Abstract
Salicylic acid (SA) may reduce the negative impact of water deficit on growth and metabolite yield of Thymus daenensis Celak subsp. daenensis Celak. The effect of foliar application of SA and reduced irrigation on growth, oil yield, chemical components, and antibacterial and antioxidant activities of T. daenensis in field condition were investigated. Treatments comprised 0.0, 1.5 and 3.0 M SA applied to plants under normal irrigation and stressed conditions. Results indicated that irrigation regime had a significant effect on growing degree days (GDD) required to reach early and full flowering. Foliar application of SA influenced GDD from early growing stage to 50 % and full flowering, minimum radius and canopy diameter. The highest values of oil content (3.2 % v/w) and yield (14.9 g m−2) were obtained from application of 3.0 M SA. Percentage of some chemical constituents in the essential oil extracted from the plants under stress was higher than non-stressed plants. Thymol content was significantly reduced under stressed conditions. Foliar application of SA significantly improved carvacrol, α-thujene, α-pinene and p-cymene contents in the oils, but reduced thymol and, β-caryophyllene amounts. Our results showed that foliar application of SA reduced the negative effect of water deficit on thymol content in the essential oil of T. daenensis. The essential oils of T. daenensis exhibited antioxidant and antibacterial activities when plants were sprayed with 1.5 and 3.0 M SA, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The genus Thymus L. belongs to the mint family (Lamiaceae), and consists of about 215 species of herbaceous perennials and small shrubs in the world. The Mediterranean region has been identified as the center of the genus (Cronquist 1988). Thymus, with the common Persian name of ‘Avishan or Azorbe’, consists of 14 species which grow wild in many regions of Iran, such as T. daenensis Celak subsp. daenensis (Nickavar et al. 2005; Ghasemi Pirbalouti et al. 2011a). The aerial parts and volatile constituents of thyme are commonly used as a medicinal herb. Thymus species are commonly used as herbal tea, flavoring agents (condiments and spices) and medicinal purposes (Stahl-Biskup and Saez 2002). Infusion and decoction of aerial parts of Thymus species are used to produce tonic, carminative, digestive, antispasmodic, anti-inflammatory, and expectorant and for the treatment of colds in Iranian traditional medicine (Nickavar et al. 2005; Ghasemi Pirbalouti 2009). The aromatic and medicinal properties of the genus Thymus have made it one of the most popular medicinal plants (Nickavar et al. 2005). T. daenensis subsp. daenensis is a main endemic subspecies of Iran and grows in high altitudes in Zagros Mountains range, western and southwestern Iran. The essential oil and extracts from the aerial parts of T. daenensis contain mainly monoterpenes, sesquiterpenes, phenolic compounds and flavonoids (Ghasemi Pirbalouti et al. 2011a). Earlier studies have identified thymol, carvacrol, p-cymene and γ-terpinene as the major constituents of the essential oils of T. daenensis (Nickavar et al. 2005; Amiri 2012; Ghasemi Pirbalouti et al. 2013a). The essential oil and extracts isolated from T. daenensis have been shown to have biological and pharmacological activities, including anti-bacterial (Ghasemi Pirbalouti et al. 2010, 2013b), anti-fungal (Ghasemi Pirbalouti et al. 2012), antioxidant (Amiri 2012), insecticide (Gavadi Elmi et al. 2007), anti-adenovirus (Saderi and Abbasi 2011) and immune-omodulatory effects (Amirghofranet al. 2012; Ghasemi Pirbalouti et al. 2011b).
Plants grown in agricultural systems are exposed to many environmental stresses that limit their quality and yield potential. The main environmental stresses are biotic (infection caused by pathogens and damage by herbivores, including pests) or abiotic (temperature, drought, high soil salinity, chilling, heat, UV light, etc.) in nature (Stevens et al. 2006). Moisture deficit has a significant influence on growth and metabolic activities of plant species. Secondary metabolites of plants can be altered by environmental factors specially water deficit and improper temperature on many aspects of plant metabolism (Charles et al. 1994). For aromatic and medicinal crops, drought may cause significant changes in their metabolites yield and compositions (Bettaieb et al. 2009). Cell membranes are the main targets of degrading processes induced by drought (Pham-Thi et al. 1987). Results of a study by Monteiro de Paula et al. (1993) indicated that drought stress decreased biosynthesis of lipids in plants under water deficit condition. Upon perceiving environmental stresses plants activate a range of defense mechanisms which may also be induced artificially or enhanced by the application of certain chemicals (Raskin 1995; Rajasekaran and Blake 1999). There are several commercially available chemical compounds that could be used as elicitors to modify plant secondary metabolites and subsequently the bioactivity of medicinal plants.
