Abstract
Endogenous levels of IAA, ABA and four types of CKs were analyzed in zygotic and indirect (ISE) and direct somatic embryogenesis of Acca sellowiana. Zygotic and somatic embryos at different developmental stages were sampled for morphological and hormonal analysis. Both embryo types showed substantial asymmetry in hormone levels. Zygotic embryos displayed a conspicuous peak of IAA in early developmental stages. The results outlined the hormonal variations occurring during zygotic and somatic embryogenesis regarding the timing, nature and hormonal status involved in both processes. The short transient pulse of IAA observed on the 3rd day in culture was suggested to be involved with the signaling for the induction of somatic embryogenesis. Fertilized ovule development was associated with increased IAA levels 21–24 days after pollination, followed by a sharp decrease in the cotyledonary stage, both in zygotic and somatic embryos. There was a prominent increase in ABA levels in cultures which generated ISE 24–30 days after pollination, a period that corresponds to the heart and torpedo stages. The levels of total CKs (Z, [9R]Z, iP and [9R]iP) were also always higher in zygotic than in somatic embryogenesis. While zygotic embryogenesis was dominated by the presence of zeatin, the somatic process, contrarily, was characterized by a large variation of the other cytokinin forms and amounts studied. The above results, when taken together, could be related to the previously observed high frequency formation of anomalous somatic embryos formed in A. sellowiana, as well as to their low germination ability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zygotic (ZE) and somatic embryogenesis (SE) are complex processes, the former initiating with gamete fusion and resulting in the formation of a mature embryo, and the latter originating from a single or a set of somatic cells. Embryo development involves distinct integrated events such as mitosis, establishment of polarity, cellular differentiation, synthesis of complex metabolites (including hormones) and storage of reserve substances (Dodeman et al. 1997).
Zygotic as well as many somatic embryos are bipolar structures, consisting essentially of an axis with short and tiny apices. From an ontogenetic view, both embryos share several developmental stages, namely globular, heart, torpedo and cotyledonary. In contrast to zygotic embryos, the somatic ones develop in the absence of vascular connections with the mother plant, indicating the existence of a genetic program within the initial cells (Zimmerman 1993). Another difference is the continuous growth of somatic embryos resulting from a lack of developmental arrest, often leading to the formation of abnormal structures with very low frequency of plant development (Faure et al. 1998; Pescador et al. 2008).
The route of SE is divided into two phases: induction and expression. In the first phase, mature cells acquire embryogenetic competence, while in the second the induced cells display their embryogenetic competence and differentiate to form somatic embryos (Jiménez 2001). In differentiated cells the processes of induction and competence are strongly influenced by two hormones: auxin and cytokinins (Christianson and Warnick 1983).
Since somatic cells are not naturally embryogenetic, an induction phase is required for these cells to acquire embryogenetic competence, while the zygote in sexual reproduction is intrinsically embryogenetic (Namasivayam 2007). In general, the induction of somatic embryogenesis has been achieved by trial and error, through the use of several plant growth regulators and/or physical shock treatments (Namasivayam 2007). A relationship between different types of imposed stress and the increase of endogenous auxin levels was proposed for the formation of embryonic cells in leaf-derived explants of alfalfa (Pasternak et al. 2002). Fluctuation of endogenous levels of IAA, ABA and cytokinins were observed in thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Murthy et al. 1995).
Many studies have been published on SE during the last five decades; however relatively little work has been carried out on the involvement of endogenous hormones during the initiation and progression of that process. Thus, the main goal of this study was to analyze and compare the endogenous levels of IAA, CKs and ABA throughout ZE and SE of A. sellowiana in order to acquire a better understanding of the embryogenetic process and potentially optimize protocols for the production of somatic embryos.
