Abstract
Chilling stress is an important constraint of global production of maize. This study was undertaken to compare the chilling responses of different maize seedling tissues and to analyze changes in polyamines as a result of chilling stress. Reponses to chilling were characterized in two maize (Zea mays L.) inbred lines, ‘HuangC’ and ‘Mo17’, that putatively differ in chilling sensitivity. Seedlings were exposed to low temperature (5°C) and chilling injury was estimated by electrical conductivity (EC), malonaldehyde (MDA) concentration, and by changes in putrescine (Put), spermidine (Spd) and spermine (Spm) concentrations in root, mesocotyl, and coleoptile tissues. Membrane permeability (as measured by EC), MDA concentrations and Put concentrations in the three tissue of maize seedlings increased after chilling stress, except for the Put concentration in roots. Spd and Spm concentrations in the three tissues of seedlings decreased after chilling stress. The EC for cold stressed tissues were lower in HuangC than Mo17. Also, the EC of coleoptile tissues were lower than for mesocotyl in both inbred lines. We suggest that mesocotyl tissue can be used to evaluate cold tolerance in maize. Stepwise regression analyses showed that chilling injury in roots was generally correlated with Spd concentration while in the mesocotyl injury was mainly correlated with Put and Spd concentrations. Spermidine and Spm concentrations in the coleoptile were correlated with chilling injury. Characteristics changes of polyamines in chill-tolerant maize seedling combined with regression analysis are a reliable method for evaluating chill tolerance in maize lines.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Maize, as is characteristic of many tropical and sub-tropical species, is susceptible to chilling injury at low non-freezing temperatures below 12°C (Stamp 1984). The chilling sensitivity of maize is particularly notable during germination and early seedling growth (Stamp 1984) but varies depending on genotype. Numerous physiological changes occur in plants in response to low temperature, particularly with cellular membranes. Membrane permeability in particular increases with chilling stress (Markowski et al. 1990). Changes in membrane permeability can be demonstrated by characterizing electrolyte leakage from tissues. Electrolyte leakage has been extensively used for evaluating cold tolerance (Markowski et al. 1990). During cold stress, cellular membranes can be attacked by free radicals, resulting in membrane lipid peroxidation (Elste 1982). Malondialdehyde (MDA) is a major component of thiobarbiturate-reactive substances and is used as an indicator of lipid peroxidation (Elste 1982). During post chilling recovery at normal temperatures, seedling growth of chill-tolerant maize lines was more rapid than that of chill-sensitive maize lines (Sowiński et al. 2005). These changes all occur before visual morphologic changes appear post-chilling stress, therefore, membrane permeability and MDA concentration may be used as physiological marker of chilling tolerance and chilling injury in maize.
Upon exposure to low temperature, plants accumulate polyamines (PAs) (Lee et al. 1995; Shen et al. 2000). Polyamines, Spd, Spm and their obligate precursor Put, are small aliphatic low-molecular-weight polycationic nitrogenous compounds that are ubiquitous in higher plants. They are important modulators of biological processes such as plant growth, development and senescence (Koetje et al. 1993). They are also involved in plant responses to environmental stress (Bouchereau et al. 1999). The concentration of endogenous polyamines has been shown to increases during chilling stress in several plant species such as rice (Lee et al. 1995), cucumber (Shen et al. 2000), maize (Szalai et al. 1997; Németh et al. 2002) and chickpea (Nayyar and Chander 2004). Because they are fully protonated and polycationic in nature at physiological pH, PAs can bind strongly to negatively charged cellular components such as nucleic acids, proteins and phospholipids (Bouchereau et al. 1999). Several mechanisms for the protective nature of PAs have been postulated, including stabilizing chromosomes, retarding lipid peroxidation (radical scavenging) and preserving membrane integrity (Nadeau et al. 1987).
