salt induced growth reduction is generally attributed to water deficit or osmotic stress, specific ion toxicities, nutritional imbalance and oxidative stress (Munns 1993; Afzal et al. 2008).
In present study, moringa seeds’ emergence was significantly affected by salt stress but seed priming improved the emergence performance of moringa seeds up to 10 dS m−1 (Table 1). The adverse effect of salinity might be due to the excessive Na+ and Cl− accumulation in root medium which induces toxic effect, nutritional imbalance and oxidative stress (Munns and Tester 2008). No doubt, moringa plants have strong self defense system to overcome abiotic stress (Nouman et al. 2012a) but this tolerance can be improved by seed priming techniques as has been reported in wheat (Afzal et al. 2008). Tedonkeng et al. (2004) and Nouman et al. (2012c) reported the improvement in moringa seeds emergence by employing hydro- and MLE priming, respectively. In primed seeds, water imbibition triggers the accumulation of sugars, minerals and organic compounds in the start of seed metabolic processes resulting in rapid and synchronized emergence (Bradford 1986; Castro and Hilhorst 2004). MLE priming has been proved for its significance in improving seed emergence of moringa seeds after hydropriming in the present investigation. MLE being rich in minerals, ascorbate and zeatin contents, activates the physiological and cellular processes in moringa seeds under saline conditions which improves stand establishment. Moreover, it might be due to the regulation of plant growth regulators like kinetin which can mitigate the abiotic stress conditions during seed emergence by inducing changes in enzymatic activities of carbohydrate metabolism (Kaur et al. 2002). So, primed seeds can overcome the adverse effects of salinity on moringa seeds emergence behaviour as found in the present investigation.
Regarding shoot attributes, hydropriming (12 h) maximally improved the shoot length and number of leaves of moringa plants under salt stress conditions but at 14 dS m−1 no priming technique could improve these attributes (Table 2). This reduction in plant biomass at 14 dS m−1 manifested the drastic effects of salinity on moringa growth behaviour. This might be due to osmotic stress, reduced cell division and ethylene production. Moreover, the salt accumulation in growing medium causes toxic ion accumulation which hinders the uptake of water and essential nutrients by roots for crop growth (Munns and Tester 2008). Maximum root length was observed when the plants were grown under moderate saline conditions (10 dS m−1), which was further reduced at 14 dS m−1 (Table 2). The increase in root length at moderate salinity level might be due to less osmotic pressure as it permits root elongation in the search of water and essential nutrients but reduces leaf area and plant height (Hsiao and Xu 2000). Such behaviour of moringa roots i.e., elongation and expansion has also been reported previously in moringa plants by Nouman et al. (2012a) at moderate saline conditions (8 dS m−1). The shoot length reduction and root elongation is a survival strategy of plants to reduce transpiration through leaves and improved water uptake through roots avoiding salt uptake (Jefferires and Rudmik 1991; Houle et al. 2001). It is obvious from the current study that hydropriming (12 h) improved the emergence of moringa seeds and the same technique increased the shoot length and number of leaves under saline conditions but MLE priming (12 h) was more effective in improving the root length and number of roots. Nouman et al. (2012b, c) also reported MLE efficacy in improving the root length of moringa plants and range grasses, respectively.