Materials and methods
Obtaining zygotic and somatic embryos
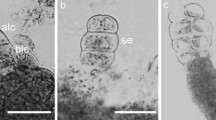
Fertilized and unfertilized ovules, as well as zygotic and somatic embryos of A. sellowiana (O.Berg) Burret were used in this study. Ovules derived from unpollinated flowers at anthesis were considered to be at time zero in the analysis of zygotic embryogenesis. For the analysis of embryos at different developmental phases, about 500 flowers were emasculated, allowing for manual pollination. During the first 30 days after pollination (DAP), plant material was collected every 3 days and subsequently every 10 days, until the physiological maturation of the fruit, which occurs about 120 DAP (Guerra et al. 1997). Each harvest, containing representative samples of different developmental stages, consisted of 10 replicates. Plant material was stored at −20 °C until the analyses were carried out. Embryos of each developmental stage were showed in Fig. 1.
For the induction of somatic embryogenesis, zygotic embryos (0.4 mm) excised from mature seeds (about 120 days old) were used. They were inoculated in test tubes (25 × 150 mm) containing 10 mL of culture medium. The medium consisted of full-strength concentrations of macro and micronutrients (von Arnold and Eriksson 1981) and Morel vitamins (Morel and Wetmore 1951), supplemented with 30 g L−1 sucrose, 0.7 % agar (Sigma A4550), 20 μM 2,4-dichlrophenoxyacetic acid (2,4-D) and 4 mM glutamine. The pH of the medium was adjusted to 5.8 before autoclaving at 120 °C. Cultures were incubated in the dark at 25 °C for 15 days, after which they were transferred to a 2,4-D-free medium for direct somatic embryogenesis (DSE) expression. In the case of indirect somatic embryogenesis (ISE) the initial zygotic embryo cultures were transferred to fresh 20 μM 2,4-D enriched medium. After 70 days, somatic embryos originated at different developmental stages from both routes (DSE and ISE, Fig. 2) were sampled under a stereomicroscope for the hormone analysis. Embryos of each developmental stage were collected from 10 test tubes to make up a final compound sample of 1 g fresh of weight.
Measurements of cytokinins and ABA
Endogenous levels of ABA and cytokinins (CKS), zeatin (Z), in both cis and trans forms, zeatin riboside ([9R], isopentenyladenine (iP) and isopentenyladenosine ([9R]iP) were measured in samples of one-gram fresh material by an immunoenzymatic method according to Peres et al. (1997), which allowed the determination of those hormones in the same extract. Freeze-dried powdered tissues were stirred and extracted with cold 80 % methanol containing butylhydroxytoluene (0.18 mM) as an antioxidant for 60 h at 4 °C in darkness. Tritiated radiolabeled standards ([3H]- IAA, Amersham® cis, trans [3H]-Z and [3H]-ABA (250 μCi in 250 μL) Isotope Laboratory® with specificity of 9.25 × 109, 6 × 1011 Bq and 100 μL, respectively) were added to samples for recovery estimation after purification, by scintillation counter. The estimation of all cytokinin types were based on the tritiated radiolabeled standards cis and trans [3H]Z. The metanolic extracts were filtered and then passed through Sep-Pak C-18 cartridge.
After filtration, the eluates were dried in a speed vacuum concentrator (Speed Vac, Heto®, CT 100) and the residues redissolved in 500 μL of acidic water (ultrapure water + 0.2 % formic acid, pH 3.0). The hormones were eluted from the reverse-phase HPLC column C18 (Prep Nova-Pak HR/60 Å, 6 μm, 7.8 × 300 mm, Waters®) with a 0.2 % formic acid (A)/methanol (B) gradient at a flow rate of 3 mL min–1. For the gradient, the following protocol was used (%B in A in each case): 0–10 min, 18 %; 10–11 min, 25 %; 11–65 min, 33 %; 65–80 min, 40 %. The hormones were then separated over 80 min by HPLC using a reverse phase, semi-preparative μBoundapak 19 × 310 mm column at a flow rate of 5 mL min−1 with a 5–50 % methanol gradient in phosphoric acid buffered to pH 3.0 with twethylamine. Fractions were collected at 30-s intervals and dried in a speed vacuum concentrator. The measurements of ABA and Z in both cis and trans forms, [9R]Z, iP and [9R]iP were carried out by the enzyme-linked immunosorbent assay (ELISA) method with monoclonal anti-ABA and, polyclonal rabbit anti-[9R]Z (for Z and [9R]Z) and anti-[9R]iP (for iP and [9R]iP) antibodies.
