Abstract
Root architecture is of key importance for plant nutrition and performance. It is known that root architecture is determined by genetics and environmental conditions. The aim of the present study was to evaluate if root exudation within a given plant has a role in the development of root architecture. We conducted a series of experiments using Arabidopsis thaliana Ler and Col grown with and without activated charcoal (AC). The addition of AC lowered the concentration of secondary metabolites in the growth media by more than 90%. Our results consistently showed that the addition of AC significantly decreased the number of lateral roots (38% in Ler and 27% in Col), but this decrease was compensated by an increase in the root length per unit of lateral root (83% in Ler and 96% in Col). This compensation resulted in a non-significant effect of AC on the total length of lateral roots. The effects of AC on root architecture were partially or totally reverted by the differential supplementation of root exudates from other plants of the same ecotype. Our results indicate a direct role of secondary metabolites present in the root exudates in the development of root architecture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Root architecture, defined as the spatial configuration of the root system, is a key factor in determining the ability of a plant to acquire soil resources (Fitter 1991; Lynch 1995). Although it is genetically determined, root architecture depends ultimately on the particular environmental conditions (i.e. mechanical impedances, nutrient patches, water content, etc) in which the root develops (Hodge 2004). The concentration of some low molecular weight compounds in the rhizosphere such as auxins (Casson and Lindsey 2003; Laskowski et al. 2006) has been recognized as another factor that contribute to determine root architecture, but mechanisms that regulate this response are basically unknown (Malamy and Benfey 1997; Fukaki et al. 2005).
Root architecture also determines the degree of competition among roots of the same plant and/or neighboring plants. Uptake of nutrients at the root surface creates a concentration gradient, which drives diffusive flux of nutrients to the root, progressively depleting the amount of nutrient in the rhizosphere (Schenk et al. 1999; Ge et al. 2000). Competition among adjacent roots occurs when their depletion volumes overlap (Ge et al. 2000), causing a reduction in the amount of nutrients acquired per unit of root mass (Rubio et al. 2001). This problem is particularly acute for the acquisition of diffusion-limited nutrients, such as phosphorus and potassium.
Recent studies suggest that the ability of roots to recognize their neighbors also determines the occupancy of soil volumes (Hess and de Kroon 2007; Semchenko et al. 2007b). Self and non-self root recognition mechanisms and ultimately root architecture may involve signaling events among roots rather than availability of nutrients in the soil (Semchenko et al. 2007a). Falik et al. (2005) found that roots of Pisum sativum are able to detect and avoid proliferation towards inanimate objects and suggested that the root secretions that accumulate in the vicinity of the physical obstacles mediate this response. Semchenko et al. (2007a) observed that the greater accumulation of self-inhibitory compounds in small rooting volumes causes chemical inhibition of root growth. These authors also observed that this response was alleviated by the addition of activated charcoal (AC), which is supposed to adsorb carbon-containing compounds present in the root exudates (e.g. Mahall and Callaway 1992) and therefore, creates a medium free of some chemicals released by roots. The aim of the present work was to evaluate if root exudates have an effect on the development of root architecture. Due to the wealth of molecular genetics information and its developing body of ecological and life history data, Arabidopsis was selected as a model plant.
Materials and methods
Plant material and growth conditions
Seeds of wild type A. thaliana ecotype Landsberg erecta (Ler) and Columbia (Col) were obtained from Dr. Javier Botto (IFEVA, Buenos Aires). Seeds were surface-sterilized using sodium hypochlorite (5% v/v) for 15 min, washed briefly in 70% (v/v) ethanol, rinsed four to five times with sterile distilled water, and placed in Petri dishes containing 0.8% agar. The Petri dishes containing the seeds were subjected to a two-day cold treatment (4°C) followed by red light treatment for 2 h and then placed in the dark for 2 days. When the seedlings were 5 days old, they were transferred to culture tubes or Petri dishes and grown in a growth room at 25 ± 2°C with continuous light (approximately 65 μmol m−2 s−1 photosynthetically active radiation).
