Abstract
Hydroponic experiments were carried out to study the role of alginate-derived oligosaccharides (ADO) in enhancing wheat (Triticum aestivum L.) tolerance to cadmium stress. Data were collected on plant biomass, chlorophyll content, photosynthetic rate, antioxidant enzyme activity and malondialdehyde (MDA) content. Under 100 μM Cd stress, plant growth was significantly inhibited. Shoot length, root length, fresh and dry weight were sharply reduced by 24.21, 34.59, 22.1 and 14.7%, respectively of the control after 10 day of Cd exposure. Superoxide dismutase (SOD), catalase (CAT) and peroxidase (POD) activities were increased and MDA content increased. Wheat seeds were soaked for 5 h in 1,000 mg L−1 ADO solution before cadmium stress. ADO pretreatment alleviated cadmium toxicity symptoms, which were reflected by increasing root and shoot lengths, fresh and dry weight, chlorophyll content and photosynthetic rate (P n ). Furthermore, ADO pretreatment significantly increased antioxidant enzyme (SOD, CAT and POD) activities and reduced MDA content in leaves and roots. The results indicated that ADO pretreatment partially protected the seedlings from cadmium toxicity during the following growth period.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium(Cd) is a toxic metal pollutant and can enter soil-plant ecology system due to discharge of industrial waste water, the excessive use of phosphate fertilizer and atmospheric sedimentation. Cadmium is not an essential element for plant growth, but it is easily taken up by plant roots and transported to above-ground tissues (Shamsi et al. 2008). High concentration of Cd is strongly phytotoxic and inhibits plant growth (Meng et al. 2009). Cadmium toxicity may be ascribed to the deterioration of many physiological processes, such as the reduction in intercellular spaces and the number of chloroplasts (Sandalio et al. 2001), inhibition of chlorophyll synthesis and photosynthesis (Hsu and Kao 2007), and generation of free radicals and reactive oxygen species (ROS) (Milone et al. 2003; Hassan et al. 2008). At high ROS concentrations, ROS promotes oxidative stress and triggers signaling associated with cell death (Alvarez et al. 1998). To avoid ROS-caused oxidative damage, plants have evolved protective mechanisms of mitigating and repairing the ROS damages (Edreva 2005). The ROS-scavenging system includes an enzymatic system consisting of superoxide dismutase (SOD), catalase (CAT), peroxidase (POD) and glutathione reductase (GR), and a non-enzymatic system consisting of reduced glutathione (GSH) and ascorbic acid (Yin et al. 2008). SOD catalyzes dismutation of superoxide anions to H2O2 which is subsequently catalyzed by CAT and POD to water and O2.
Under normal physiological conditions, plants keep the balance between ROS production and quenching activity of antioxidants. However, under environmental stress, the balance is upset, often resulting in oxidative damage (Weckx and Clijsters 1997). It is important to develop practical techniques for alleviating the Cd stress. Hassan et al (2008) reported that nitrogen affected Cd uptake and accumulation in rice. The application of sulfur helped reducing Cd toxicity in mustard (Anjum et al. 2008). Some species or genotypes such as durum wheat (Penner et al. 1995), rice (Cheng et al. 2008) and barley (Wu and Zhang 2004) are Cd tolerant and are used for phytoremediation as they can accumulate high amounts of heavy metals. Alternatively, crops that do not accumulate Cd in grains or fruits can be planted on Cd-contaminant soil (DalCorso et al. 2008). Hydrogen peroxide (H2O2) pretreatment alleviated Cd toxicity in rice seedlings (Hu et al. 2009). Plant growth regulators, such as jasmonic acid (JA), abscisic acid (ABA), gibberellins (GA) and salicylic acid (SA) can also alleviate Cd toxicity (Guo et al. 2007; Meng et al. 2009; Metwally et al. 2003).
Biologically active oligosaccharides act as signal molecule, regulating growth and development as well as defense mechanisms in plants by regulating gene expression (Albersheim and Darvill 1985). Alginate-derived oligosaccharides (ADO) with 4-deoxy-Lerythro-hex-4-ene pyranosyluronate structures function as endogenous elicitors (Akimoto et al. 1999). Many researchers have reported that ADO enhances seed germination, shoot elongation, and root growth (Hu et al. 2004; Natsume et al. 1994). Akimoto et al (1999) had reported that ADO also promoted the production of certain enzymes. However, no information is available about effects of ADO on wheat seedlings stressed by Cd. Therefore, in the current research, we investigated the growth, chlorophyll content, photosynthetic rate, lipid peroxidation and the activities of some antioxidant enzymes (SOD, POD and CAT) in Cd stressed wheat seedlings.