The hormone level in each sample was measured four times and the standard error was calculated. Calculations were made by reference to a calibration curve established on each microtitration plate with a fourth-order polynomial regression obtained from four experimental standard curves.
Indoleacetic acid (IAA) quantification was carried out by GC–MS–SIM based on Chen et al. (1988). HPLC fractions corresponding to IAA were methylated with 1 mL etheric diazomethane for 5 min and dried at room temperature. The methylated extracts were redissolved in 100 mL ethyl acetate and analyzed in a Hewlett-Packard® 6,890 gas chromatograph connected to a mass spectrometer detector model 5973. The column used for separation was an HP-1701 (30 m, internal diameter (ID) 0.25 mm, internal thickness (IT) 0.5 μm) and helium was the carrier gas, with a flux of 4 mL min−1 The injected volume for each sample was 5 μL. Ions with a mass/charge (m/z) ratio between 130 and 189 (endogenous IAA), and between 136 and 195 (internal standard) were monitored. The endogenous IAA concentration was obtained by comparing the peak areas of the chromatograms extracted in m/z 130–136 and 189–195.
Results
Hormonal status during induction and expression of ZE and SE
The amounts of IAA during the initial time course of ovule-embryo development and ISE are displayed in Fig. 3. Following pollination, no immediate detectable changes in auxin levels were observed in the ovules. Auxin was observed at very low levels and nearly constant up to the 21st day when fertilization took place. After this, its concentration increased rapidly to about 60 times (24th day), then decreased until the 30th day. On the other hand, in the ISE process, a conspicuous IAA pulse of about 23 times above normal occurred faster, on the 3rd day of explant incubation, decreasing and disappearing by the 6th day in culture. From the 6th day on, no more auxin was detected.
The levels of total CKs were expressively higher in the sexual process than in the asexual one during the first 30 days (Fig. 4). Three days after pollination CKs levels dropped until the 15th DAP, and exhibited a significant increment on the 18th day, followed by a second increase 3 days after fertilization (24th day). By the 30th day total CKs levels reached 10,980 pmol g−1 FM. As for the ISE, the total CKs in initial explants (mature somatic embryos) decreased from 192.12 pmol g−1 FM to 34.16 pmol g−1 FM after 6 days of incubation, a reduction of 82 %. A small and transient level increase occurred between the 21st and 27th day, which coincided with the presence of globular, heart and torpedo developmental phases (Fig. 15).
The distribution of the four types of CKs found in the ovules before and for the first 30 DAP in ZE is shown in Fig. 5. Clearly, the abundance of zeatin largely surpasses all other forms of detectable CKs, representing more than 90 % of the total amount. It is interesting to note that the amounts of zeatin were practically the same in unpollinated (time zero) and pollinated ovules up to the 30th day, when the embryos in the globular stage were already present.
However, a distinct hormonal profile was observed in the ISE. The levels of CKs found in this route (Fig. 6) varied during the first 30 days of culture incubation, which greatly differed from what was observed in the ZE process (Fig. 5). The predominant presence of iP in the explants (time zero) was drastically reduced just after the 3rd day in culture and was maintained at a steady level up to the 30th day. On the other hand, zeatin reached higher levels on the 9th day and from the 18th to 24th day, closely coinciding with the globular, heart and torpedo stages, respectively. In a certain way, the variation in the levels of ZR was rather opposite to that observed for zeatin. Only two peaks were reached by iPR.