Salicylic acid (SA) naturally occurs in plants at a very low concentration. It is a common plant-produced signal molecule that is responsible for inducing tolerance to a number of biotic (infection caused by fungi, bacteria and viruses) (Delaney et al. 1994; Yalpani et al. 1994) and abiotic stresses (drought, high soil salinity, chilling, heat, UV light and ozone and heavy metal) (Yalpani et al. 1994; Senaratna et al. 2003; Wang et al. 2004; Idrees et al. 2010). For example, SA has been identified as an important signaling element which is involved in establishing the local and systemic disease resistance response of plants after pathogen attack (Delaney et al. 1994; Loake and Grant 2007). The ameliorative effect of SA on plant growth under abiotic stress conditions have been related to its role in nutrient uptake, membrane stability, water relations, stomatal regulation, photosynthesis, growth and inhibition of ethylene biosynthesis (Glass and Dunlop 1974; Srivastava and Dwivedi 2000; Khan et al. 2003; Stevens et al. 2006; Arfan et al. 2007).
Limited studies have been documented to identify the effects of foliar application of SA on the accumulation of secondary metabolites in medical plants under field conditions. The present study was performed to evaluate the effect of various concentrations of SA on growth, essential oil, essential oil composition, and antibacterial and antioxidants activities of T. daenensis under two moisture regimes.
Materials and methods
Chemicals
Alkan standard solution C5–C24, 1,1-Diphenyl-2-picrylhydrazyl (DPPH) and SA were purchased from Sigma–Aldrich Co. (Steineheim, Germany). Buffered peptone water (BPW), mueller–hinton agar (MHA), dimethyl sulfoxide (DMSO), glycerol phosphate buffered saline (PBS), ethanol and anhydrous sodium sulphate were obtained from Merck Co. (Darmstadt, Germany).
Plant material and experimental site description
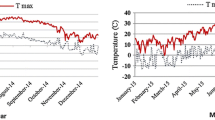
Thymus daenensis subsp. daenensis (local) seeds were obtained from the Research Center of Agricultural and Natural Resources (RCANR), Isfahan, Iran. In the spring 2011, the seedlings of T. daenensis were transplanted to research farm at Shahid–Fozveh Research Station, RCANR, Iran (latitude, 32°36′N; longitude 51°26′E, altitude, 1,612 m above sea level). According to multivariate statistical method, Isfahan is considered as cold and arid region (Yaghmaei et al. 2009), where its average annual rainfall and temperature are 140 mm and 14.5 °C, respectively. The meteorological data recorded during the trial period in each growing season is given in Fig. 1. A composite soil sample was collected from 0 to 30 cm depth. The soil sample was air-dried, and tested for pH, electrical conductivity (EC), organic carbon (through sulfuric acid method), soil texture (hydrometer method), total N (Kjeldahl method), available P (Olsen procedure) and available K after extraction with ammonium acetate. Details of soil and water properties are shown in Table 1. In this study, no inorganic fertilizer and systemic pesticide was used during the entire experiment, and weed control was done manually.
Experimental design and treatments
The experiment was conducted in a split plot design with three replications. Two irrigation regimes, viz., I1 (unstressed or control) and I2 (irrigation in 50 % field capacity when 50 % of maximum total available soil water was depleted in the upper 30 cm of the soil profile from early flowering until complete bloom) were assigned to the main plots. Subplots comprised three SA treatments (0.0, 1.5 and 3.0 M) sprayed twice at early bloom and two weeks later. SA solution (adjusted to pH 7.0 with NaOH) was sprayed using an atomizer onto the leaves until it completely ran off. The control plants were sprayed with distilled water, also adjusted to pH 7.0 with NaOH. Each experimental plot was 3 × 5 m, and plants were grown in 5 rows, with a spacing of 25 cm in rows 50 cm apart per replication. Adjacent subplots, main plots and replications were 1.0, 1.5 and 2.0 m apart, respectively. In 2011, no treatments were applied to the experimental plots. SA spray and moisture regimes were applied in spring 2012.
Measurements
The aerial parts of T. daenensis were harvested at full flowering stage (June 2012), and following data was collected: number of days and growing degree days (GDD) to onset of flowering, 50 % flowering, full bloom, terminal spike length, terminal spike weight, aerial size (maximum and minimum radius), canopy diameter, plant height, 1000-seed weight, herbage wet weight and dry weight, chlorophyll content, leaf width, leaf length and leaf are index (LAI). Chlorophyll content (SPAD unit) was measured using a portable chlorophyll meter (Minolta model SPAD 502) on five randomly selected leaves per plant. Phenological stages were recorded and GDD was calculated for selected growth stage using the following equation (Snyder 1985):
where Tmax and Tmin are daily maximum and minimum temperatures, respectively and Tb is the base temperature was set to 4 °C.
The main stem height was measured from the soil level to the tip of the tallest inflorescence. Harvested tissues were weighed and dried at 40 °C in a forced air oven for 72 h. Essential oil content based on dry matter (v/w), oil yield (g m−2), chemical compositions, antibacterial and antioxidant activities of the essential oils were estimated.
Essential oil isolation
Dried plant material (50 g) was powdered and subjected to hydro-distillation for 3 h using a Clevenger-type apparatus. The essential oils were dried with anhydrous sodium sulphate and kept in amber vials at 4 °C prior to use.