Materials and methods
Plant culture and treatments
The experiments were carried out with wheat (Triticum aestivum L. Liaochun10) seeds, surface sterilized in 2% sodium hypochlorite solution for 10 min and subsequently triple-rinsed in sterile distilled water. Seeds were soaked for 5 h either in 1,000 mg L−1 ADO (supplied by Ocean University of China) solution or in distilled water. A preliminary study showed that 1,000 mg L−1 ADO solution was optimum for enhancing Cd tolerance in wheat seedlings. Plants were grown hydroponically on Hoagland’s nutrient solution in a growth chamber at a day/night cycle of 12 h/12 h, at 25°C/20°C, respectively, a relative humidity 65% and a light intensity of 800 μmol m−2 s−1. When the second leaf of wheat seedlings was fully expanded, CdCl2 was added into the solution to obtain two Cd levels, 0 and 100 μM. Hence, four treatments were established, including a control (neither ADO nor Cd-stressed), Cd stressed, ADO treated and ADO-Cd stressed (pretreatment with 1,000 mg L−1 ADO and then 100 μM Cd stressed). There were three replications for each treatment. The nutrient solution was renewed every 3 days.
Growth parameters
Plants were harvested after 10 day of Cd exposure. Shoot and root lengths and fresh weight were determined immediately after harvesting. Samples were oven-dried at 105°C for 30 min and kept at 70–80°C for 24 h to obtain a constant dry weight. The data were obtained from three replicates (10 plants in each).
Chlorophyll content and photosynthetic rate
After 10 day of Cd exposure, the second fresh leaves were collected for the determination of photosynthetic pigments. Samples (0.1 g) were extracted in 10 ml 80% acetone and ethanol (v/v = 1:1) for 24 h under dark conditions. The absorbance of the supernatant was recorded at 645 (A645) and 663 (A663) nm, respectively. Photosynthesis rate (P n ) was measured with a portable photosynthesis system (LI-6400, Lincoln, NE, USA) in the open circuit mode. Atmospheric conditions consisted of photosynthetic photon flux density of 800 ± 50 μmol CO2 m−2 s−1 and CO2 concentration of 400 μmol CO2 mol−1 air. Chlorophyll measurements were taken for three independent experiments.
Antioxidant enzyme activities and lipid peroxidation
After 5 and 10 days of Cd exposure, enzymes were extracted at 4°C from about 0.5 g tissue from the second leaves and the roots, using a mortar and pestle, with 5 ml of extraction buffer, containing 0.1 M phosphate buffer (pH 7.8), 0.1 mM EDTA, 1 g PVP. Extracts were centrifuged at 10,000 × g for 15 min, and the supernatants were used for the assays. SOD activity was assayed by the inhibition of the photochemical reduction of β-nitro blue tretrazolium chloride (NBT). One unit of SOD was defined as the amount of enzyme, which produced a 50% inhibition of NBT reduction at 560 nm (Costa et al. 2002). CAT activity was determined by the decomposition of H2O2 that was followed by the decline in absorbance at 240 nm (Cakmak and Horst 1991). POD activity was measured with guaiacol as the substrate. Increase in the absorbance due to oxidation of guaiacol was measured at 470 nm. Protein concentration was estimated by the method of Lowry et al. (1951) using bovine albumin as standard. MDA content was determined as 2-thiobarbituric acid (TBA) reactive metabolites according to Heath and Packer (1968). The level of lipid peroxidation was measured as nmol g−1 fresh weight by using an extinction coefficient of 155 mM cm−1. The results presented are the mean of three independent experiments.
Statistical analysis
Differences among treatments were analyzed taking P < 0.05 as significance according to Duncan’s multiple range test. Statistical procedures were carried out with the software package SPSS11.0 for windows.
Results
Plant growth and biomass accumulation
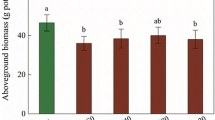
Shoot and root lengths and biomass were significantly inhibited under cadmium exposure (P < 0.05) (Fig. 1). However, growth inhibition was considerably alleviated (P < 0.05) when plants were pretreated by 1,000 mg L−1 ADO before Cd stress. A similar trend was also observed for fresh (Fig. 1c) and dry weight (Fig. 1d). The alleviation of root growth was more evident than that of shoot growth.
