Abstract
Sawgrass (Cladium jamaicense) is the predominant plant and vegetation community in the Florida Everglades. Germination of sawgrass seeds in the laboratory or nursery has been difficult and problematic, yet little is known about the physiological mechanistic regulation of the sawgrass seed germination process. In the present study, we examined the factors and mechanisms that influence sawgrass seed germination. We found that removal of seed husk and bracts, pre-soaking with bleach (hypochlorite), breaking the seed coat, or combinations of these treatments promoted the rate and success of germination, whereas presence of seed-encasing structures or treatment with husk/bract extract inhibited germination. We further detected the presence of abscisic acid (ABA) in the husk and bract. Experiments with ABA and gibberellin biosynthesis inhibitors fluridone and tetcyclacis suggested that ABA already presented in the pre-imbibed seeds, and not derived through post-dormancy de novo synthesis, contributed to the inhibition of seed germination. Examination of bleach and mechanical treatments indicated the physical barrier presented by the seed-encasing structures provided additional mechanism for the long-term delay of seed germination. Based on the results of this study and others, we discussed the implications of sawgrass seed dormancy and germination in relation to its natural habitat and proposed a hypothesis that the protracted seed dormancy in sawgrass offered an adaptive advantage in the pre-anthropogenic Everglades environment, but may become a liability in the current man-managed Everglades water system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The timing and speed of seed germination are vital developmental processes that correlate directly with the success of a plant species. The process of germination begins when a dormant seed takes up water (imbibition). Imbibition triggers the cellular and metabolic events that set the course for germination, but the entire process is further regulated by a myriad of external signals such as light and temperature. Plant hormones, especially abscisic acid (ABA) and gibberellic acids (GAs), are important in mediating the germination process (Bewley 1997; Finkelstein et al. 2008). The breaking of dormancy and the initiation of seed germination are, in general, restricted in two ways. First, the seed coat acts as a physical barrier that prevents imbibition and progression towards germination (Schütz 2000). Second, inhibitory compounds including ABA present in seed-encasing structures such as the coat, husk, and bract/glume as well as in the endosperm/cotyledons and embryos may further prevent or delay germination of imbibed seeds. In nature, protracted dormancy provides a mechanism for preventing germination “out-of-season” or under inappropriate environmental conditions. In this way, seed dormancy is an adaptive trait with the capacity to provide a species with an ecological advantage under specific environmental conditions (Jain 1982).
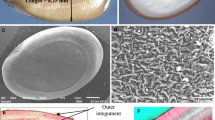
Sawgrass (Cladium jamaicense Crantz) is a sedge species abundantly distributed in the Florida Everglades wetlands. Sawgrass seeds are relatively large achene (ca. 4 mm × 2 mm) and have a thick protective seed coat (Miao et al. 1998). A fibrous husk surrounds the seed coat and the entire assembly is encased in modified leaves, or bracts (Fig. 1a; Richards 2002). In recent years, sawgrass communities in the Florida Everglades have been invaded by a native species of cattail (Typha domingensis). Cattail invasion is seemingly a result of recent anthropogenic changes, most notably changes in hydrology and nutrient (namely phosphorous) levels (Miao 2002). Cattail seeds are practically the antithesis of sawgrass. They are considerably smaller (ca. 1 mm × 0.2 mm) with a hairy stalk that aids in dispersal by wind and water, possess only a thin seed coat, and germinate readily under a wide variety of conditions (our observations; Flora of North America Editorial Committee 1993). Although both species also propagate vegetatively through clonal propagation, sexual reproduction is thought to play a key role in the establishment of new stands (Miao et al. 1998). Despite the vast difference in seed morphology, the genetic and molecular mechanisms underlying how and if these phenotypic differences contribute to cattail invasion into the sawgrass habitat are largely speculative.