Identification of the oil components
The essential oils were analyzed using an Agilent 7890A gas chromatograph (Agilent Technologies, Palo Alto, CA, USA) with a HP-5MS 5 % phenylmethylsiloxane capillary column (30.00 m × 0.25 mm, 0.25 μm film thickness). Oven temperature was kept at 60 °C for 4 min initially, and then raised at the rate of 4 °C min−1 up to 260 °C. Injector and detector temperatures were set at 290 and 300 °C, respectively. Helium was used as a carrier gas at a flow rate of 2 ml min−1, and 0.1 μl samples were injected manually in the split mode. Peaks area percents were used for obtaining quantitative data. The gas chromatograph was coupled to an Agilent 5975 C (Agilent Technologies, Palo Alto, CA, USA) mass selective detector. The EI-MS operating parameters were: ionization voltage, 70 eV; ion source temperature, 200 °C. Retention indices were calculated for all components using a homologous series of n-alkanes (C5–C24) injected in conditions as described above. Identification of oil components was accomplished based on comparison of their retention times with those of authentic standards and by comparison of their mass spectral fragmentation patterns (WILLEY/ChemStation data system (Adams 2007).
Antibacterial test
Clinical isolates of two positive-Gram (Bacillus cereus and Listeria monocytogenes) and two negative-Gram (Proteus vulgaris and Salmonella typhimurium) bacteria strains obtained from Food Microbiology Laboratory, Veterinary Medicine Faculty (I.A.U.), Iran. They were identified using polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) and conventional morphological as well as biochemical tests. Stock cultures of bacteria were kept in 20 % glycerol PBS at −70 °C. The density of bacteria culture required for the test was adjusted to 1.0 McFarland standards (1.0 × 107 CFU/ml) measured using a spectrophotometer (Eppendorf, AG, Germany). The minimum inhibitory concentration (MIC) values were evaluated using the serial dilution method, according to standard methods (NCCLS 2003). Stock solutions of the essential oil and antibiotic standards (ampicillin and ciprofloxacin) were prepared in 5.0 % (v/v) DMSO. Dilution series, using BPW, were prepared from 0.016 to 0.25 mg ml−1. After incubation at 37 °C for 24 h, the microorganism growth inhibition was evaluated by measuring absorbance at 630 nm, using a spectrophotometer. Experiments were performed in triplicate at three different times. The minimum bactericidal concentrations (MBC) of essential oil were determined according to the MIC values. Five micro-liters from MIC tubes were transferred to agar plates (MHA) and incubated at 37 °C for 24 h. The MBC was referred to as the minimum concentration of essential oils with no viable bacteria.
Antioxidant test
The antioxidant capacity of the essential oils was evaluated by the method of Wang et al. (1998). The essential oils at different concentrations (16–500 μg ml−1) were mixed with the same volume of 0.2 mM methanol solution of DPPH. The disappearance of DPPH by essential oils after 30 min of incubation at room temperature was determined spectrophotometrically at 515 nm. Methanol was used to zero spectrophotometer. The absorbance of the DPPH radical without antioxidant, i.e. the control was measured daily using a Perkin–Elmer Lambda UV/VIS spectrophotometer at 515 nm against a blank, i.e. without DPPH. All tests were run in triplicate and an average was used. Decreasing of DPPH solution absorbance indicates an increase of DPPH radical scavenging activity. The amount of sample necessary to decrease the absorbance of DPPH by 50 % (IC50) was calculated graphically. This activity is given as % DPPH inhibition that is calculated as follows:
where AC(0) is the absorbance of the control at t = 0 min; and AA(t) is the absorbance of the antioxidant at t = 15 min.
Statistical analysis
Main and interaction effects of experimental factors were derived from two-way analysis of variance (ANOVA) based on the GLM procedure of the SAS statistical package (SAS/STAT® v.9.2. SAS Institute Inc., Cary, NC). The assumptions of variance analysis were tested by ensuring that the residuals were random and homogenous, with a normal distribution about a zero mean. The significance of differences among treatment means was tested using Duncan’s multiple range test (DMRT) at P ≤ 0.05.
Results and discussion
Growth characteristics
Results indicated that only irrigation treatment had a significant main effect on number of days and GDD required to reach early and full flowering (Tables 2, 3). Foliar application of SA influenced only number of days and GDD from early growing stage to 50 % and full flowering, minimum radius and canopy diameter.
SA at 3.0 M significantly increased the above-mentioned traits in T. daenensis. For example, GDD for early growing to 50 % and full flowering stages were increased to 1,115 and 1,348, respectively (Tables 2, 3). T. daenensis plants re-grew on 10–20 February 2012 and flowering began 100–103 days after re-growth (GDD = 872–925). It took 110–113 and 118–124 days for re-growing to reach 50 % (GDD = 1,055–1,115) and full flowering (GDD = 1,227–1,348), respectively. The highest values for minimum radius (46.4 cm) and canopy diameter (126.9 cm) were observed under foliar spray of 3.0 M SA (Tables 2 and 3).