Effect of ADO pretreatment on growth parameters of wheat seedlings. The data of shoot (a) and root (b) length as well as fresh (c) and dry (d) weight are mean ± SE from three independent experiments. Different letters indicate significant differences at P < 0.05. CK: plants were grown in neither ADO nor Cd; Cd: plants were stressed by 100 μM CdCl2; ADO: plants were pretreated with 1,000 mg L−1 ADO; ADO-Cd: plants were pretreated with 1,000 mg L−1 ADO and then stressed by 100 μM CdCl2
Chlorophyll content and photosynthetic rate
Under Cd stress, Chl-a and Chl-b (Fig. 2) content were significantly reduced (P < 0.05). The application of 1,000 mg L−1 ADO resulted in an increase of the Chl-a and Chl-b contents of plants grown either in the 0 μM Cd control or in the 100 μM Cd medium. The Chl-a content increase was more apparent than that of Chl-b. Under Cd stress, photosynthetic rate was significantly reduced (P < 0.05) compared to the control (Fig. 3). However, ADO had a significant (P < 0.05) effect on the photosynthetic rate of plants under Cd stress, where ADO increased the photosynthetic rate (Fig. 3).
Effect of ADO pretreatment on Chlorophyll content of wheat leaves. Each value represents the mean ± SE. Different letters indicate significant differences at P < 0.05. CK: plants were grown in neither ADO nor Cd; Cd: plants were stressed by 100 μM CdCl2; ADO: plants were pretreated with 1,000 mg L−1 ADO; ADO-Cd : plants were pretreated with 1,000 mg L−1 ADO and then stressed by 100 μM CdCl2
Effect of ADO pretreatment on photosynthetic rate of wheat leaves. Each value represents the mean ± SE. Different letters indicate significant differences at P < 0.05. CK: plants were grown in neither ADO nor Cd; Cd : plants were stressed by 100 μM CdCl2; ADO: plants were pretreated with 1,000 mg L−1 ADO; ADO-Cd : plants were pretreated with 1,000 mg L−1 ADO and then stressed by 100 μM CdCl2
Activities of antioxidant enzymes
Cadmium (Cd) stress induced higher SOD activity in leaves and roots than that in the control (Fig. 4a, b). SOD activity was higher in the leaves after 10 days Cd stress compared to the SOD activity after 5 days Cd stress. However, SOD activity in roots showed the opposite trend. Pretreatment with ADO further resulted in an increase of SOD activities in leaves and roots both in the control and in Cd stressed plants.
Effect of ADO pretreatment on activities of SOD (leaf, a; root, b), POD (leaf, c; root, d), CAT (leaf, e; root, f). Each value represents the mean ± SE. CK: plants were grown in neither ADO nor Cd; Cd : plants were stressed by 100 μM CdCl2; ADO: plants were pretreated with 1,000 mg L−1 ADO; ADO-Cd : plants were pretreated with 1,000 mg L−1 ADO and then stressed by 100 μM CdCl2
Cadmium (Cd) stressed plants had higher POD activity in leaves and roots than the control (Fig. 4c, d). Under ADO-Cd stress, POD activity in leaves and roots increased significantly. Furthermore, POD activity increased with increasing ADO-Cd stress time.
When comparing the Cd stressed plants with the control, there was obvious decrease in leaf CAT, while it showed no significant difference on root CAT activity (Fig. 4e, f). CAT activity was increased (P < 0.05) when seeds were soaked with 1,000 mg L−1 ADO.
Lipid peroxidation
Lipid peroxidation levels in leaves and roots of wheat plants were determined as the content of MDA. MDA content in leaves (Fig. 5a) and in roots (Fig. 5b) was increased significantly to nearly double responding to 100 μM Cd stressed without ADO pretreatment. MDA content of ADO pretreated plants decreased in both the control and Cd stressed plants. In comparison with Cd stressed plants, MDA content in leaves and roots of ADO-Cd stress decreased significantly.
Effect of ADO pretreatment on MDA content (leaf, a; root, b). Each value represents the mean ± SE. CK: plants were grown in neither ADO nor Cd; Cd : plants were stressed by 100 μM CdCl2; ADO: plants were pretreated with 1,000 mg L−1 ADO; ADO-Cd : plants were pretreated with 1,000 mg L−1 ADO and then stressed by 100 μM CdCl2
Discussion
In the present study, the growth parameters of Cd stressed plants which were indicated by the height of seedling, the root length, fresh weight and dry weight, were significantly reduced compared with the control (Fig. 1). This is in agreement with the literature reports (Kumar et al. 2008). However, these parameters increased markedly when seeds were soaked in 1,000 mg L−1 ADO solution before Cd stress. Growth increase is clearly ascribed to the promotion of carbon fixation as reflected by chlorophyll content (Fig. 2) and photosynthetic rate (Fig. 3). The same results had been reported by Tomoda et al. (1994) in barley.