Studies of the habitat competition between sawgrass and cattail have experimentally required seed germination. There have also been interests and requirements in cultivating large quantities of sawgrass plants for habitat restoration. While cattail seeds readily germinate, sawgrass seeds demonstrate slow, incomplete, and non-uniform germination under laboratory or nursery conditions. Early studies observed a high variability of germination ranging between 0.4 and 30.2% (Alexander 1971; Ponzio et al. 1995; Sleszynski 1991). While examining the effects of various pretreatments on germination, Ponzio (1998) observed between 50 and 79.3% germination. Interestingly, the highest performing pretreatment was a 3-day soak in 2–3% hypochlorite. Insight concerning the effect of temperature on sawgrass germination was provided in a study demonstrating an increase from 0% germination in seeds maintained at constant temperatures to 42% under a fluctuating temperature regime (Lorenzen et al. 2000). This study also addressed the regulation of germination in the context of cattail invasion into sawgrass habitat. We have observed a rate of germination between 0 and 60%, with considerable variation between seed batches and pretreatments.
There is no published study addressing the molecular or physiological mechanisms underlying sawgrass seed germination, hence control of germination in sawgrass is not fully understood. For example, it is not clear what causes the variability in seed germination rate for sawgrass. Knowledge of how physical and chemical factors regulate seed dormancy and germination would help improve the consistency, uniformity, and rate of seed germination, therefore facilitate studies of lab-cultivated sawgrass. Furthermore, a mechanistic insight of the interaction between seed morphology and recent anthropogenic environmental change will contribute to our understanding of the strategies different plants use to compete for resources and space. Information concerning sawgrass seed biology is particularly relevant to Everglades restoration efforts. Here we report studies aimed at understanding how the physical barrier associated with husk, bract and seed coat as well as chemical factors such as phytohormones present in the husk and bract may contribute to regulation of the seed germination process in sawgrass. We also discuss the implication of the sawgrass seed germination process in the context of its natural and anthropogenic wetland habitats.
Materials and methods
Seed collection and germination
Seeds of sawgrass (Cladium jamaicense Crantz) were collected during the summer of 2006 from the Water Conservation Areas (WCA) 2A in the Florida Everglades and stored at 4°C with the husk and bract intact until use. Seed husk and bract were removed by placing approximately 100 seeds and 3 ml of water in a mortar. Seeds were ground with a pestle using only light pressure. Seeds were rinsed with water and the husk (floating) and seed (sinking) separated via their different densities. The study comparing husked and dehusked seeds utilized 200 seeds per treatment germinated en masse. Individual seed germination in this comparison was monitored and recorded daily with a digital camera linked stereomicroscope (1274ZH, VanGuard). All other studies including phytohormones, inhibitors and enzymatic/mechanical seed coat treatments (see below) were conducted using 100 seeds sown in four replicate treatments of 25 seeds. Following pretreatments, seeds were rinsed in deionized water and sown on moist paper towels. Seeds were placed in a growth chamber (AR-36, Percival) under a 16-h light, 8-h dark photoperiod and a 25°C light, 15°C dark diurnal temperature cycle. Daytime light intensity was approximately 250 μmol/m2/s. Humidity was maintained near 100%. All germination rates were reported as the mean ± SE of the four replicate treatments at 6 weeks post imbibition. Germination rates were compared using a univariate analysis of variance with a Tukey post hoc test (Ρ ≤ 0.05).
Treatments with hypochlorite, phytohormones and their inhibitors
The effect of seven different pretreatments administered via a 7-day imbibition period on the germination rate of sawgrass was examined. For studies comparing the germination rate of husked and dehusked seeds, imbibition in 10% household bleach (Clorox) equivalent of 0.3–0.6% sodium hypochlorite was used. For studies comparing the effect of pretreatment on germination rate, 10% bleach, 30 μM fluridone (Sonar, Chem Service), 50 μM gibberellic acid (GA3, Sigma), 25 μM tetcyclacis, deionized water, and water-soluble bract extract were tested respectively. The water-soluble bract extract was made by soaking bract and husk from 200 seeds in 100 ml of water for 7 days.