As shown in Table 3, lower supplied irrigation resulted in reduced vegetative growth period and therefore plants bloomed earlier. The decrease in days and GDD of T. daenensis due to water deficiency has also been reported by other researchers (Desclaux and Roumet 1996). A more recent report by Tabrizi et al. (2011) indicated that increasing the irrigation intervals had no significant effect on developmental duration in Khorasan thyme (Thymus transcaspicus Klokov). Similarly, Bannayan et al. (2008) applied different irrigation intervals and reported that irrigation regimes did not show any effect on the duration of the developmental stages up to seed formation in black cumin (Nigella sativa L.) and isabgol (Plantago ovata Forsk.). In our study, SA application had no significant effect on measured growth indices of T. daenensis. However, Gharib (2006) concluded, the foliar application of SA increased growth in terms of plant height, number of branches, spikes and leaves per plant, leaf area and fresh and dry weights of herb in two species of sweet basil (Ocimum basilicum) and marjoram (Majorana hortensis) during three cuttings. In the present study, no interaction effects between irrigation regime and SA on measured growth and physiological traits were detected. Our results are not in agreement with an earlier report (Bahreininejad et al. (2013) that indicated that plant height, leaf area and total dry matter in T. daenensis cultivated in the same location were significantly reduced under drought stress.
Essential oil
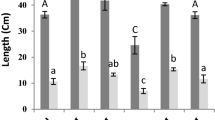
The color of oil extracted from aerial parts of T. daenensis in all treatments was yellow. Results showed that there was a significant difference between the two irrigation regimes for essential oil content. SA also increased essential oil content and oil yield of T. daenensis (Fig. 2a, b). Similarly, Bahreininejad et al. (2013) reported an increase in essential oil content, but a decrease in essential oil yield of T. daenensis increased under water stress. In addition, Simon et al. (1992) reported that water stress increased essential oil accumulation via higher density of oil glands due to the reduction in leaf area. Opposing results, however, indicated that optimum irrigation either resulted in higher essential oil accumulation (Figueiredo et al. 2008) or had no effect on essential oil content (Khazaie et al. 2008). Different results in water deficit effects on essential oil content could be related to stress level, thyme species as well as environmental conditions.
The effect of foliar application of SA on oil content of T. daenensis was influenced by irrigation regime (Table 3). The highest oil content (3.3 % v/w) was achieved by foliar spray of 1.5 M SA under reduced irrigation (I2). The lowest oil content (2.0 % v/w) was obtained when SA was not applied under normal irrigation (I1). There was no significant SA × irrigation interaction effect on oil yield. Our results indicated that SA at 3.0 M produced maximum oil content (3.2 % v/w) and yield (14.9 g m−2). Idrees et al. (2010) stated that improvement in essential oil content by foliar application of SA might be due to the increase in cycle growth, nutrients uptake or changes in leaf oil gland population and monoterpenes biosynthesis. Improvement of essential oil percentage and yield per plant in response to SA application has been reported in other plant species (Gharib 2006; Idrees et al. 2010).
Chemical compositions of oil
The chemical constituents of the essential oil identified by GC–MS are presented in Table 4. GC–MS analysis identified 32 essential oil constituents, detecting some major compounds, viz., thymol, carvacrol, p-cymene, β-myrcene, γ-terpinen, α-pinene, α-thujene, 1,8-cineol, borneol, carvacrol methyl ether, α-terpinene and β-caryophyllene. Results also indicated that there were significant differences between two irrigation treatments in terms of thymol, carvacrol, α-pinene, β-myrcene and β-caryophyllene (Fig. 3). Percentages of carvacrol, α-pinene, β-myrcene and β-caryophyllene in oils were higher in stressed plants than in non-stressed plants, whereas thymol percentage decreased under water deficit stress. The ANOVA showed that different levels of the foliar application of SA had significant effects on the major constitutes of T. daenensis essential oils (Fig. 4a, b). Different levels of SA and irrigation regimes had significant impacts on thymol, carvacrol, 1,8-cineol and β-myrcene contents (Table 4).
Our results demonstrated that essential oils obtained from T. daenensis contained oxygenated monoterpenes, monoterpene hydrocarbons and sesquiterpenes, confirming earlier reports that major volatile constituents obtained from the aerial parts of T. daenensis were thymol, carvacrol, p-cymene, γ-terpinene and β-caryophyllene (Nickavar et al. 2005; Amiri 2012; Bahreininejad et al. 2013; Ghasemi Pirbalouti et al. 2013a). The highest percentage of thymol (64.3 %), an important constituent of T. daenensis essential oil, was extracted from non-stressed plants. Monoterpenes are secondary metabolites formed in chloroplasts from freshly fixed carbon (Bohlmann et al. 1998) and their levels may, therefore, depend on CO2 acquisition and formation of photosynthesis intermediates (Loreto et al. 1996). Our results are in agreement with those from a study by Aziz et al. (2008) in T. vulgaris. In addition, carvacrol contents were reported to be reduced in T. vulgaris (Letchamo et al. 1995) and T. hyemalis (Aziz et al. 2008) under water deficit stress.