Substantial differences were also observed in ABA between zygotic and somatic embryogenetic cultures. In the unpollinated ovules the levels of ABA (4,216.67 pmol g−1 FM) rapidly decreased (10-fold) up to the 6th DAP, and no other changes in the concentration of that hormone was observed up to the 30th DAP. Regarding somatic embryogenesis (Fig. 7), relatively low levels of ABA were found in the initial explants (673.54 pmol g−1 FM−1), which dropped to 10.4 nmol g−1 FM on the 9th day of culture (a 64-fold reduction), which was maintained until the 21th day. This period coincides with cell differentiation and intense proliferation of the cultures. However, from the 24th to the 30th a highly significant increase in ABA (96-fold) was observed, a period that coincides with the establishment of at least three developmental stages of somatic embryogenesis of A. sellowiana: heart, torpedo and cotyledonary. Intriguingly, in contrast to ZE, this ABA accumulation occurred rather early in the ontogeny of somatic embryos.
Hormonal levels
Figure 8 shows the amounts of IAA in globular, heart, torpedo and cotyledonary stages obtained from zygotic embryos (ZE), as well as ISE and DSE. The results showed clear differences in their respective ontogeny. As a rule, all ZE stages exhibited substantially higher auxin levels than the equivalent SE phases; the globular ZE phase yielding the highest amount of this hormone. Its level was 10 and 14-fold higher in ZE than in DSE and ISE, respectively. Moreover, while the concentration of IAA gradually decreased (5-fold) from globular to cotyledonary ZE phases, no differences were observed in SE until the torpedo phase, regardless of their origin (through either direct or indirect embryogenesis). Virtually no IAA was detected in the cotyledonary SE phase.
Comparing the total CK levels in the globular phases of both ZE and SE (Fig. 9), it was observed that they reached 4,617.91 pmol g−1 FM in the former, while in the latter, the levels dropped to 1,004.37 and 185.33 pmol g−1 FM in ISE and DSE, respectively. It must still be emphasized that this phase represents the only one in which significant differences between both types of somatic embryos were observed. After that point, only traces of total CKs were detected in SE phases. As for zygotic embryos, a decrease of about 16-fold occurred along the embryogenesis process as a whole, mainly caused by a drastic drop of this hormone in the cotyledonary phase. Heart and torpedo developmental stages did not show any significant differences.
A strong predominance of zeatin during ZE developmental stages was detected (Fig. 10). In view of the abundance of zeatin observed during ovule and embryo establishment (Fig. 5), it is plausible to consider a relationship between this CK and sexual reproduction of A. sellowiana.
However, in contrast to ZE, the corresponding SE was characterized by a lack of CKs regardless of their origin (Figs. 11 and 12). In the two SE processes the CK amounts varied both within each type as well as between them. Thus, for instance, in the globular phase the iP form was the predominant form in SE (Fig. 11), while zeatin proved to be the most important in DSE (Fig. 12). In a certain way, iP and zeatin decreased along somatic embryogenesis.
Similarly to that observed for auxin and CKs, differences in ABA levels were found also between ZE and SE developmental stages (Fig. 13). As might be expected, this hormone was totally absent during the globular, heart and torpedo developmental stages even though at the cotyledonary phase a conspicuous increment of ABA concentration took place, reaching 8,817,03 pmol g−1 FM on the 30th day. Although significantly smaller, an ABA peak in somatic embryos was also observed at the cotyledonary phase.