Enzymatic and mechanical treatments of seed coat
Seeds were treated enzymatically and mechanically with the intention of weakening the seed coat. For enzymatic treatment, dehusked seeds were incubated in a solution containing 1% Cellulysin® (Calbiochem Co.), 0.5% macerase® pectinase (Calbiochem Co.), 1.5% Driselase® (Sigma), 0.7 mM KH2PO4, 6 mM CaCl2, 1.5 mM sorbitol, 0.15 M mannitol, 0.1 M glucose, and 3 mM 2-(N-morpholino) ethanesulfonic acid, pH 5.6, at 25°C with gentle shaking for 7 days. Seeds were monitored daily for visible signs of seed coat removal. For mechanical treatment, the endosperm cap was removed by making a cut parallel to the axial plate at the point where the radicle emerges. Dehusked seeds that had been water-imbibed or 10% bleach-incubated for 7 days were used. Seeds were sown as described above.
Measurements of abscisic acid (ABA)
Plant hormones were extracted from dry and pre-germination seeds, using a modified procedure of Lewis and Visscher (1982). A total of 25 seeds were weighed, ground in liquid nitrogen, and extracted in 2 ml of 80% methanol for 24–40 h at −20°C. Extracts were centrifuged at 14,000 RPM for 10 min and the supernatant was removed. The supernatant was then extracted in 1 ml of 80% methanol for 24–40 h at −20°C. The extract was centrifuged as above and the supernatant combined with the first extract. Extracts were diluted to 10% methanol (pH 2.8) using water and HCl. Extracts were purified using C18 preparatory columns (WAT020515, Waters) using the following process.
-
1.
Column conditioned with 10 ml 100% methanol.
-
2.
Column equilibrated with 10 ml of 10% methanol (pH 2.8).
-
3.
Extract (~20 ml) loaded onto the column.
-
4.
Column washed with 10 ml of 20% methanol (pH 2.8).
-
5.
Column washed with 10 ml of 32% methanol (pH 2.8)
-
6.
Column eluted with 5 ml of 32% methanol (pH 8.0)
-
7.
Column stripped with 10 ml of 100% methanol
-
8.
Column washed with 10 ml of 100% ethyl acetate
Elution fractions were vacuum dried and re-suspended in 1 ml of water. The pH was adjusted to 2–3 before extracting three times with ethyl acetate. Organic phases from the three extractions were combined, vacuum dried, and re-suspended in 300 ml of 20 mM Tris and 50 mM NaCl (pH 7.5) buffer. ABA was measured using the competitive enzyme-linked immunosorbent assay (ELISA) kit (Agdia) according to the manufacturer’s protocol. ABA from Sigma was used a standard. ABA concentration was expressed as ng ABA per g of fresh weight. Three replicate extractions and ELISA reactions were performed for each treatment. Mean ABA concentrations were compared using a univariate analysis of variance with a Tukey post hoc test (Ρ < 0.05).
Results
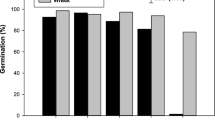
Sawgrass seeds in their natural form are encased with husk, bract and seed coat (Fig. 1a). Dehusked seeds had a germination rate nearly four times that of husked seeds (56% vs. 16%) as shown in Fig. 2. Germination synchrony was also improved (50% germination over 6 days vs. 12 days) and the time to germination reduced by approximately 1 week (50% germination after 23 days vs. 29 days). These results suggested that the presence of bracts and husks hindered germination. The mechanism underlying this improvement was examined by determining whether husk/bract inhibition was a result of physical and/or chemical factors.