Experiment 1: effect of charcoal on secondary metabolite secretion by roots
A first experiment was conducted to determine the effect of AC on the adsorption of compounds released by Arabidopsis roots, such as proteins and carbon-containing phenolic compounds. To rule out the effect of any accompanying nutrients or other chemical compounds that possibly affect plant growth both positive or a negative way (Lau et al. 2008; Weißhuhn and Prati 2009), the AC (obtained from Sigma, St. Louis) was washed thoroughly with distilled water before it was added to the growth media. Treatments were arranged in a factorial experiment with two factors (AC and ecotype) and 20 replicates. Five days old seedlings of A. thaliana ecotypes Ler and Col were transferred into 20 ml culture tubes with 10 ml of Arabidopsis thaliana salts growth media (ATS; Williamson et al. 2001) with or without AC at a dose of 5 g L−1. Preliminary tests was conducted to determine the optimum concentration of AC. ATS contains 5 mM KNO3, 2.5 mM KH2PO4 buffered with 2.5 mM K2HPO4 to pH 5.5, 2 mM MgSO4, 2 mM Ca(NO3)2, 70 μM H3BO4, 50 μM FeEDTA, 14 μM MnCl2, 10 μM NaCl, 1 μM ZnSO4, 0.5 μM CuSO4, 0.2 μM NaMoO4 and 0.01 μM CoCl2. Plant cultures were maintained on an orbital shaker set at 90 rpm. It should be noted that the addition of AC to the media darkens it; therefore, all tubes were covered with aluminum foil in order to give the roots of both treatments the same dark environment. After 20 days, plants were removed and the liquid culture media from both treatments was filtered, freeze-dried and the concentrate dissolved in 3 ml of sterile distilled water. Proteins were measured by the Lowry method (Lowry et al. 1951) and phenolic compounds by the Folin-Ciocalteu method (Singleton et al. 1999). Both are colorimetric methods and use the same reagent (Folin reagent; FR), whose principal component is fosfomolibdotungstic acid. Phenolic compounds react with FR in an alkaline solution. When the reaction is completed, the solution changes color from yellow to blue due to the reduction of W+6 and Mo+6 ions to W+5 and Mo+5. Absorbance was read at 760 nm. For measuring protein concentration, tyrosine’s residues react with FR as described above and absorbance was read at 580 nm. This procedure served as the control representing the natural growth of plants with AC in the growth media.
Experiment 2: effect of AC on root architecture
A second experiment was conducted to determine the effect of AC on the ability of roots to form its own architecture. Treatments were arranged in a factorial experiment with two factors (AC and ecotype) and 10 replicates. The AC factor was composed of two levels (control and AC) and the ecotypes evaluated were Col and Ler. Germinated plants were transferred to 15 cm diameter Petri dishes containing 1.2% (w/v) agar and 100 ml ATS. In the AC treatment, 4 ml of a solution containing 5 g L−1 AC was distributed homogeneously to the solidified agar containing ATS growth media, and then allowed to evaporate in a transfer hood. The top part of the agar in the Petri dish (1.5 cm from the top) was cut to provide support for the seedling and to impede contact between the leaves and the agar. Seedlings were placed at the center of this artificial ledge in the Petri dish. Petri dishes were sealed with Parafilm®, arranged in a vertical position and were separated by cardboard supports. These cardboard stands provide all the Petri dishes with a similar dark environment. After 20 days of growth, plants were harvested, dried at 60°C for 48 h and weighed. Before harvesting, we drew the root trajectories by hand on a clear film. The drawings were scanned and the images analyzed with the public domain software Image J (http://rsbweb.nih.gov/ij/). The measured parameters were primary root length, total root length, lateral root length, vertical growth index (VGI; Grabov et al. 2005), total number of contacts among roots, and number of contact among roots per lateral root. VGI lies in the range between 0 and 1. Geometrically VGI represents the cosine of the angle between the vector of gravity and straightened root. If the root shape is close to a straight line, a VGI of 1 describe the response of a primary root with positive gravitropism. Physical root contact was defined as the number of times that lateral roots crossed each other or the primary root per number of lateral roots.