Previous researchers have indicated that maize mesocotyl tissues were the most sensitive to low temperature injury during seed germination (Anderson et al. 1995; Gao et al. 2006). PAs are generally distributed in maize embryonic axis (Sepulveda and Sanchez-de-Jimenez 1988) but Put mainly distributed in the base of both the coleoptile and root during seed germination at normal temperatures (Dumortier et al. 1983). These reports showed that sensitivity to low temperature, as well as the PAs distribution, varied in different maize seedling tissues. Earlier investigations of PAs metabolism, in chilling stressed maize seedlings mainly used callus tissues (Songstad et al. 1990) and leaves (Szalai et al. 1997; Németh et al. 2002; Páldi et al. 2002). Furthermore, these tissues were usually derived from common maize varieties (Szalai et al. 1997; Németh et al. 2002). However, by using chill-tolerant and chill-sensitive maize lines, it should be possible to characterize differences in PA profiles following chilling stress that more effectively characterize relationships between PA metabolism and chilling tolerance in maize. By partitioning maize seedling, the differences in the metabolism of PA between different tissues could be investigated. However, to our knowledge, there are no data using maize inbred lines with different chilling tolerance to study changes in PA concentrations in different tissues of seedlings under chilling stress, and no reports on the relationship between the changes of PA concentrations and chilling injury parameters such as membrane permeability and MDA concentration.
In the present study, chill-tolerant and chill-sensitive maize inbred lines are used to determine tissue difference (i.e. root, mesocotyl plus coleoptile node and coleoptile plus leaves), in polyamine concentrations in response to chilling tolerance in maize seedlings. Moreover, we attempt to assess which PAs are more closely correlated with chilling tolerance in three different maize seedling tissues.
Materials and methods
Growth of plants and stress application
Two maize (Zea mays L.) inbred lines, HungC (chill-tolerant) (Zheng et al. 2006), Mo17 (chill-sensitivity) (Páldi et al. 2002; Zheng et al. 2006), were used. All seeds were stored at 4°C until used. Maize seeds were surface sterilized in 0.5% NaOCl for 5 min (Joseph et al. 1992) and germinated for 4 days in darkness at 25°C (control temperature) on paper moistened with water. The low temperature stress treatments were accomplished by transferring them to 5°C (chilling temperature) for 3 days. Seedlings were then transferred back to 25°C for a recovery period of 3 days. Seedlings were grown in growth chambers with a 12 h light: dark period, illumination at a constant 250 μmol m−2 s−1 during both chilling and the recovery period. Each of the three replicates was comprised of 50 seeds.
Seedlings were harvested before the chilling treatment (day 4), immediately after the chilling treatment (day 7) and at the end of the recovery growth period (day 10). Tissues were sectioned into: (a) coleoptile plus leaves (coleoptile); (b) mesocotyl plus coleoptile node (mesocotyl); (c) root. MDA concentration, membrane permeability and PA concentrations were measured in three replicates each consisting of 30 seedlings for each treatment.
MDA concentration assay
MDA concentration was determined by the thiobarbituric acid (TBA) reaction as described by Li (2000). Briefly, 0.3 g fresh weight (FW) of seedling tissue was collected, and homogenized in 3 ml of 0.1% trichloroacetic acid (TCA). The homogenate was centrifuged at 3,600g for 10 min. Two ml of 0.67% TBA was added to the 2 ml of supernatant. The mixture was heated at 100°C for 30 min, then cooled to room temperature and centrifuged at 1,800g for 5 min. The optical density of the supernatant was measured at 450, 532 and 600 nm. The MDA concentration was calculated according to the formula:
then the MDA (nmol g Fw−1) concentration was calculated.
Membrane permeability assay
Electrolyte leakage was used to measure membrane permeability using the method of Liu et al. (2000). The three different seedling tissues were sectioned into segments of about 5 mm. Each tissue sample (0.2 g FW) was washed three times with deionized water, then it was placed in a test tube with 10 ml deionized water and covered with a plug. After incubation at 25°C for 6 h, the conductivity (E1) was measured with conductivity meter DDS-11A (Made in Shanghai, China). Subsequently, the tissue was placed in a 100°C water bath for 30 min, and then cooled to 25°C. A second conductivity measurement was made (E2). The electrical conductivity of deionized water was also measured (E0). The relative electrolyte leakage (REL) was calculated as follows:
Polyamine assay
Free polyamine concentrations were measured according to the method of Flores and Galston (1982) with slight modification. Each 100 mg of tissue was homogenized with 1 ml 5% (w/v) cold perchloric acid using a cooled mortar and pestle. The homogenates were kept in an ice bath for 1 h, and then centrifuged at 23,000g for 30 min at 4°C, the supernatant was transferred to new plastic vials and were stored at −70°C for PA quantification.