SA application significantly increased carvacrol, α-thujene, α-pinene and p-cymene, while decreased thymol and β-caryophyllene contents in the thyme essential oil (Fig. 4a, b). SA may convert thymol to its isomer (carvacrol), and is thought to play an important signaling role in the activation of various plant defense responses, such as the biosynthesis of special secondary metabolites, which function as phytoalexins in plants (Sirvent and Gibson 2002; Kang et al. 2004). The induction mechanism of plant defense is generally thought to be related to the elevation of H2O2 and other reactive oxygen species (ROS), which can then serve as second messengers in the defense signaling pathway (Lamb and Dixon 1997; Ebel and Mithöfer 1998; Shi et al. 2006). We earlier reported that the foliar application of jasmonic acid increased thymol and carvacrol contents in the essential oil obtained from T. daenensis aerial parts (Ashrafi et al. 2012). Pu et al. (2009) also suggested that exogenous application of SA to A. annua leaves induced artemisinin biosynthesis in at least two ways via increasing the conversion of dihydro-artemisinic acid into artemisinin caused by the burst of ROS and up-regulating the expression of genes involved in artemisinin biosynthesis.
Our results further indicated that foliar application of SA reduced the effect of water deficit stress on amount of thymol, a major compound in the essential oil of T. daenensis (Table 4). This demonstrated that SA at 1.5 M produced the highest level of carvacrol (5.6 %), but at 3.0 M produced the highest levels of 1,8-cineol (2.2 %) and β-myrcene (1.9 %) under reduced irrigation (Table 4). Similar interactive effect of SA and water stress on secondary metabolites production has been reported in other plant species (Idrees et al. 2010).
Antibacterial activity
The antibacterial activity of the essential oil of T. daenensis was assessed in vitro by the serial dilution methods against major bacterial pathogens (B. cereus, L. monocytogenes, P. vulgaris and S. typhimurium). Antibacterial activities were expressed as MIC and MBC values (Table 5). The essential oils exhibited varying levels of antibacterial activities against the investigated bacteria. The MICs of the essential oils were within concentration ranges of 0.062–0.5 mg ml−1, with corresponding ranges of 0.125–0.5 mg ml−1 MBCs (Table 5). In general, the thyme essential oil had higher antibacterial activity against B. cereus and P. vulgaris under normal than under reduced irrigation conditions. On the other hand, the oil extracted from stressed plants had higher antibacterial activity against S. typhimurium and L. monocytogenes than non-stressed plants (Table 5). Probably, this variation in the antibacterial activity could be attributed to the effects of soil moisture conditions on amounts of phenolic compounds, such as thymol, carvacrol, γ-terpinene, etc. (Bettaieb et al. 2011). In addition, carvacrol amount was found to be increased, with corresponding decline in the concentration of thymol under water deficit stress. However, Bahreininejad et al. (2013) reported an increment in thymol content in T. daenensis under water stress. Nevertheless, foliar application of SA was found to mitigate the negative effect of water deficit on the amount of thymol. The mechanisms by which essential oils can inhibit microorganisms vary. The essential oils containing phenolic compounds, such as thymol, carvacrol, γ-terpinene and p-cymene, are widely reported to possess high levels of antibacterial activities (Burt 2004).
Results of antibacterial activity of the essential oils of T. daenensis under foliar application of SA and irrigation treatments are shown in Table 5. The essential oils extracted from plants treated with 3.0 M SA exhibited highest antibacterial activities against B. cereus, P. vulgaris and L. monocytogenes, whereas that extracted from control plants had the highest antibacterial activity against S. typhimurium (Table 5). SA, being a natural phenolic compound, is an important component in the signal transduction pathway (Qin et al. 2003). It is also involved in local and systemic resistance to pathogens (Meena et al. 2001). Exogenous application of SA at nontoxic concentrations to susceptible plants could enhance their resistance against pathogens (Murphy et al. 2000). Taken together, results of the antibacterial activities of the thyme essential oils under foliar SA application and irrigation treatments indicate that SA does not exhibit higher antibacterial activities against four bacteria strains under normal irrigation condition as compared to other treatments (Table 5).