Experiment 3: effect of external addition of root exudates on root architecture
In a third experiment, we tried to exacerbate the effects of root exudates on root architecture by adding root exudates released by other plants of the same ecotype to the growth medium. Seedlings of A. thaliana ecotype Col were grown on liquid nutrient media as described for the first experiment. Twenty-day-old plants were transferred to 20 ml culture tubes with 1 ml of distilled water (one plant per tube). Water rather than media was used to collect root exudates to avoid media nutrients becoming a confounding factor. Plants were kept in these tubes for 24 h. Then, plants were removed and the root exudates were collected from the aqueous media. The collected exudates were pooled and used in an agar-plate factorial experiment with two factors (AC, exudates) and six replicates. The AC factor was composed of two levels (control and 5 g L−1 AC added to the culture media). The root exudates factor had four levels (control with no exudates, exudates collected from three and six plants added once, and exudates of six plants added twice; at day 0 and 10 of transplanting) and six replicates for each treatment. Root secretions were added to the agar media immediately before transplanting and plants were allowed to grow for 20 days. Growth conditions and measured parameters were similar to those described for Experiment 2.
Experiment 4: effect of AC and root exudates in modifying the trajectory of roots
This experiment was designed to force the path of growth of a given lateral root to cross a neighboring lateral root. Seedlings of A. thaliana were grown for 10 days on culture tubes as described for the first experiment. Then, plants were transferred to 15 cm diameter Petri dishes containing 1.2% (w/v) agar and 100 ml ATS with and without AC added into the growth media as described in experiment 2. At this time, plants had 4–5 lateral roots. Roots were transplanted in such a way that two lateral roots per plant (“target roots”) were oriented by hand in a collision direction with another lateral root of the same plant (“targeted roots”). The distance between the tip of the “target root” and the “targeted root” was 2 cm. Treatments involving addition of 1 ml for liquid growing were included to ensure that the “target root” explore a medium rich in root exudates in the vicinity of the “targeted root”. These exudates were distributed in the proximity of the “targeted root”. Treatments were three: (1) control without AC or root exudates’ additions; (2) root exudates added: 1 ml of root exudates added and no AC; and (3) 5 g L−1 AC without root exudates addition. The number of replicates was 10. The measurements were performed 3 and 7 days after transplanting. At day 3 only total number of contacts among roots were measured.
At day 7, lateral root number, lateral root length, total root length per lateral root number, total number of contacts among roots, and number of contact among roots per lateral root was measured.
Statistical analysis
Data from the three experiments were arranged in a completely randomized design and ANOVA and mean separation tests (LSD) were performed. The assumption of equality of variances was rejected (P < 0.05) only for contacts among roots per unit of lateral root. In this case, a square root transformation prior to ANOVA was conducted.
Results
Experiment 1: effect of charcoal on root exudates
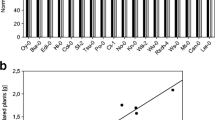
Total protein concentration measured by the Lowry method was not significantly modified by the addition of AC (Fig. 1a). In contrast, the addition of AC diminished the concentration of phenolic compounds in the media by more than 90% in both ecotypes (P < 0.01; Fig. 1b). The roots of control Col plants released around 38% fewer secondary metabolites than did those of Ler (P < 0.05; Fig. 1).
Data from Experiment 1. Effect of 5 g L−1 AC on the concentration of different compounds exuded by A. thaliana ecotypes Ler and Col in the growth media: a total proteins in growth media and b total phenolic compounds in growth media. Factors were ecotype (A. thaliana ecotypes Ler and Col) and AC [control (−AC) and 5 g L−1 AC added to the growth media (+AC)]. Unfilled bars represent the treatment without AC; filled bars represent the treatment with AC. Bars represent average of 20 plants for every configuration +/− SE. Different letters correspond to significant differences between treatments (P < 0.05, LSD procedure)
Experiment 2: effect of AC on root architecture
Figure 2 shows plants of Arabidopsis thaliana ecotype Ler grown in a Petri dish with (Fig. 2a) and without (Fig. 2b) AC into the growth media. The effect of AC was similar in Col and Ler in all plant measurements as indicated by the non-significant interaction between factors, ecotype and AC, (Online Resource 1). Biomass accumulation was not significantly affected by AC but was 25% higher in Col than in Ler (Online Resource 1). Neither AC nor ecotype affected the root to shoot ratio (Online Resource 1).