Discussion
Hormonal changes during zygotic and somatic embryogenesis
Contrarily to ZE which is intrinsically embryogenetic, somatic cells are not naturally embryogenetic, and thus an induction phase is required for cells to acquire embryogenetic competence (Dodeman et al. 1997; Namasivayam 2007). Both induction and expression phases appear to be independent of each other, and are influenced by different factors (Jiménez 2001). Auxin has so far been considered the most prominent plant hormone related to cell division and differentiation, as well as in the triggering of SE. The pulse of IAA observed in A. sellowiana (Fig. 3) is consistent with the induction phase, and a close correlation between an increase in transient IAA and early activation of cell division was also observed. The importance of polar distribution and transport of IAA was essential for the development of zygote and embryo differentiation in Nicotiana tabacum (Chen et al. 2010). Specifically for the induction phase, the existing explants’ gene expression pattern appears to be reprogrammed for commitment to embryogenesis, during which enhanced endogenous auxin levels and DNA methylation seem to play important roles (Lo Schiavo et al. 1989).
Several genes have been proposed for the control of SE (Fehér et al. 2003; Barton 2010). Amongst them Wuschel (WUS) appears to be a prominent candidate. In transgenic Arabidopsis plants WUS expression responded to auxin treatment giving rise directly to ectopic embryo formation even in the roots (Gallois et al. 2004). A close relationship between WUS expression and an endogenous gradient of PIN1-mediated polar auxin transport was demonstrated for SE in the callus of Arabidopsis thaliana (Su et al. (2009). This gene family is required for both formation and maintenance of stem cells in the shoot apical meristem of this model plant (Haecker et al. 2004), and both organogenetic events are considered critical for zygotic embryo development (Meinke 1991).
In Vitis vinifera, the WUS-related homeobox genes, VvWOX3 and VvWOX11, were strongly activated in correspondence to torpedo and cotyledonary phases of somatic embryos (Gambino et al. 2011). These results, if taken together, seem to indicate that ZE as much as SE processes depends on a tuned and complex interaction of endogenous auxin and some genes for correct embryo formation and development. If so, it is plausible to consider that disturbances in this hormonal-molecular-biochemical status could cause deleterious effects on both embryogenesis and embryo development processes. In view of this, it is also plausible to believe that the relatively high frequency of anomalous somatic embryos connected to the very low percentage of germination observed in A. sellowiana (Pescador et al. 2008), could be seen as a consequence of these disturbances.
The conspicuous predominance of zeatin—more than 90 % of total CKs—found both before and after pollination, seems to point out that A. sellowiana ZE (Fig. 5) occurred under a stable status of endogenous CK type, a condition greatly different from that found for the SE (Fig. 6). Despite the importance of the balance of CKs and auxins on the overall plant development, relatively little is known about the role of the former in the triggering of SE. In the explants of A. sellowiana, the initial relatively high level of total CKs found on the 3rd day of culture (Fig. 4), represented mainly by ZR and iP forms (Fig. 6), coincided with a peak of IAA (Fig. 3) which, in turn, could have synergistically triggered the first steps of SE.
In Leymus chinensis seed development, only ZR levels differed significantly from the other monitored hormones—ZR, IAA, GA and ABA—(Ma et al. 2010). Although the physiological importance of CK riboside forms on the control of developmental events is still controversial, the AHK3 receptor showed to be activated in plants of A. thaliana treated with ZR (Spíchal et al. 2004). Taking that into account, it is reasonable to believe that in A. sellowiana explants, the induction of embryogenesis should have resulted mainly from high levels of IAA and ZR. These in turn, by synergism, favored cell division about 9 days later (data not shown), while the total CKs and IAA levels were significantly decreased. A relationship between reduced endogenous hormone levels and cell division initiation was also observed during SE from cotton hypocotyls (Zing et al. 2007). It is important to emphasize the fact that some SE stages, such as proembryo cluster cells as well as globular, cotyledonary and torpedo phases (Fig. 13), also occurred under a relatively stable low CKs-auxin status, marked by a slight predominance of total CKs (Fig. 4) over IAA levels (Fig. 3).