In the present study, moringa leaves showed decreasing K+ and Mg2+ contents with increasing salinity levels while Na+ contents were found increasing (Table 3). K+ is an essential nutrient for plant growth and survival while Na+ concentration results in cell death and ionic imbalance. K+:Na+ ratio is a good indicator to assess the plant growth rate and K+ homeostasis which is also important to activate enzymatic reactions in cytoplasm. Moreover, the addition of external K+ supply to Jatropha curcas plants, showed stability in photosynthesis and reduced Na+ toxicity to plants (Rodrigues et al. 2013). Low K+ and high Na+ contents in plants represent stress conditions which affects the plant growth and vigour (Luan et al. 2009). Higher Na+ and lower K+ contents in plants under saline conditions might be attributed to the uptake competition specifically through high-affinity potassium transporters (HKTs) and nonselective cation channels (NSCCs). Na+ toxicity causes membrane depolarization which hinders in K+ uptake by K+ inward-rectifying channels (KIRs) which increases cell K+ leakage by activating potassium outward-rectifying channels (KORs) (Wakeel 2013). Moreover, Chlorophyll a and b contents were adversely affected under saline conditions. Maximum chlorophyll contents were recorded in hydroprimed (12 h) seedlings at 10 dS m−1 salinity level (Fig. 1). Akram et al. (2002) reported the reduction in crop yield under saline conditions due to damage of photosynthetic system. It might be due to more Na+ accumulation in leaves which degrade the chlorophyll or reduce the synthesis of magnesium (Mg2+) (Rubio et al. 1995). Mg2+ has active role in plant metabolism, maintaining the integrity of plant ribosomes and triggering about 300 enzymes to perform their catalytic reactions. Its deficiency may cause damage to photosynthetic system and vascular tissues (Hannick et al. 1993). The export of carbohydrates from the source tissues was also affected due to Mg2+ deficiency (Mehne-Jakobs 1995; Sun and Payn 1999). ATP (adenosine triphosphate) is considered as biologically active when it is bound to Mg2+ ion. The increase in Na+ accumulation in leaves may inhibit the uptake of Mg2+ by roots which disturb the protein synthesis and inhibit the phosphorous elimination from plant cells (Li et al. 2008). The decrease in chlorophyll contents and Mg2+ contents in moringa leaves with increase in phosphorous contents has been previously reported as growth retarding agents (Nouman et al. 2012a). In the present study, chlorophyll a and b contents exhibited different trends under saline conditions and in the response of seed priming treatments. The researchers are still trying to find out the factors which are basically responsible for photosynthetic system damage (Steduto et al. 2000).
Salinity causes oxidative stress in plants resulting in the production of reactive oxygen species (ROS) like hydrogen peroxide (H2O2), singlet oxygen (1O2) and hydroxyl radical (OH·) imbalancing the antioxidant activities (Hernández et al. 1993). ROS cause the damage to protein and lipid molecules resulting in cell death. It was also reported that the plants use most of their sources in improving their antioxidant defence mechanism to avoid the damage caused by oxidative stress (Athar et al. 2008; kolbert et al. 2012). The improvement in antioxidant activities in response to increased levels of ROS generation under salt stress have been reported in wheat, cotton, maize and rice (Vaidyanathan et al. 2003). Under stress conditions, moringa also enhances its antioxidant activities to overcome oxidative damage (Nouman et al. 2012a). In this experiment, seed priming techniques improved the antioxidants’ activities but at 14 dS m−1, these were badly damaged (Figs. 3, 4, 5). It shows that moringa plants cannot withstand at higher salinity level even when seeds were primed. The reduction in plant growth and development might be due to the damage of antioxidant system which could not scavenge ROS and induce tolerance against salinity (Mittler 2002). It is evident from the present study that MLE (12 h) primed seeds effectively improved SOD, POD and CAT activities when grown at 10 dS m−1. Moringa leaves are rich in antioxidants which can mitigate the effects of salinity and improve the defence system of plants. Improvement in creeping bent grass under stress conditions was recorded with increased SOD level when seaweed extract was applied containing good concentration of cytokinins (Zhang and Ervin 2008). The efficacy of MLE to mitigate salinity effects on plants can be attributed to higher K+, Mg2+, ascorbate, auxin, antioxidants and protein contents (Makkar and Becker 1996; Nagar et al. 2006). The analysis report presented in this study also supports this information (Tables 4, 5). Moreover, moringa leaves have been reported as a rich source of zeatin (5–200 μg g−1 of fresh matter basis) by Fuglie (1999), so its effectiveness may also be attributed to cytokinin mediated stay green effect. Hence, it has the potential to induce tolerance against abiotic stress in plants. Previously, MLE has been used as an effective natural plant growth enhancer for rangeland grasses (Nouman et al. 2012b), late-sown wheat (Yasmeen et al. 2012) and maize plants under stress conditions (Afzal et al. 2012).