Accumulation of active oxygen species (AOS), including superoxide radical (O ·−2 ), hydroxyl radical (·OH) and hydrogen peroxide (H2O2) are the major reason of Cd injury to plants, leading to the occurrence of oxidative stress (Romero-Puertas et al. 2004; Shah et al. 2001). In general, plant cells may develop the defense mechanisms against AOS. One of the protective mechanisms is the enzymatic antioxidant system, which involves the sequential and simultaneous action of a number of enzymes. SOD, POD and CAT are major enzymes used to demonstrate the status of antioxidant capacity. SOD is a key enzyme which catalyzes dismutation of superoxide anions to H2O2. In the present study, SOD activity increased in wheat leaves (Fig. 4a) but decreased in roots (Fig. 4b) when under Cd stress. This result is in accordance with that of Molina et al. (2008), but differs from the report of Hu et al. (2009). Shah et al. (2001) reported that increased SOD activity protected the plants from oxidative damage. Produced H2O2 is subsequently scavenged by CAT which is a key enzyme for the defense responses against oxidative stress (DalCorso et al. 2008). In the present study, CAT activity decreased in Cd stressed leaves and roots (Fig. 4e, f). POD also eliminates H2O2. POD activity increased both in leaves and in roots of Cd stressed plants (Fig. 4c, d). MDA, which is a peroxidation product of plant cell membrane exposed to stress, is an indicator of lipid peroxidation and demonstrates the extent of oxidative stress in plants (Chaoui et al. 1997). The results of the present study indicated that MDA content of Cd stressed plants significantly increased in comparison with that of the control, indicating the existence of oxidative stress (Fig. 5). Plant growth was eventually inhibited (Fig. 1).
The adverse symptoms caused by Cd can be partially corrected by applying exogenous chemicals (Anjum et al. 2008; Meng et al. 2009) and by phytoremediation (Cheng et al. 2008). So, the plant-biotechnology research has great implications both from theoretical and practical perspectives. In the present study, we firstly used wheat to show that ADO application markedly ameliorated Cd-caused plant growth inhibition. Similar to plant growth regulators (PGRs) which were used to improve plant defense by acting as a signaling molecule, ADO resembles an endogenous elicitor that functions as a signal to trigger the synthesis of the enzymes and to activate stress-response gene expression. When seeds were soaked in 1,000 mg L−1 ADO before Cd stress, SOD, POD and CAT activity was significantly increased (Fig. 4), and MDA content (Fig. 5) showed a remarked reduction in leaves and roots of the seedling. This showed that the oxidative stress caused by Cd addition may be alleviated, thus allowing the plants to adapt by actively increasing antioxidant enzyme activity and decreasing MDA content.
Taken together, our study confirmed the positive effects of ADO in alleviating Cd toxicity, which were reflected by increased fresh weight, dry weight, Chlorophyll content and photosynthetic rate, higher antioxidant enzyme activities and less MDA content in leaves and roots.
References
Akimoto C, Aoyagi H, Tanaka H (1999) Endogenous elicitor-like effects of alginate on physiological activities of plant cells. Appl Microbiol Biotechnol 52:429–436
Albersheim P, Darvill AG (1985) Oligosaccharins: novel molecules that can regulate growth, development, reproduction, and defense against disease in plants. Sci Am 253:58–64
Alvarez M, Pennell R, Meijer P, Ishikawa A, Dixon R, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 92:773–784
Anjum NA, Umar S, Ahmad A, Iqbal M, Khan NA (2008) Sulphur protects mustard (Brassica campestris L.) from cadmium toxicity by improving leaf ascorbate and glutathione. Plant Growth Regul 54:271–279
Cakmak I, Horst WJ (1991) Effect of aluminum on lipid peroxidation, superioxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Chaoui A, Mazhoudi S, Ghorbal MH, Ferjani EL (1997) Cadmium and zinc induction of lipid peroxideation and effects on antioxidant enzyme activities in bean (Phaseolus ulgaris L.). Plant Sci 127:139–147
Cheng W, Zhang G, Yao H, Zhang H (2008) Genotypic difference of germination and early seedling growth response to Cd stress and its relation to Cd accumulation. J Plant Nutr 11:702–715
Costa H, Gallego SM, Tomaro ML (2002) Effects of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Sci 162:939–945