In fact, under a hormonal point of view, the levels of CKs, IAA and ABA found in A. sellowiana consistently corroborate the conspicuous differences in the initial events of ZE and SE (Figs. 3, 4 and 7). Thus, while IAA levels in SE occurred just 3 days after explant inoculation (Fig. 3), in the zygotic process it was observed 21 days after pollination, sharply coinciding with ovule fertilization. Considering that an increase in CKs was also observed just after fertilization (Fig. 4), it is reasonable to assume the importance of the initial zygotic phases as an important source of this hormone. The increase in CKs around the 18th DAP (2 days before fertilization), probably arose from the unfertilized ovule tissues. It is interesting to note that in A. sellowiana, fertilization takes place under an ovule hormonal status predominantly marked by higher levels of CKs [4- fold] (Fig. 4), mainly Z, when compared to IAA (Fig. 3). In spite of the differences in the peaks of IAA, the steps of both embryogenesis systems share a common initial feature, characterized by an intense and short endogenous auxin pulse.
In A. sellowiana cultures, proembryos were observed close to the 12th day of culture (Fig. 13) when low levels of total CKs (Fig. 4), represented mainly by Z, in both cis and trans forms, and ZR forms, were detected (Fig. 6). The changes in the levels of ABA in A. sellowiana along the first 30 days of culture (Fig. 12) could be expected, considering their presence in the explants (mature zygotic embryos) as well as their fast increase observed from the 25th to the 30th day of culture, a period corresponding to cotyledonary and torpedo developmental stages (Fig. 13). The rapid increase and magnitude (7-fold) of ABA present on the 6th day of culture (Fig. 12) also needs to be emphasized. Similar increases in ABA levels were observed during the embryogenesis of alfalfa (Ivanova et al. 1994). These results show that the increment in ABA levels coincided with the heart-shaped phase (early embryo development). On the 30th day of incubation, when torpedo embryos were already formed, high levels of ABA were detected (Fig. 13).
Increased ABA levels have frequently been associated with zygotic embryo dormancy to prevent precocious germination. Although somatic embryos of V. vinifera were able to accumulate ABA and IAA throughout their development, no peak in ABA was detected during embryogenesis (Gillaspy et al. 1993). Faure et al. (1998) proposed that prevention of germination could be attained by the presence of endogenous ABA followed by slow desiccation. Application of ABA and partial desiccation were used in other species favoring SE development (Kamada and Harada 1981). According to Nakagawa et al. (2001), the endogenous ABA contents detected in carrot cultures were markedly higher in the embryogenetic callus than in non-embryogenetic ones at any given period. According to Kikuchi et al. (2006), the induction of embryogenesis in carrot culture was not only caused by the presence of ABA, but also as a result of physiological responses directly controlled by different stresses. However, in A. sellowiana cultures, only a small and transient increase in the level of endogenous ABA was observed on the 3rd day of incubation (Fig. 7). After that, no ABA variation took place until the 21st day, when a rapid and significant increase in this hormone occurred, a period that coincided with the presence of globular, heart-shape, torpedo and cotyledonary stages (Figs. 13, 14 and 15).
Schematic overview of both structural events and hormone levels during 75 days after pollination in A. sellowiana. The zero time represents not pollinated ovules at flowers anthesis. The size and the thickness of arrows indicate hormonal fluctuations of endogenous levels. Legends: Zyg (zygotic); Glo (globular); H (heart) (cordiform); Torp (torpedo); Cot (cotyledonary)
Schematic overview of the structural events during 30 days of cultivation, to obtain indirect somatic embryos (1) and direct somatic embryos in globular stage, cordiform, torpedo and cotyledonary; (2) A. sellowiana arrows and their thickness indicate hormonal fluctuations of endogenous levels. Legends: Glo (globular); H (Heart); Torp (torpedo); Cot (cotyledonary)
In ZE, no ABA variation occurred until the last performed measurement (30th day), a period corresponding to the globular phase (Fig. 14). The initial detection of ABA occurred only in the torpedo stage reaching the highest peak at the cotyledonary phase, which was at least two times higher than that detected in ISE (Fig. 13). Low levels of ABA were related to incomplete maturation of oil palm somatic embryos (Aberlenc-Bertossi et al. 2008). Thus, it is possible that low ABA levels account for the small amount of viable somatic embryos formed in A. sellowiana, according to what was demonstrated by Pescador et al. (2008). Unfortunately, our present data are not enough to explain this critical, challenging and persistent question found in the experimental and applied studies of plant SE.