Seed priming duration play a vital role in its effectiveness. Lee and Kim (2000) and Farooq et al. (2006) reported poor germination and plant vigour when rice seeds were primed for longer duration. Moringa seeds manifested good germination and plant vigour when its seeds were primed with less duration (Nouman et al. 2012b). In the present investigation, similar trend was also recorded i.e., seed priming for minimum duration (12 h) was found more effective than 24 h priming.
Conclusion
Moderate to higher NaCl salinity has detrimental effect on moringa germination and seedling growth and vigour as well as on photosynthesis and antioxidants’ activities. Moringa seedlings can grow till 10 dS m−1 salinity level with a mild decrease in its vigour by executing its self defense system. This reduction in biomass can be overcome by employing seed priming techniques like hydro- and MLE priming. Hydropriming was proved as the best priming technique in improving emergence and shoot vigour while MLE priming improved root length, number of roots and accelerated antioxidant system to overcome saline conditions. These both seed priming techniques are environment friendly and can be easily adapted by farmers.
References
Afzal I, Rauf S, Basra SMA, Murtaza G (2008) Halopriming improve vigor, metabolism of reserves and ionic contents in wheat seedling under salt stress. Plant Soil Environ 54:382–388
Afzal I, Hussain B, Basra SMA, Rehman H (2012) Priming with moringa leaf extract reduces imbibitional chilling injury in spring maize. Seed Sci Technol 40:271–276
Akram M, Hussain M, Akhtar S, Rasul E (2002) Impact of NaCl salinity on yield components of some wheat accession/variety. Int J Agric Biol 4:156–158
AOSA (1983) Association of official seed analysts. Seed vigour testing handbook. Contribution No. 32 to the handbook on seed testing. Association of official seed analysts, Springfield, IL, USA
AOSA (1990) Association of official seed analysts. Rules for testing seeds. J Seed Technol 12:1–112
Athar H, Khan A, Ashraf M (2008) Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat. Environ Exp Bot 63:224–231
Basra SMA, Zia MN, Mehmood T, Afzal I, Khaliq A (2002) Comparison of different invigoration techniques in wheat (Triticum aestivum L.) seeds. Pak J Arid Agric 5:11–16
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bradford KJ (1986) Manipulation of seed water relations via osmotic priming to improve germination under stress conditions. Hort Sci 21:1105–1112
Castro RD, Hilhorst HWM (2004) Embebição e reativação do metabolismo. In: Ferreira AG, Borghetti F (eds) Germinação: do básico ao aplicado. Artmed, Brasil, pp 149–162
Chance M, Maehly AC (1955) Assay of catalases and peroxidases. Methods Biochem Anal 2:764–817
Chapman HD, Pratt PF (1961) Methods of analysis for soils, plants and water. University of California, Berkeley
Ellis RA, Roberts EH (1981) The quantification of ageing and survival in orthodox seeds. Seed Sci Technol 9:373–409
El-Tayeb MA (2005) Response of barley grains to the interactive effect of salinity and salicylic acid. Plant Growth Regul 45:215–224
Farooq M, Basra SMA, Hafeez K, Ahmad N (2005) Thermal hardening: a new seed vigour enhancement tool in rice. J Integr Plant Biol 47:187–193
Farooq M, Basra SMA, Afzal I, Khaliq A (2006) Optimization of hydropriming techniques for rice seed invigoration. Seed Sci Technol 34:507–512
Foidl N, Makkar HPS, Becker K (2001) The potential of Moringa oleifera for agricultural and industrial uses. In: Proceedings of International Workshop What development potential for Moringa products? October 29th to November 2nd 2001. Dar-es-Salaam, Tanzania
Fuglie LJ (1999) The miracle tree: Moringa oleifera: natural nutrition for the tropics. Church World Service, Dakar, pp 68; revised in 2001 and published as the miracle tree: the multiple attributes of Moringa, pp 172