No significant differences in water uptake were observed between husked and dehusked seeds following a 7 day imbibition, suggesting that the husk/bract inhibition was not a direct result of altered water penetration into the seed. As shown in Fig. 3, husk/bract removal resulted in an approximate 20% reduction (423 ± 13 to 328 ± 36 ng ABA per g FW) in seed ABA content as determined by ELISA, although the difference was not statistically significant (Ρ = 0.174). Imbibition significantly (Ρ ≤ 0.05) reduced the ABA content of seeds but bleach did not significantly reduce the content in comparison to water (Fig. 3). These results indicate that chemical factors may have been at least partly responsible for husk/bract inhibition. The involvement of chemical factors in husk/bract inhibition was also suggested by a reduction in percent germination from 45 ± 3% in water-imbibed seeds to 35 ± 2% in seeds imbibed in water-soluble bract extract, although the difference was not statistically significant (Fig. 4).
To examine the roles of the phytohormones ABA and gibberellin (GA) in sawgrass seed germination, two inhibitors were tested: fluridone, an inhibitor of ABA biosynthesis and tetcyclacis, an inhibitor of cytochrome P450 enzymes involved in pathways leading to ABA and GA biosynthesis (Fig. 5). Surprisingly, sawgrass germination was not improved in 30 μM fluridone-imbibed seeds, even though fluridone action was evident in the reduced pigmentation of emerging radicles (Fig. 1b). Treatment with 25 μM tetcyclacis also did not affect seed germination. Furthermore, neither 30 μM fluridone nor 50 μM GA3 treatments had a significant effect on germination rate (Fig. 4). These results suggest that de novo synthesis of ABA or GA may not play a major role in regulation of sawgrass seed germination.
While removal of the husk/bract appears to stimulate germination by decreasing the level of chemical inhibitors, our results could not fully explain the mechanism underlying increased germination in dehusked bleach-imbibed seeds. The germination rate of bleach-imbibed seeds (60 ± 6.3%) was significantly (Ρ = 0.044) higher than that of water-imbibed seeds (45 ± 2.5%) (Fig. 4). Interestingly, ELISA analysis indicated that in comparison to water, bleach had no significant effect on the post-imbibition level of ABA (Fig. 3), suggesting that bleach-imbibition did not increase the rate or magnitude of ABA degradation.
The inability of chemical treatment to elevate germination to bleach-imbibed levels suggests that the physical barrier presented by the seed coat may be the main inhibiting force. In this scenario, we hypothesized that the bleach pretreatment would promote germination by weakening the seed coat. An examination of the effect of a 1-week pretreatment in 0, 10, 20, 30, 40, and 50% bleach on germination demonstrated that 10% bleach was the optimum concentration in our hands. An attempt to weaken the seed coat by digestion with an enzyme cocktail commonly used for protoplast preparation (Zhang et al. 2001) resulted in no observable change to the seed coat or germination rate following a 7 day incubation period. These observations indicated that the sawgrass seed coat is resistant to cellulose- and pectin-degrading enzymes. Finally, mechanical removal of the endosperm cap by surgical nicking following a 7 day imbibition resulted in a significant increase (Ρ = 0.05) in germination rate, as compared to that of untreated seeds under the same condition (Table 1), which supported our notion that the physical barrier of the seed coat provided additional, perhaps major, mechanism in delaying germination of sawgrass seeds.
Discussion
It is well known that ABA normally inhibits seed germination whereas GAs generally promote the germination process (Bewley 1997; Cutler and Krochko 1999; Hedden and Phillips 2000; Finkelstein et al. 2008). The biosynthesis of both ABA and GAs is originated from geranylgeranyl diphosphate in the carotenoid pathway (Ajjawi and Shintani 2004; Cutler and Krochko 1999; Hedden and Phillips 2000; Sawada et al. 2008; Fig. 5). Fluridone is an inhibitor of phytoene desaturase (a key enzyme in the formation of the ABA precursor carotene, Fig. 5) and is commonly used as an inhibitor of ABA synthesis to dissect the role of hormones in seed germination. Our studies show that inhibition of ABA synthesis by fluridone during imbibition had not improved seed germination, which indicated that unlike some other plants (Grappin et al. 2000), post-dormancy de novo ABA synthesis does not contribute significantly to the inhibition of germination in sawgrass.