Plant extracts were benzoylated. One ml of 2 mol/l NaOH was mixed with 500 μl supernatant. After the addition of 10 μl benzoyl chloride, samples were vortexed for 20 s, incubated for 20 min at 37°C. Following the high temperature incubation, 2 ml of saturated NaCl was added. Benzoyl-polyamines were extracted in 2 ml diethyl ether and vortexed for 10 s. After centrifugation at 1,500g for 5 min at 4°C, 1 ml of the ether phase was collected, evaporated to dryness under a stream of warm air, and redissolved in 100 μl methanol.
The benzoylated extracts were filtered through a 0.22 μm membrane filter, then eluted at room temperature through a 3.9 × 150 mm, 4 μm particle size reverse-phase (C18) column (Waters Nova-Pak), and detected at 254 nm. HPLC analysis was carried out using a Waters 2487 dual λ absorbance detector, 515 HPLC pump. The mobile phases consisted of methanol: water (64:36), at a flow rate of 1 ml/min. Three polyamine standards (Sigma Chemical Co.) of Put, Spd and Spm were prepared at different concentrations for the production of standard curves.
Calculation of the percent change of PAs
If the percent change of PAs was more than 100% following exposure to stress, it means that the concentration of this polyamine component increases during chilling stress.
Statistical analysis
Analysis of variance (ANOVA) was performed using SAS software (before analysis, percentage data were arcsin transformed y = arcsin[sqr(x/100)]. Membrane permeability and MDA concentration were dependent variables in regression analyses against the three kinds of polyamine (independent variables). Their correlation was analyzed by stepwise regression procedure of SAS software (Hu 2001). The influence of an experimental variable was considered significant for P values ≤0.05.
Results
Membrane permeability and MDA concentration
There were no significant differences in membrane permeability among tissues or between maize line (HuangC or Mo17) prior to stress (day 4). However, after stress (day 7), membrane permeability in all three tissues of Mo17 was higher than that of HuangC (Table 1). These differences in membrane permeability were still apparent after 3 days of warming (day 10) except for mesocotyl tissue where there were no difference between the lines.
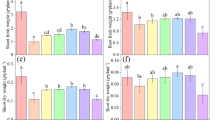
The maize lines had the same MDA concentrations in all the tissues at day 4 (Table 2). However, MDA concentrations of HuangC were lower than those of Mo17 at day 7 and day 10. MDA concentrations in the three tissues of both HuangC and Mo17 seedlings at day 7 were higher than those on both day 4 and 10. MDA concentrations in all three tissues of both lines at day 10 were higher than those at day 4, except for MDA concentration in the coleoptile of HuangC which were not significant between day 4 and day 10 (Table 2).
Polyamines in the roots
After growth for 4 days (day 4) before chilling, there were no differences in Put concentrations in roots of HuangC or Mo17. Spd concentration in HuangC roots was higher than that of Mo17 (Table 3), however, Spm concentration in HuangC was lower than that in Mo17. After 3 days at chilling temperatures (day 7), Put and Spm concentrations in Mo17 roots were higher than those in HuangC, however, there were no differences in Spd concentration between HuangC and Mo17. After recovering at control temperature for 3 days (day 10), Put concentrations in Mo17 roots was higher than that in HuangC, however, there was no difference in either Spd or Spm concentration in roots of either HuangC or Mo17 (Table 3).
Put concentrations in root of Mo17 and HuangC were not different between day 4 and 7, however, Put concentration on day 10 was lower than that on both day 4 and 7 (Table 3). Spd concentration in roots of Mo17 on day 4 was higher than that on both day 7 and 10, however, there was no difference between day 7 and 10. Spd concentration of HuangC root declined from day 4. The change in Spm concentration in roots of Mo17 over time was similar to that of Spd concentration. Spm concentration of HuangC roots on day 4 was higher than that on day 7 and 10, however, Spm concentration in root of HuangC on day 7 was lower than that on day 10 (Table 3).
Polyamines in the mesocotyl
For mesocotyls, Put and Spm concentrations of Mo17 were higher than those of HuangC on day 4, however, there was no difference in Spd concentration between HuangC and Mo17. After 3 days of chilling temperatures (day 7), Put, Spd and Spm concentrations of both lines were similar to day 4. After recovery, Put, Spd and Spm of Mo17 were higher than those of HuangC (Table 4).