Col and Ler showed a parallelism in most root parameters evaluated in this study. Root dry weight was the only parameter that showed a significant influence of ecotype and AC (Fig. 3a; Online Resource 1). In contrast, there was a strong effect of AC on most parameters. AC decreased the number of lateral roots by 38% in Ler and 27% in Col (P < 0.05; Fig. 3b), but this decrease was compensated by an increase in the root length per unit of lateral root (83% in Ler and 96% in Col) (Fig. 3d; Online Resource 1). This compensation resulted in a non-significant effect of AC on the total length of lateral roots (Fig. 3c; Online Resource 1).
Data from Experiment 2. Effect of adding 5 g L−1 AC to the growth media of A. thaliana ecotypes Ler and Col: a root dry weight; b lateral root number; c total lateral root length; and d lateral root length. Factors were ecotype (A. thaliana ecotypes Ler and Col) and AC [control (−AC) and AC at 5 g L−1 added to the growth media (+AC)]. Unfilled bars no AC in the growth media. Filled bars treatment with AC in the growth media. Bars represent average of 10 plants for every configuration +/− SE
When plants were grown without AC, total contacts among roots were around three in both ecotypes (Fig. 4a). AC addition significantly increased these contacts to approximately eight in Ler and 10 in Col (Fig. 4a; Online Resource 1). If the number of contacts are expressed on a per unit of lateral root basis, AC provoked an increase of about 300% in both ecotypes (Fig. 4b). AC effects on root architecture were restricted to lateral roots, since no effects were observed on the parameters related to the primary root (total root length and VGI; Online Resource 1).
Data from Experiment 2. Effect of 5 g L−1 AC: a total number of contacts among roots and b number of contacts among roots per unit of lateral root for plants of A. thaliana ecotypes Ler and Col for 20 days of growth. Treatments were ecotype (A. thaliana ecotypes Ler and Col) and AC [control (−AC) and 5 g L−1 AC added to the growth media (+AC)]. Unfilled bars treatment without AC. Filled bars plants growing with AC added to the growth media. Bars represent average of 10 plants for every configuration +/− SE
Experiment 3: effect of the addition of root exudates of other plants of the same ecotype
Total biomass, primary root length, lateral root length and VGI of Col plants were not affected by AC or the addition of exudates of other Col plants (Online Resource 2). Other root parameters were affected by both factors and, in many cases, the interaction of AC vs. exudates was significant at 0.01 level. The general observation was that the addition of root exudates of other Col plants to Col roots partially or totally reversed the effect of AC on root architecture (Fig. 5). In general, the addition of exudates of three plants was enough to compensate for the effects of AC. The addition of exudates coming from more plants (6 or 12) did not exert any additional effect. The number of lateral roots was slightly diminished when root exudates were added to plants grown without AC, but a substantial reduction in the number of lateral roots was observed when AC was added to the growth media (Fig. 5a). This reduction in the number of lateral roots due to AC was partially reversed when root exudates were added. Lateral root length was not significantly different between treatments (Online Resource 2). However, when this parameter was analyzed on a per unit lateral root basis, we observed a significant and positive effect of the addition of AC, as observed in the previous experiment (Fig. 5b; Online Resource 2). This effect was almost completely reversed by the addition of root exudates. The addition of root exudates to plants grown with AC reduced lateral root length per unit of lateral root number by 40% (P < 0.05; Fig. 5b). The addition of root exudates did not produce any modification in the number of physical root contacts (Fig. 5c) in plants grown without AC, but had a strong effect on AC-grown plants. Plants grown without AC did not change the number of root-root contacts when the concentration of exudates was increased. Plants treated with AC showed a notorious increase in the number of contacts. Interestingly, the addition of root exudates reversed completely the effect of AC on the number of contacts among roots (Fig. 5c, d).
Data from Experiment 3. Effect of the addition of root exudates from 0, 3, 6 or 12 plants of the same ecotype on the root architecture of A. thaliana ecotype Col growing with and without 5 g L−1 AC in the growth media: a lateral root number; b lateral root length; c total contacts among roots; d contacts among roots per unit of lateral root. Unfilled bars correspond to the treatment control. Filled bars indicate the addition of root exudates from three plants, horizontal lines to the addition of root exudates from six plants and vertical lines to the addition of root exudates from six plants added two times. Bars represent average of 6 plants for every configuration +/− SE. Different letters correspond to significant differences between treatments (P < 0.05, LSD test)
Experiment 4: effect of AC and root exudates in modifying the trajectory of roots
When the trajectory of target roots towards targeted roots was modified, the number of contacts among roots was not affected by the imposed treatments (Fig. 6a). The number of contacts among roots was slightly lower in the treatment −AC + exudates, but this difference was not significant (P > 0.05; Fig. 6b).