Giannopolitis CN, Ries SK (1977) Superoxide dismutase occurrence in higher plants. Plant Physiol 59:309–314
Gomez KA, Gomez AA (1983) Statistical procedures for agricultural research. Wiley-Interscience Publication, New York
Hannick AF, Waterkeyn L, Weissen F, van Praag HJ (1993) Vascular tissue anatomy of Norway spruce needles and twigs in relation to magnesium deficiency. Tree Physiol 13:337–349
Hernández JA, Francisco FJ, Corpas GM, Gómez LA, Del Río FS (1993) Salt induced oxidative stresses mediated by activated oxygen species in pea leaves mitochondria. Physiol Plant 89:103–110
Houle G, Morel L, Reynolds CE, Siégel J (2001) The effect of salinity on different developmental stages of n endemic annual plant, Aster laurentianus (Asteraceae). Am J Bot 88:62–67
Hsiao TC, Xu LK (2000) Sensitivity of growth of roots versus leaves to water stress: biophysical analysis and relation to water transport. J Exp Bot 51:1595–1616
Jafar MZ, Farooq M, Cheema MA, Afzal I, Basra SMA, Wahid MA, Aziz T, Shahid M (2012) Improving the performance of wheat by seed priming under saline conditions. J Agron Crop Sci 198:38–45
Jefferires RL, Rudmik T (1991) Growth, reproduction and resource allocation in halophytes. Aquat Bot 39:3–16
Jisha KC, Vijayakumari K, Puthur JT (2013) Seed priming for abiotic stress tolerance: an overview. Acta Physiol Plant 35:1381–1396
Kaur S, Gupta AK, Kaur N (2002) Effect of osmoand hydropriming of chickpea seeds on seedling growth and carbohydrate metabolism under water deficit stress. J Plant Growth Regul 37:17–22
Kolbert Z, Peto A, Lehotai N, Feigl G, Erdei L (2012) Long-term copper (Cu2+) exposure impacts on auxin, nitric oxide (NO) metabolism and morphology of Arabidopsis thaliana L. Plant Growth Regul. doi:10.1007/s10725-012-9701
Lee SS, Kim JH (2000) Total sugars, α-amylase activity and germination after priming of normal and aged rice seeds. Korean J Crop Sci 45:108–111
Li M, Zhang LJT, Li LW (2008) Ecophysiological responses of Jussiaea rapens to cadmium exposure. Aquat Bot 88:347–352
Luan S, Lan W, Chul LS (2009) Potassium nutrition, sodium toxicity, and calcium signaling: connections through the CBL-CIPK network. Curr Opin Plant Biol 12:339–346
Makkar HPS, Becker K (1996) Nutritional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves. Anim Feed Sci Technol 63:211–228
Mehne-Jakobs B (1995) The influence of magnesium deficiency on carbohydrate concentrations in Norway spruce (Picea abies) needles. Tree Physiol 15:577–584
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trend Plant Sci 7:405–410
MSTAT Development Team (1989) Mstat user’s guide: a microcomputer program for the design management and analysis research experiments. Michigan State University East Lansing, USA
Munns R (1993) Physiological processes limiting plant growth in saline soils: some dogmas and hypotheses. Plant Cell Environ 16:15–24
Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25:239–250
Munns R (2011) The impact of salinity stress. The environmental and physiological nature of salinity web. http://www.plantstress.com/Articles/salinity_i/salinity_i.htm. Accessed January 11, 2013
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nagar PK, Iyer RI, Sircar PK (2006) Cytokinins in developing fruits of Moringa pterigosperma Gaertn. Physiol Plant 55:45–50
Nagata M, Yamashta I (1992) Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J Jpn Soc Food Sci Technol 39:925–928
Nambiar VS (2006) Nutritional potential of drumstick leaves: an Indian perspective. In: Proceedings of Moringa and other highly nutritious plant resources: Strategies, standards and markets for a better impact on nutrition in Africa. Accra, Ghana, November 16–18, 2006