Antioxidant activity
The DPPH is a stable free radical, which has been widely accepted as a tool for estimating the free radical scavenging activities of antioxidants (Hu et al. 2004). The antioxidant activity in thyme is attributed both to its essential oil and soluble phenolic fractions (Amiri 2012). The lower IC50 value indicates a stronger ability of the extract to act as a DPPH scavenger, while the higher IC50 value indicates a lower scavenging activity of the scavengers as more scavengers are required to achieve 50 % scavenging reaction. The present study showed that the essential oil of T. daenensis had higher antioxidant activity under normal irrigation (Table 5), indicating further that different levels of SA significantly affected antioxidant activities of T. daenensis oil (Table 5). The antioxidant activity of phenolic compounds in plants is mainly due to their redox properties and chemical structure, which can play an important role in neutralizing ROS, such as free radicals, singlet and triplet oxygen and peroxides (Wang and Zheng 2001). Pérez-Tortosa et al. (2012) reported that SA decreased rosmarinic acid in methanol extract of Thymus membranaceus shoots. They also suggested that a single application of SA on culture media resulted in an increase in rosmarinic acid and phenolic levels, which in turn can improve antioxidant properties of the extracts. A direct physiological effect of SA is the alteration of antioxidant enzyme activities in vivo. SA is also involved in activation of the stress-induced antioxidant system when plants are exposed to stress (Borsani et al. 2001), and is now considered to be a hormonal substance that plays a key part in regulating plant growth and development. Exogenous SA could regulate the activities of antioxidant enzymes and increase plant tolerance to the abiotic stress (He et al. 2002).
Conclusions
Foliar application of 1.5 and 3.0 M SA increases essential oil content and yield in T. daenensis by 27 and 60 %, respectively. SA also significantly increases secondary metabolites, such as carvacrol, α-thujene, α-pinene and p-cymene. It mitigates the deleterious effect of water deficit stress on thymol content of the thyme essential oil. The essential oils extracted from T. daenensis plants sprayed with 1.5 and 3.0 M SA have antioxidant and antibacterial activities, respectively. In conclusion, SA is found to positively affect biological activities, essential oil yield and its major constituents in T. daenensis under water deficit stress.
Abbreviations
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- GDD:
-
Growing degree days
- MBC:
-
Minimum bactericidal concentration
- MIC:
-
Minimum inhibitory concentration
- SA:
-
Salicylic acid
References
Adams RP (2007) Identification of essential oil components by gas chromatography/mass spectrometery, 4th edn. Allured Publishing Corporation, Carol Stream, IL, USA
Amirghofran Z, Ahmadi H, Karimi MH (2012) Immunomodulatory activity of the water extract of Thymus vulgaris, Thymus daenensis, and Zataria multiflora on dendritic cells and t cells tesponses. J Immunoass Immunochem 33:388–402
Amiri H (2012) Essential oils composition and antioxidant properties of three Thymus species. Evid Based Complement Altern Med 2012:1–8
Arfan M, Athar HR, Ashraf M (2007) Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J Plant Physiol 164:685–694
Ashrafi M, Ghasemi Pirbalouti A, Rahimmalek M, Hamedi B (2012) The effect of foliar application of jasmonic acid on Thymus daenensis Celak. Planta Med 78:35
Aziz EE, Hendawy SF, Ezz El-Din AA, Omer EA (2008) Effect of soil type and irrigation intervals on plant growth, essential oil yield and constituents of Thymus vulgaris plant. Am Euras J Agric Environ Sci 4:443–450
Bahreininejad B, Razmjoo J, Mirza M (2013) Influence of water stress on morpho-physiological and phytochemical traits in Thymus daenensis. Int J Plant Prod 7:155–166
Bannayan M, Nadjafi F, Azizi M, Tabrizi L, Rastgoo M (2008) Yield and seed quality of Plantago ovata and Nigella sativa under different irrigation treatments. Ind Crops Prod 27:11–16
Bettaieb I, Zakhama N, Wannes WA, Kchouk ME, Marzouk B (2009) Water deficit effects on Salvia officinalis fatty acids and essential oils composition. Sci Hortic 120:271–275
Bohlmann J, Meyer-Gauen G, Croteau R (1998) Plant terpenoid synthases: molecular biology and phylogenetic analysis. Proc Natl Acad Sci USA 95:4126–4133
Borsani O, Valpuesta V, Botella MA (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol 126:1024–1030
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods. Int J Food Microbiol 94:223–253
Charles O, Joly R, Simon JE (1994) Effect of osmotic stress on the essential oil content and composition of peppermint. Phytochemistry 29:2837–2840