Data from Experiment 4 at day 3 after transplant. Effect of the addition of 5 g L−1 AC or 1 ml of root exudates in the Petri dishes in the root architecture of A. thaliana ecotype Col: a total contacts among roots; b contact among roots per unit of lateral root. Bars represent average of 10 plants for every configuration +/− SE
Measurements performed 1 week after transplanting verified the significant effect of AC on root architecture (Fig. 7); AC plants had 40% less lateral roots (P < 0.01; Fig. 7a), 73% higher lateral root length (P < 0.01; Fig. 7b), twice the number of contacts (P < 0.05; Fig. 7c) and finally three times more contacts among roots per unit of lateral root (P < 0.01; Fig. 7d).
Data from Experiment 4 at day 7 after transplant. Effect of the addition of 5 g L−1 AC (+AC) or 1 ml of root exudates (+ex) in the Petri dishes in the root architecture of A. thaliana ecotype Col: a lateral root number; b lateral root length; c total contacts among roots; d contact among root length per unit of lateral root. Bars represent average of 10 plants for every configuration +/− SE
Discussion
Many studies have employed treatments involving the addition of AC to the growth media to study the effect of secondary metabolites exuded by plant roots (e.g., Mahall and Callaway 1992; Falik et al. 2005; Semchenko et al. 2007a; Goldwasser et al. 2008; Lau et al. 2008; Weißhuhn and Prati 2009) under the assumption that AC adsorbs carbon-containing compounds present in the root secretions and thus neutralizes their activity in the rhizosphere. Those experiments were designed to test the effects of root exudates and lack thereof on targeted plants but not on the producing plant. Unlike reports presented by Lau et al. (2008) and Weißhuhn and Prati (2009) in which AC affected plant growth, in our studies we did not observe these effects; biomass accumulation did not differ between control and AC-treated plants. A possible explanation for this observation is that in our case AC was thoroughly washed with distilled water to remove excess of nutrients that could affect plant nutrient availability. Another possible explanation is that Arabidopsis thaliana is not as susceptible to AC treatment as Festuca idahoensis, Nassella lepida, Elymus glaucus and Centaurea solstitialis as reported by Lau et al. (2008). In the present study, we found that AC retained the phenolic compounds exuded by Arabidopsis roots without affecting the presence of other high molecular weight compounds in the root exudates such as proteins (Fig. 1). On the other hand, the fact that no differences were found in biomass accumulation between control and +AC plants in the several experiments done for this work suggests that AC did not promote nutrient shortage. Therefore, we used the comparison of treatments with and without the addition of AC to the growth media to evaluate the role of secondary metabolites in the development of root architecture within a single plant.
It is known that root architecture is genetically determined but ultimately defined by the chemical and physical characteristics of the soil environment (Fitter 1991; Lynch 1995). The growth media employed in our studies was porous and homogenous allowing root exploration and belowground resource uptake at optimal rates. Therefore, the root architecture observed in our experimental system was defined by the genetic background of the plant. Here, we tested if the intrinsic biochemistry of the roots to exude secondary metabolites and the ability of roots to sense and to respond to the presence of these compounds were involved in determining root architecture. Our studies indicate that if root exudates are absent then the final expression of root architecture is re-programmed (Fig. 2). When AC was added to the growth media, plants consistently produced fewer but longer lateral roots. Both responses compensated each other, resulting in a non-significant effect of AC on the total length of lateral roots. These results suggest that the presence of secondary metabolites in the vicinity of the roots reduced the length of the lateral roots, and that this effect was diminished by the AC treatment. The addition of AC did not have any relevant effect on the growth of primary roots, which suggests that the target of action of AC was specific to lateral roots. Since lateral roots are the ones directly affected by the secondary metabolites present in the root exudates, it is possible that these roots are also the ones secreting the compounds. For example, there might be a feedback inhibition loop related to the secretion and sensing of secondary metabolites in these roots, leading to the determination of the physical architecture of the root system.