Relatively high levels of IAA were observed after fertilization. Also, the induction phase of SE was clearly accompanied by an increase in IAA, probably in response to culture conditions, in particular to the presence of 2,4-D. This endogenous signal seems to be important for cells to dedifferentiate and acquire the necessary competence for embryogenesis. The development of somatic embryos of A. sellowiana with cotyledon fusion, malformed apical meristems, or even their complete absence (Pescador et al. 2008), could involve disturbances in IAA distribution within the embryo or lower levels of IAA found in embryos derived from ISE and DSE compared to those found in zygotic embryos.
The occurrence of high and practically stable levels of CKs, especially zeatin, after pollination, as well as in the different stages of ZE development, compared to ISE, indicate rather consistently that this hormone plays an essential role in this process. The highly significant levels of ABA found in zygotic embryos at the cotyledonary stage when compared to the corresponding somatic ones, appear to be indicative of a strong signal for their maturation.
Conclusion
In summary, the hormone concentrations observed in somatic embryogenesis do not corroborate the common and widespread view according to which SE steps would recapitulate the general events found in the zygotic counterpart. The results clearly show that both types of embryogenesis in A. sellowiana are not synchronous; somatic embryogenesis occurs faster than in the zygotic version. These results, when taken together, suggest that the temporal and spatial changes in the levels of endogenous auxin, CKs and ABA act as important tuning signals involved in the control of both ZE and SE in A. sellowiana.
References
Aberlenc-Bertossi F, Chabrillange N, Duval Y, Tregear J (2008) Contrasting globulin and cystein proteinase gene expression patterns reveal fundamental developmental differences between zygotic and somatic embryos of oil palm. Tree Physiol 28:1157–1167
Barton MK (2010) Twenty years on: the inner workings of the shoot apical meristem, a developmental dynamic. Dev Biol 341:95–113
Chen KH, Miller AN, Patterson GW, Cohen JD (1988) A rapid and simple procedure for purification of indole-3-acetic acid prior to GC-SIM-MS analysis. Plant Physiol 86:822–825
Chen D, Ren Y, Deng Y, Zhao J (2010) Auxin polar transport is essential for the development of zygote and embryo in Nocotiana tabacum L. and correlated with ABP1 and PM H+- ATPase activities. J Exp Bot 61:1853–1867
Christianson ML, Warnick DA (1983) Competence and determination in the process of in vitro shoot organogenesis. Dev Biol 95(2):288–293
Dodeman VL, Ducreux G, Kreis M (1997) Zygotic embryogenesis versus somatic embryogenesis. J Exp Bot 48:1493–1509
Faure O, Dewitte W, Nougarede A, Van Onckelen H (1998) Precociously germinating somatic embryos of V. vinifera have lower ABA and IAA levels than their germinating zygotic counterparts. Physiol Plant 102:591–595
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74:201–228
Gallois JL, Nora FR, Mizukami Y, Sablowiski R (2004) WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev 18:375–380
Gambino G, Minuto M, Boccacci P, Perrone I, Vallania R, Gribaudo I (2011) Characterization of expression dynamics of WOX homeodomain transcription factors during somatic embryogenesis in V. vinifera. J Exp Bot 62:1089–1101
Gillaspy G, David HB, Gruissem W (1993) Fruits: a developmental perspective. Plant Cell 5(10):1439–1451