Cronquist A (1988) The evolution and classification of flowering plants. The New York Botanical Garden, New York, USA
Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J (1994) A central role of salicylic acid in plant disease resistance. Science 266:1247–1250
Desclaux D, Roumet P (1996) Impact of drought stress on the phenology of two soybean (Glycine max L. Merr) cultivars. Field Crop Res 46:61–70
Ebel J, Mithöfer A (1998) Early events in the elicitation of plant defense. Planta 206:335–348
Figueiredo AC, Barroso JG, Pedro LG, Scheffer JJ (2008) Factors affecting secondary metabolite production in plants: volatile components and essential oils. Flavour Fragr J 23:213–226
Gavadi Elmi M, Karami J, Bandani AR (2007) Fumigant toxicity of three plant essential oils on the adults of Callosobruchus maculates (Col: Bruchidae) in vitro. New Find Agric 2:71–78
Gharib FAE (2006) Effect of salicylic acid on the growth, metabolic activities and oil content of basil and marjoram. Int J Agric Biol 4:485–492
Ghasemi Pirbalouti A (2009) Medicinal plants used in Chaharmahal and Bakhtyari districts Iran. Herba Polon 55:69–75
Ghasemi Pirbalouti A, Malekpoor F, Enteshari S, Yousefi M, Momtaz H, Hamedi B (2010) Antibacterial activity of some folklore medicinal plants used by Bakhtiari tribal in Southwest Iran. Int J Biol 2:55–63
Ghasemi Pirbalouti A, Rahimmalek M, Malekpoor F, Karimi A (2011a) Variation in antibacterial activity, thymol and carvacrol contents of wild populations of Thymus daenensis subsp. daenensis Celak. Plant Omics J 4:209–214
Ghasemi Pirbalouti A, Pirali E, Pishkar GH, Jalali SMA, Reyesi M, Jafarian Dehkordi M, Hamedi B (2011b) The essential oils of some medicinal plants on the immune system and growth of rainbow trout (Oncorhynchus mykiss). J Herb Drugs 2:149–155
Ghasemi Pirbalouti A, Malekpoor F, Hamedi B (2012) Ethnobotany and antimicrobial activity of medicinal plants of Bakhtiari Zagross mountains Iran. J Med Plants Res 6:675–679
Ghasemi Pirbalouti A, Hashemi M, Taherian Ghahfarokhi F (2013a) Essential oil and chemical compositions of wild and cultivated Thymus daenensis Celak and Thymus vulgaris L. Ind Crop Prod 48:43–48
Ghasemi Pirbalouti A, Neshat SH, Rahimi E, Hamedi B, Malekpoor F (2013b) Chemical composition and antibacterial activity of essential oils of Iranian herbs against Staphylococcus aureus isolated from milk. Int J Food Prop (in press)
Glass AD, Dunlop J (1974) Influence of phenolic acids on ion uptake IV. Depolarization of membrane potentials. Plant Physiol 54:855–858
He CY, Zhang JS, Chen SY (2002) A soybean gene encoding a proline-rich protein is regulated by salicylic acid, an endogenous circadian rhythm and by various stresses. Theor Appl Genet 104:1125–1131
Hu FL, Lu RL, Huang B, Ming L (2004) Free radical scavenging activity of extracts prepared from fresh leaves of selected Chinese medicinal plants. Fitoterapia 75:14–23
Idrees M, Khan MMA, Aftab T, Naeem M, Hashmi N (2010) Salicylic acid-induced physiological and biochemical changes in lemongrass varieties under water stress. J Plant Interact 5:293–303
Jordán MJ, Martínez RM, Maria A, Sotomayor JA (2003) Watering level effect on Thymus hyemalis Lange essential oil yield and composition. J Agric Food Chem 51:5420–5427
Kang SM, Jung HY, Kang YM, Yun DJ, Bahk JD, Yang JK, Choi MS (2004) Effects of methyl jasmonate and salicylic acid on the production of tropane alkaloids and the expression of PMT and H6H in adventitious root cultures of Scopolia parviflora. Plant Sci 166:745–751
Khan W, Prithiviraj B, Smith DL (2003) Photosynthetic responses of corn and soybean to foliar application of salicylates. J Plant Physiol 160:485–492
Khazaie HR, Nadjafi F, Bannayan N (2008) Effect of irrigation frequency and planting density on herbage biomass and oil production of thyme (Thymus vulgaris) and hyssop (Hyssopus officinalis). Ind Crop Prod 27:315–321
Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Biol 48:251–275
Letchamo W, Xu HL, Gosselin A (1995) Variations in photosynthesis and essential oil in thyme. J Plant Physiol 147:29–37
Loake G, Grant M (2007) Salicylic acid in plant defense—the players and protagonists. Curr Opin Plant Biol 10:466–472
Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Fabozzi C, Tricoli D (1996) Evidence of the photosynthetic origin of monoterpenes emitted by Quercus ilex L. leaves by 13C labeling. Plant Physiol 110:1317–1322
Meena B, Marimuthu T, Velazhahan R (2001) Salicylic acid induces systemic resistance in groundnut against late leaf spot caused by Cercosporidium personatum. J Mycol Plant Pathol 31:139–145