Mechanistically, it is possible that AC retains auxins or other compounds that stimulate or mimic auxin activity that are found in the root exudates and that promote the production of new lateral roots. However, our results do not correspond with the observed phenotypes of auxin mutants (minor number of lateral roots without length compensation, alteration of the VGI, decrease plant growth; Overvoorde et al. 2010). Therefore, other compounds present in the root exudates might have an effect on direct inhibition of growth of lateral roots; thus providing for an auto-inhibitory role. We propose that these two mechanisms happening concurrently might account for the effect of root exudates on root architecture within a given plant.
To test the direct effect of root exudates on root architecture, the root secretions of other individuals of the same Arabidopsis ecotype were artificially added to the substrate where the target plants were growing (Fig. 5). It was observed that the changes produced by the addition of AC were reversed and that the roots tended to recover the root architecture characteristic of control plants. In other words, when the supplementary exudates were added to plants grown in the presence of AC, the lateral root length was reduced and the lateral root numbers increased compared to the treatment with no supplementary exudates added. The addition of root exudates to plants grown in media with no AC had not effect supposedly because those roots were already sensing their endogenous exudates.
We further examined whether secondary metabolites had any effect in avoiding contacts among lateral roots. In our experimental system, the number of contacts was determined by counting the number of times a given lateral root crossed other roots. It is believed that roots of the same plant tend to avoid competing among themselves for resources and that is why their architecture is spread out (Mahall and Callaway 1996). Having this in mind, a higher number of contacts between lateral roots might indicate an increased level of competition for resources among roots of the same plant possibly due to a lack of self recognition. In such sense, a dramatic increase in the number of physical contacts was observed among self-roots when plants were grown in the presence of AC. Whereas lateral roots of control plants tended to avoid themselves and grew parallel to each other; roots growing with AC did not show this parallelism and tended to contact each other. The external addition of root exudates to the experimental system reinforced this evidence. The magnitude of the increase in root contacts (exceeding three fold) could be due to: (a) AC by trapping the root exudates diminished the aptitude of roots to recognize and avoid them; (b) the increase in the number of contacts in AC-grown plants is a direct consequence that these roots are longer and thus more prone to hit each other. To add light to these issues and to specifically determine if root exudates had an effect on root recognition we developed an experiment in which we modified the trajectory of the lateral root to put it in the direct path to contact a different lateral root (Fig. 6). In all the treatments related to this last study, we did not see a significant effect of root exudates in avoiding contacts when the roots were placed in direct impact trajectories. However, at this point we cannot reject the possibility that root exudates are involved in the self-recognition process. The added root exudates did not belong to the tested plants and we can’t conclude if a given plant is able to recognize their own root exudates as compared to the exudates of another plant from the same species. Certainly, additional studies are needed to determine the role of root exudates in self recognition of roots.
A complete understanding of plant root architecture involves both unraveling how it is regulated and determining the signaling and sensing processes that take place. Lateral root formation in Arabidopsis has high sensitivity to environmental factors, such as nitrogen or phosphorus supply (Linkohr et al. 2002), mechanical injury (Ditengou et al. 2008), internal nutrient status (Zhang and Forde 1998), and auxin signaling (Casson and Lindsey 2003; Malamy 2005; Ditengou et al. 2008). Our studies provide an additional layer of complexity by indicating that root exudation is involved in this process. Additional studies are needed to determine the exact mechanistic nature of how root exudates regulate root architecture.