DalCorso G, Farinati S, Maistri S, Furini A (2008) How plants cope with cadmium: staking all on metabolism and gene expression. J Integr Plant Biol 50:1268–1280
Edreva A (2005) Generation and scavenging of reactive oxygen species in chloroplasts: a submolecular approach. Agric Ecosyst Environ 106:119–133
Guo B, Liang YC, Li ZJ, Guo W (2007) Role of salicylic acid in alleviating cadmium toxicity in rice roots. J Plant Nutr 30:427–439
Hassan MJ, Shfi M, Zhang GP, Zhu ZJ, Qaisar M (2008) The growth and some physiological responses of rice to Cd toxicity as affected by nitrogen form. Plant Growth Regul 54:125–132
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Hsu YT, Kao CH (2007) Toxicity in leaves of rice exposed to cadmium is due to hydrogen peroxide accumulation. Plant Soil 298:231–241
Hu XK, Jiang XL, Hueymin H, Liu SL, Guan HS (2004) Promotive effects of alginate-derived oligosaccharide on maize seed germination. Appl Phys 16:73–76
Hu YL, Ge Y, Zhang CH, Ju T, Cheng WD (2009) Cadmium toxicity and translocation in rice seedling are reduced by hydrogen peroxide pretreatment. Plant Growth Regul 58:47–59
Kumar P, Tewari PK, Sharma PN (2008) Cadmium enhances generation of hydrogen peroxide and amplifies activities of catalase, peroxidases and superoxide dismutase in maize. J Agron Crop Sci 194:72–80
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin phenol reagent. J Biol Chem 193:265–275
Meng HB, Hua SJ, Shamsi IH, Jilani G, Li YL, Jiang LX (2009) Cadmium- induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regul 58:47–59
Metwally A, Finkemeier I, Georgi M, Dietz KJ (2003) Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol 132:272–281
Milone MT, Sgherri C, Clijsters H, Navari-Izzo F (2003) Antioxidative responses of wheat treated with realistic concentrateion of cadmium. Environ Exp Bot 50:265–276
Molina AS, Nievas C, Chaca MVP, Garibotto F, Gonza’lez U, Marsá SM, Luna C, Gime’nez MS, Zirulnik F (2008) Cadmium- induced oxidative damage and antioxidantive defense mechanisms in Vigna mungo L. Plant Growth Regul 56:285–295
Natsume M, Kamo Y, Hirayama M, Adachi T (1994) Isolation and characterization of alginate-derived oligosaccharides with root growth-promoting activities. Carbohydr Res 258:187–197
Penner GA, Clarke J, Bezte LJ, Leisle D (1995) Identification of RAPD markers linked to a gene governing cadmium uptake in durum wheat. Genome 38:543–547
Romero-Puertas MC, Rodriguez-Serrano M, Corpas FJ, Gómez M, del Rio LA, Sandalio LM (2004) Cadmium-induced subcellular accumulation of O2 − and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Sandalio LM, Dalruzo HC, Gomez M, Romero-Puetras MC, del-Rio LA (2001) Cadmium- induced changes in the growth and oxidative metabolism of pea plants. J Exp Bot 52(364):2115–2126
Shah K, Kumar RG, Verma S, Dubey RS (2001) Effect of cadmium on lipid peroxidation, superoxide anion generation and activities of antioxidant enzymes in growing rice seedlings. Plant Sci 161:1135–1144
Shamsi IH, Jilani G, Zhang GP, Kang W (2008) Interactive effects of cadmium and aluminum on growth and antioxidative enzymes in soybean. Biol Plant 52:165–169
Tomoda Y, Umemura K, Adachi T (1994) Promotion of barley root elongateion under hypoxic conditions by alginate lyase-lysate. Biosci Biotechnol Biochem 58:202–203
Weckx JEJ, Clijsters HMM (1997) Zinc phytotoxicity induces oxidative stress in primary leaves of Phaseolus vulgaris. Plant Physiol Biochem 35:405–410
Wu FB, Zhang GP (2004) Effect of cadmium on free amino acids, glutathione and ascorbic acid content in two barley genotypes differing in Cd tolerance. Chemosphere 57:447–454
Yin H, Chen Q, Yi M (2008) Effects of short-term heat stress on oxidative damage and responses of antioxidant system in lilium longiflorum. Plant Growth Regul 54:45–54
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30870205), the Department of Education of Liaoning province Foundation (L2010516) and the Liaoning province Natural Science Foundation (20052053) and Director Foundation of Experimental Center, Shenyang Normal University (sy200705).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, L.J., Li, X.M., Bu, N. et al. An alginate-derived oligosaccharide enhanced wheat tolerance to cadmium stress. Plant Growth Regul 62, 71–76 (2010). https://doi.org/10.1007/s10725-010-9489-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-010-9489-2