Guerra MP, Pescador R, Dal Vesco LL, Nodari RO, Ducroquet JPHJ (1997) In vitro morphogenesis in Feijoa sellowiana: somatic embryogenesis and plant regeneration. Acta Horticulturae 452:27–36
Haecker A, Gross-Hardt R, Geiges B et al (2004) Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131:657–666
Ivanova A, Velcheva M, Denchev P, Atanassov A, Van Onckelen HA (1994) Endogenous hormone levels during direct somatic embryogenesis in Medicago falcata. Physiol Plant 92(1):85–89
Jiménez M (2001) Regulation of in vitro somatic embryogenesis with emphasis on the of role endogenous hormone. Rev Bras Fisiol Veg 13(2):196–223
Kamada H, Harada H (1981) Changes in endogenous level and effect of abscisic acid during somatic embryogenesis of Daucus carota L. Plant Cell Physiol 22:1423–1429
Kikuchi A, Sanuki N, Higashi K, Koshiba T, Kamada H (2006) Abscisic acid and stress treatment are essential for the acquisition of embryogenic competence by carrot somatic cells. Planta 223:637–645
Lo Schiavo F, Pitto L, Giuliano G et al (1989) DNA methylation of embryogenic carrot cell cultures and its variations as caused by mutation, differentiation, hormones and hypomethylating drugs. Theor Appl Genet 77:325–331
Ma H, Liang Z, Wu H, Huang L, Wang Z (2010) Role of endogenous hormones, glumes, endosperm and temperature on germination of Leymus chinensis (Poaceae) seeds during development. J Plant Ecol 3:269–277
Meinke DW (1991) Perspectives on genetic analysis of plant embryogenesis. Plant Cell 3:857–866
Morel G, Wetmore RM (1951) Fern callus culture. Am J Bot 38:141–143
Murthy BNS, Murch SJ, Saxena PK (1995) Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): endogenous growth regulator levels and significance of cotyledons. Physiol Plant 94(2):268–276
Nakagawa H, Saiyo T, Yamauchi N, Shygyo M, Kako S, Ito A (2001) Effects of sugars and abscisic acid on somatic embryogenesis from melon (Cucumis melo L) expanded cotyledon. Scientia Horticult 90:85–92
Namasivayam P (2007) Acquisition of embryogenic competence during somatic embryogenesis. Plant Cell Tissue Organ Cult 90(1):1–8
Pasternak T, Prinsen E, Ayaydin F et al (2002) The role of auxin, pH and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa (Medicago sativa L). Plant Physiol 129:1807–1819
Peres LEP, Mercier H, Kerbauy GB, Zaffari GR (1997) Níveis endógenos de AIA, citocininas e ABA em uma orquídea acaule e uma bromélia sem raiz, determinados por HPLC e Elisa. Revista Brasileira de Fisiologia Vegetal 9:169–176
Pescador R, Kerbauy GB, Viviani D, Kraus JE (2008) Anomalous somatic embryos in A. sellowiana (O. Berg) Burret (Myrtaceae). Revista Brasileira de Botânica 31(1):155–164
Spíchal L, Rakova NY, Riefler M et al (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45(9):1299–1305
Su YH, Zhao XY, Liu YB, Zhang CL, O`Neil SD, Zhang XS (2009) Auxin-induced WUS expression is essential for embryonic stem cells renewal during somatic embryogenesis in Arabdopsis. Plant J 59:448–460
von Arnold S, Eriksson T (1981) In vitro studies of adventitious shoot formation in Pinus cordata. Can J Bot 59:870–874
Zimmerman JL (1993) Somatic embryogenesis: a model for development in higher plants. Plant Cell 5:1411–1423
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pescador, R., Kerbauy, G.B., de Melo Ferreira, W. et al. A hormonal misunderstanding in Acca sellowiana embryogenesis: levels of zygotic embryogenesis do not match those of somatic embryogenesis. Plant Growth Regul 68, 67–76 (2012). https://doi.org/10.1007/s10725-012-9694-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-012-9694-2