References
Casson SA, Lindsey K (2003) Genes and signaling in root development. New Phytol 158:11–38
Ditengou FA, Teale WD, Kochersperger P, Flittner KA, Kneuper I, van der Graaff E, Nziengui H, Pinosa F, Li X, Nitschke R, Laux T, Palme K (2008) Mechanical induction of lateral root initiation in Arabidopsis thaliana. PNAS 105:18818–18823
Falik O, Reides P, Gersani M, Novoplansky A (2005) Root navigation by self-inhibition. Plant Cell Environ 28:561–569
Fitter A (1991) The ecological significance of root system architecture. Plant root growth. An ecological perspective, D. Atkinson. Oxford, Blackwell Scientific Publications, pp 229–246
Fukaki H, Okushima Y, Tasaka M (2005) Regulation of lateral root formation by auxin signaling in Arabidopsis. Plant Biotechnol 22:393–399
Ge Z, Rubio G, Lynch JP (2000) The importance of root gravitropism for inter-root competition and phosphorus acquisition efficiency: results from a geometric simulation model. Plant Soil 218:159–171
Goldwasser Y, Yoneyama K, Xie X, Yoneyama K (2008) Production of strigolactones by Arabidopsis thaliana responsible for Orobanche aegyptiaca seed germination. Plant Growth Regul 55:21–28
Grabov A, Ashley MK, Rigas S, Hatzopoulos P, Dolan L, Vicente-Agullo F (2005) Morphometric analysis of root shape. New Phytol 165:641–652
Hess L, de Kroon H (2007) Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination. J Ecol 95:241–251
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Laskowski M, Biller S, Stanley K, Kajstura T, Prusty R (2006) Expression profiling of auxin-treated Arabidopsis roots: toward a molecular analysis of lateral root emergence. Plant Cell Physiol 47:788–792
Lau JA, Puliafico KP, Kopshever JA, Steltzer H, Jarvis EP, Schwarzländer M, Strauss SY, Hufbauer RA (2008) Inference of allelopathy is complicated by effects of activated carbon on plant growth. New Phytol 178:412–423
Linkohr BI, Williamson LC, Fitter AH, Leyser HMO (2002) Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J 29:751–760
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Lynch JP (1995) Root architecture and plant productivity. Plant Phys 95:7–13
Mahall BE, Callaway RM (1992) Root communication mechanisms and intracommunity distributions of two Mojave desert shrubs. Ecology 73:2145–2151
Mahall BE, Callaway RM (1996) Effects of regional origin and genotype on intraspecific root communication in the desert shrub Ambrosia dumosa (Asteraceae). Am J Bot 83:93–98
Malamy JE (2005) Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ 28:67–77
Malamy JE, Benfey P (1997) Down and out in Arabidopsis: the formation of lateral roots. Trend Plant Sci 2:390–396
Overvoorde P, Fukaki H, Beeckman T (2010) Auxin control of root development. Cold Spring Harb Perspect Biol 2:a001537
Rubio G, Walk T, Ge Z, Yan X, Liao H, Lynch JP (2001) Root gravitropism and belowground competition among neighboring plants: a modeling approach. Ann Bot 88:929–940
Schenk HJ, Callaway RM, Mahall BE (1999) Spatial root segregation: are plants territorial? Adv Ecol Res 28:145–180
Semchenko M, Hutchings MJ, John EA (2007a) Challenging the tragedy of the commons in root competition: confounding effect of neighbor presence and substrate volume. J Ecol 95:252–260
Semchenko M, John EA, Hutchings MJ (2007b) Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytol 176:644–654
Singleton VE, Orthofer R, Lamuela-Raventós RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Method Enzymol 299:152–178
Weißhuhn K, Prati D (2009) Activated carbon may have undesired side effects for testing allelopathy in invasive plants. Basic Appl Ecol 10:500–507
Williamson LC, Ribrioux SPCP, Fitter AH, Leyser HMO (2001) Phosphate availability regulates root system architecture in Arabidopsis. Plant Phys 126:875–882
Zhang H, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279:407–409
Acknowledgments
This research was supported by grants from ANPCYT and CONICET to GR, and a grant from National Science Foundation to JMV (MCB-0950857). We thank Dr. Javier Botto and colleagues at CSU who contributed initial ideas towards the development of this project.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
F values, significance levels (between brackets) and LSD mean comparisons for data collected in Experiment 2. Factors were ecotype (A. thaliana ecotypes Ler and Col) and AC [control (−AC) and 5 g L−1 AC added to the growth media (+AC)] (PDF 32 kb)
Online Resource 2
F values, significance levels (between brackets) and LSD mean comparisons for data collected in Experiment 3. Factors were addition of exudates from 0, 3, 6 and 12 plants of A. thaliana ecotype Col and AC [control (−AC) and 5 g L−1 AC added to the growth media (+AC)]. (PDF 31 kb)
Rights and permissions
About this article
Cite this article
Caffaro, M.M., Vivanco, J.M., Gutierrez Boem, F.H. et al. The effect of root exudates on root architecture in Arabidopsis thaliana . Plant Growth Regul 64, 241–249 (2011). https://doi.org/10.1007/s10725-011-9564-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-011-9564-3