Monteiro de Paula F, Pham Thi AT, Zuily Fodil Y, Ferrari-Iliou R, Vieira da Silva J, Perry NB, Anderson RE, Brennan NJ, Douglas MH, Heanney AJ, McGimpsey JA, Smallfield BM (1993) Essential oils from dalmatian sage (Salvia officinalis L.): variations among individuals, plant parts, seasons, and sites. J Agric Food Chem 47:2048–2054
Murphy AM, Holcombe LJ, Carr JP (2000) Characteristics of salicylic acid-induced delay in disease caused by a necrotrophic fungal pathogen in tobacco. Physiol Mol Plant Pathol 57:47–54
NCCLS (2003) National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—Sixth Edition: Approved Standard M7-A6. Wayne, PA, USA
Nickavar B, Mojab F, Dolat-Abadi R (2005) Analysis of the essential oils of two Thymus species from Iran. Food Chem 90:609–611
Pérez-Tortosa V, López-Orenes A, Martínez-Pérez A, Ferrer MA, Calderón AA (2012) Antioxidant activity and rosmarinic acid changes in salicylic acid-treated Thymus membranaceus shoots. Food Chem 130:362–369
Pham-Thi AT, Borrel-Flood C, Vieira da Silva J, Justin AM, Mazliak P (1987) Effects of drought on [1-14C]-oleic and [1-14C]-linoleic acid desaturation in cotton leaves. Physiol Plant 69:147–150
Pu GB, Ma DM, Chen JL, Ma LQ, Wang H, Li GF, Liu BY (2009) Salicylic acid activates artemisinin biosynthesis in Artemisia annua L. Plant Cell Rep 28:1127–1135
Qin GZ, Tian SP, Xu Y, Wan YK (2003) Enhancement of biocontrol efficacy of antagonistic yeasts by salicylic acid in sweet cherry fruit. Physiol Mol Plant Pathol 62:147–154
Rajasekaran LR, Blake TJ (1999) New plant growth regulators protect photosynthesis and enhance growth under drought of jack pine seedlings. J Plant Growth Regul 18:175–181
Raskin I (1995) Salicylic acid. In: Davies PJ (ed) Plant hormones and their role in plant growth and development, 2nd edn. Kluwer Academic Publishers, Dordrecht, p 205
Saderi H, Abbasi M (2011) Evaluation of anti-adenovirus activity of some plants from Lamiaceae family grown in Iran in cell culture. Afr J Biotechnol 10:17546–17550
Senaratna T, Merritt D, Dixon K, Bunn E, Touchell D, Sivasithamparam K (2003) Benzoic acid may act as the functional group in salicylic acid and derivatives in the induction of multiple stress tolerance in plants. Plant Growth Regul 39:77–81
Shi Q, Bao Z, Zhu Z, Ying Q, Qian Q (2006) Effects of different treatments of salicylic acid on heat tolerance, chlorophyll fluorescence, and antioxidant enzyme activity in seedlings of Cucumis sativa L. Plant Growth Regul 48:127–135
Simon JE, Reiss-Bubenheim D, Joly RJ, Charles DJ (1992) Water stress-induced alterations in essential oil content and composition of sweet basil. J Essent Oil Res 4:71–75
Sirvent T, Gibson D (2002) Induction of hypericins and hyperforin in Hypericum perforatum L. in response to biotic and chemical elicitors. Physiol Mol Plant Pathol 60:311–320
Snyder RL (1985) Hand calculating degree-days. Agric Forest Meteorol 35:353–358
Srivastava MK, Dwivedi UN (2000) Delayed ripening of banana fruit by salicylic acid. Plant Sci 158:87–96
Stahl-Biskup E, Saez F (2002) Thyme the genus Thymus. Taylor & Francis, NY, NJ
Stevens J, Senaratna T, Sivasithamparam K (2006) Salicylic acid induces salinity tolerance in tomato (Lycopersicon esculentum cv. Roma): associated changes in gas exchange, water relations and membrane stabilisation. Plant Growth Regul 49:77–83
Tabrizi L, Koocheki A, Moghaddam PR, Mahallati MN, Bannayan M (2011) Effect of irrigation and organic manure on Khorasan thyme (Thymus transcaspicus Klokov). Arch Agron Soil Sci 57:317–326
Wang SY, Zheng W (2001) Effect of plant growth temperature on antioxidant capacity in strawberry. J Agric Food Chem 49:4977–4982
Wang M, Li J, Rangarajan M, Shao Y, La Voie EJ, Huang CT, Ho CT (1998) Antioxidative phenolic compounds from sage (Salvia officinalis). J Agric Food Chem 46:4869–4873
Wang YD, Yuan YJ, Wu JC (2004) Induction studies of methyl jasmonate and salicylic acid on taxane production in suspension cultures of Taxus chinensis var. mairei. Biochem Eng J 19:259–265
Yaghmaei L, Soltani S, Khodagholi M (2009) Bioclimatic classification of Isfahan province using multivariate statistical methods. Int J Climatol 29:1850–1861
Yalpani N, Enyedi AJ, León J, Raskin I (1994) Ultraviolet light and ozone stimulate accumulation of salicylic acid, pathogenesis-related proteins and virus resistance in tobacco. Planta 193:372–376
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi Pirbalouti, A., Rahmani Samani, M., Hashemi, M. et al. Salicylic acid affects growth, essential oil and chemical compositions of thyme (Thymus daenensis Celak.) under reduced irrigation. Plant Growth Regul 72, 289–301 (2014). https://doi.org/10.1007/s10725-013-9860-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9860-1