On the other hand, improvement in germination through removal of the bract, glume or husk has been observed in other grasses (Fandrich and Mallory-Smith 2006; Wurzburger and Leshem 1969). We also observed an increase in both germination and synchrony for dehusked seeds (Fig. 2) and an apparent decrease in germination for seeds soaked in bract/husk extracts (Fig. 4). Together, these findings lead us to propose that ABA and probably other compounds synthesized prior to dormancy and stored in the husk and bract contribute, although to a lesser extent (see below), to the regulation of germination in sawgrass. The presence of ABA and other germination inhibitors in seed-encasing structures may provide a mechanism for the long-term delay of seed germination. In nature, the erosion of the bracts, husk, and seed coat would require months to years depending on the seed-encasing structure and environmental conditions. For example, to achieve seed germination for field work we routinely store sawgrass seeds in field sediment for three to four months before sowing (Miao, unpublished data). One of the consequences of this long period weathering is probably the degradation of germination inhibiting compounds, as well as the break down of the seed coat.
More importantly, our studies suggest that dormancy and seed germination in sawgrass is regulated by the innate characteristics of its seed-encasing structures. Surgically removal of part of the seed coat significantly increased germination rate (Table 1), supporting our notion that physical barrier may be a major factor controlling sawgrass seed germination. Conceivably, the chemical inhibitors in the husk/bract and the physical restriction of the seed coat apparently function synergistically to maintain dormancy and delay germination in nature. These findings may reflect evolutionary and ecological implications for sawgrass adapted to the Everglades.
Sawgrass seed dispersal normally occurs between July and August during the wet season when daytime temperatures can be as high as 35°C. The seed encasing structures maintain the level of inhibiting chemicals, thus physically and physiologically prevent early season germination. Studies have shown that high temperature promotes ABA biosynthesis and represses GA synthesis and signaling in seed germination (Toh et al. 2008). During the Everglades dry season (between November and April), there is a marked difference in day/night temperatures (around 20°C day and 10°C to as low as 5°C night). This temperature condition would favor a decrease in ABA content and an increase in GA synthesis thus promoting seed germination in late winter/early spring when water condition becomes favorable. Erosion of the seed coat over the wet months, degradation of inhibitory compounds and water and temperature conditions during the dry period all favor sawgrass seed germination. The ample nutrient reserves in the large seeds would help sawgrass grow into robust seedlings and quickly establish themselves at the start of the wet season in the Everglades, since sawgrass seedlings are not tolerant to deep water (Miao 2002). Thus, the seed-encasing structures and the inhibitory chemical compounds in the husk, bract, and probably endosperm as well, may be an adaptation to the natural Everglades climate and hydrological rhythm. In the modern Everglades, however, anthropogenic changes in hydrology (e.g., year-round elevated water level) and soil nutrition (e.g., increased phosphate level) may have diminished the adaptive advantage of protracted seed dormancy in sawgrass.