Nouman W, Siddiqui MT, Basra SMA, Khan RA, Gull T, Olson ME, Munir H (2012a) Response of Moringa oleifera to saline conditions. Int J Agric Biol 14:757–762
Nouman W, Siddiqui MT, Basra SMA (2012b) Moringa oleifera leaf extract: an innovative priming tool for rangeland grasses. Turk J Agric For 36:65–75
Nouman W, Siddiqui MT, Basra SMA, Afzal I, Rehman H (2012c) Enhancement of emergence potential and stand establishment of Moringa oleifera Lam. by seed priming. Turk J Agric For 36:227–235
Nouman W, Siddiqui MT, Basra SMA, Farooq H, Zubair M, Gull T (2013) Biomass production and nutritional quality of Moringa oleifera as field crop. Turk J Agric For 37:410–419
Nouman W., Basra SMA, Siddiqui MT, Yasmeen A, Gull T, Alcayde MAC (2014) Potential of Moringa oleifera L. as livestock fodder crop: a review. Turk J Agric For 38:1–14
Rashid A (1986) Mapping zinc fertility of soils using indicator plants and soils-analyses. Ph.D. dissertation, University of Hawaii, HI, USA
Rodrigues CRF, Evandro NS, Sergio LFS, Eduardo LV, Ricardo AV, Joaquim AGS (2013) High K+ supply avoids Na+ toxicity and improves photosynthesis by allowing favorable K+: Na+ ratios through the inhibition of Na+ uptake and transport to the shoots of Jatropha curcas plants. J Plant Nutr Soil Sci 176:157–164
Rubio F, Gassman W, Schroeder J (1995) Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science 270:1660–1663
Santos ARFD, Renata SM, Roberio AF, Alexandro SB (2011) Water pre-hydration as priming for Moringa oleifera Lam. seeds under salt stress. Trop Subtrop Agroecosyst 14:201–207
Sattar S, Hussnain T, Javaid A (2010) Effect of NaCl salinity on cotton (Gossypium arboreum L.) grown on MS medium and in hydroponic cultures. J Anim Plant Sci 20:87–89
Singleton VL, Rossi JA (1965) Colourimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Am J Enol Viticul 16:144–158
Steduto PR, Albrizio PG, Sorrentino G (2000) Gas exchange response and stomatal and non-stomatal limitations to carbon assimilation of sunflower under salinity. Environ Exp Bot 44:243–255
Steel RCD, Torrie JH, Deekey DA (1997) Principles and procedures of statistics a biometric approach, 3rd edn. McGraw Hill Book Co. Inc., New York, pp 400–428
Sun OJ, Payn TW (1999) Magnesium nutrition and photosynthesis in Pinus radiata: clonal variation and influence of potassium. Tree Physiol 19:535–540
Tedonkeng PE, Boukila B, Solefack MMC, Kana JR, Tendonkeng F, Tonfack LB (2004) Potentiel de germination de Moringa oleifera Lam. sous différents traitements a Dschang dans les Hautes terres de l’Ouest- Cameroun [Germination potential of Moringa oleifera Lam. under different treatments in Dschang in the highlands of western Cameroon]. J Cameroon Acad Sci 4:199–203
Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G (2003) Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.) differential response in salt-tolerant and sensitive varieties. Plant Sci 165:1411–1418
Wakeel A (2013) Potassium-sodium interactions in soil and plant under saline-sodic conditions. J Plant Nutr Soil Sci 176:344–354
Waterhouse AL (2001) Determination of total phenolics in current protocols. In: Food and analytical chemistry. Wrolstad, R. E. Wiley, pp 11.1–11.1.8
Yasmeen A, Basra SMA, Ahmad R, Wahid A (2012) Performance of late sown wheat in response to foliar application of Moringa oleifera Lam. leaf extract. Chil J Agric Res 72:92–97
Zhang X, Ervin EH (2008) Impact of seaweed extract-based cytokinins and zeatin riboside on creeping bentgrass heat tolerance. Crop Sci 48:364–370
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nouman, W., Basra, S.M.A., Yasmeen, A. et al. Seed priming improves the emergence potential, growth and antioxidant system of Moringa oleifera under saline conditions. Plant Growth Regul 73, 267–278 (2014). https://doi.org/10.1007/s10725-014-9887-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9887-y