References
Ajjawi I, Shintani D (2004) Engineered plants with elevated vitamin E: a nutraceutical success story. Trends Biotechnol 22:104–107. doi:10.1016/j.tibtech.2004.01.008
Alexander T (1971) Sawgrass biology related to the future of the Everglades ecosystem. Proc Soil Crop Sci Soc Fla 31:72–74
Bewley JD (1997) Seed germination and dormancy. Plant Cell 9:1055–1066. doi:10.1105/tpc.9.7.1055
Cutler AJ, Krochko JE (1999) Formation and breakdown of ABA. Trends Plant Sci 4:472–478. doi:10.1016/S1360-1385(99)01497-1
Fandrich L, Mallory-Smith CA (2006) Factors affecting germination of jointed goatgrass (Aegilops cylindrica) seed. Weed Sci 54:677–684. doi:10.1614/WS-05-104R.1
Finkelstein R, Reeves W, Ariizumi T, Steber C (2008) Molecular aspects of seed dormancy. Annu Rev Plant Biol 59:387–415. doi:10.1146/annurev.arplant.59.032607.092740
Flora of North America Editorial Committee (ed) (1993) Flora of North America. Oxford, New York, p 213
Grappin P, Bouinot D, Sotta B, Miginiac E, Jullien M (2000) Control of seed dormancy in Nicotiana plumbaginifolia: post-imbibition abscisic acid synthesis imposes dormancy maintenance. Planta 210:279–285. doi:10.1007/PL00008135
Hedden P, Phillips AL (2000) Gibberellin metabolism: new insights revealed by the genes. Trends Plant Sci 5:523–530. doi:10.1016/S1360-1385(00)01790-8
Jain SK (1982) Variation and adaptive role of seed dormancy in some annual grassland species. Bot Gaz 143:101–106. doi:10.1086/337276
Lewis RW, Visscher SN (1982) A simplified purification method for the analysis of abscisic acid. Plant Growth Regul 1:25–30. doi:10.1007/BF00024219
Lorenzen B, Brix H, McKee KL, Mendelssohn IA, Miao S (2000) Seed germination of two Everglades species, Cladium jamaicense and Typha domingensis. Aquat Bot 66:169–180. doi:10.1016/S0304-3770(99)00076-5
Miao SL (2002) Ecological studies on species replacement and restoration in the Florida Everglades, USA. In Wu J, Han X and Huang J (eds) Lectures in modern ecology (II) from basic science to environmental issues, Science and Technology Press, Beijing, China, pp 159–177
Miao SL, Kong L, Lorenzen B, Johnson RR (1998) Versatile modes of propagation in Cladium jamaicense in the Florida Everglades. Ann Bot (Lond) 82:285–290. doi:10.1006/anbo.1998.0690
Ponzio KJ (1998) Effects of various treatments on the germination of sawgrass, Cladium jamaicense Crantz, seeds. Wetlands 18:51–58
Ponzio KJ, Miller SJ, Lee MA (1995) Germination of sawgrass, Cladium jamaicense Crantz, under varying hydrologic conditions. Aquat Bot 51:115–120. doi:10.1016/0304-3770(95)00461-8
Richards JH (2002) Flower and spikelet morphology in sawgrass, Cladium jamaicense Crantz (Cyperaceae). Ann Bot (Lond) 90:361–367. doi:10.1093/aob/mcf197
Sawada Y, Aoki M, Nakaminami K, Mitsuhashi W, Tatematsu K, Kushiro T, Koshiba T, Kamiya Y, Inoue Y, Nambara E, Toyomasu T (2008) Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiol 146:1386–1396. doi:10.1104/pp.107.115162
Schütz W (2000) Ecology of seed dormancy and germination in sedges (Carex). Perspect Plant Ecol Evol Syst 3:67–89. doi:10.1078/1433-8319-00005
Sleszynski PA (1991) The significance of soil seed reserves in the seasonal wetlands of south Florida and their possible applications for habitat restoration. M.S. Thesis, University of Florida, Gainesville
Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, Hanada A, Aso Y, Ishiyama K, Tamura N, Iuchi S, Kobayashi M, Yamaguchi S, Kamiya Y, Nambara E, Kawakami N (2008) High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol 146:1368–1385
Wurzburger J, Leshem Y (1969) Physiological action of the germination inhibitor in the husk of Aegilops kotschyi Boiss. New Phytol 68:337–341. doi:10.1111/j.1469-8137.1969.tb06445.x
Zhang X-H, Widholm JM, Portis ARJ (2001) Photosynthetic properties of two different soybean suspension cultures. J Plant Physiol 158:357–365. doi:10.1078/0176-1617-00233
Acknowledgements
This work was supported in part by a fund from the South Florida Water Management District. We thank Robert Johnson and Manuel Tapia for their help with seed collections and Dr. Wilhelm Rademacher of BASF, Germany, for the gift of tetcyclacis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Webb, J., Miao, S. & Zhang, XH. Factors and mechanisms influencing seed germination in a wetland plant sawgrass. Plant Growth Regul 57, 243–250 (2009). https://doi.org/10.1007/s10725-008-9341-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-008-9341-0