Put concentrations in mesocotyl of both Mo17 and HuangC on day 7 were higher than those on both day 4 and 10. Put concentrations of Mo17 on day 10 were lower than on day 4. The Put concentration of HuangC did not change between day 4 and 10. The Spd concentration of Mo17 on day 7 was lower than that on day 4 or 10, which were similar. Spd concentration in HuangC declined throughout. The change in Spm concentration of both lines over time was similar to that in Spd concentration of HuangC (Table 4).
Polyamines in the coleoptile
Prior to chilling (day 4), Put concentrations of Mo17 coleoptiles were higher than those of HuangC while the Spd and Spm concentrations were lower (Table 5). After chilling, as comparing Mo17 and HuangC, Put and Spd concentrations in coleoptiles were similar on day 4 at normal temperatures. However, Spm concentration showed no difference between HuangC and Mo17. After recovery at control temperatures for 3 days, the Put and Spd concentrations of HuangC were higher than those of Mo17 while the Spm concentrations were similar.
Put concentration in coleoptiles of Mo17 on day 7 was higher than either day 4 or 10. However, after 3 days at the control temperature (day 10), they were lower than on day 4 (Table 5). The Put concentration of HuangC on day 4 was lower than that on day 7 or 10, but there was no difference between day 7 and 10. Spd concentrations of both Mo17 and HuangC decreased throughout the sampling period. Spm concentrations of both lines on day 4 were higher than those on day 7 or 10, which were not different from each other.
Regression analysis between the chilling injury parameters and polyamine components in different tissues of maize seedlings under chilling stress
Stepwise regression analysis was used to select polyamine components which had significant effect (α = 0.05 significant level) on chilling injury markers. The results showed that all regression equations were highly significant level (<0.0001) (Table 6).
For root tissues, Spd (X 2) affected membrane permeability \( (\hat{y}_{1} ), \) while Spd (X 2) and Spm (X 3) affected MDA \( (\hat{y}_{2} ) \) concentration. Spd was highlighted by two of the regression equations, indicating that Spd was a significant factor influencing chilling injury in roots. MDA concentration in roots appears to be a more reliable descriptor of chilling injury due to its relatively larger R 2 value (Table 6).
For mesocotyl tissue, both Put and Spd affected membrane permeability \( (\hat{y}_{1} ), \) whereas all polyamines affected MDA \( (\hat{y}_{2} ) \) concentration. Put and Spd were the main factors influencing chilling injury. Moreover, the R 2 value in \( \hat{y}_{2} \) (0.939) was larger than that in \( \hat{y}_{1} \) (0.889), indicating that MDA concentration was again a key indicator of chilling injury.
Both Spd and Spm affected the membrane permeability of coleoptile tissues \( (\hat{y}_{1} ), \) all polyamines also affected MDA \( (\hat{y}_{2} ) \) concentration. Spd and Spm were selected into the two regression equations, showing that Spd and Spm were the main factors influencing chilling injury in coleoptiles. However, the membrane permeability (R 2 = 0.880) as a chilling injury marker in coleoptiles might be more reliable than MDA (R 2 = 0.773) according to their R 2 value.
Discussion
Chilling injury affected membrane permeability and MDA concentration in all tissues of both lines. The apparent injury caused by chilling in Mo17 was greater than HuangC. Cellular membrane damage was induced by chilling stress, and the degree of chilling injury in HuangC was lower than that of Mo17, which confirms earlier results (Zheng et al. 2006). Furthermore, the present study showed that mesocotyl tissue was more easily damaged during chilling stress than other tissues. Mesocotyl tissue appears suitable for evaluating cold tolerance in maize. This is consistent with the results using measures of chilling injury such as catalase and peroxidase activities and proline concentration (Gao et al. 2006).
The value of Put concentration as an indicator of chilling damage varied with seedling tissue. There was no significant change in Put concentrations in root tissue after chilling. However, it increased in both mesocotyl and coleoptile of both maize lines. During chilling stress, Put can be bound to antioxidant enzymes such as superoxide dismutase or be conjugated to small antioxidant molecules allowing them to permeate to sites of oxidative stress within cells (Bouchereau et al. 1999). Therefore, Put can alleviate chilling injury. Put concentration in chilling-tolerant HuangC was lower than that of chilling-sensitive Mo17 under chilling stress, however, the percent change of Put levels in both mesocotyl and coleoptile of HuangC were higher than those of Mo17, moreover, the percent change of Put concentration in coleoptiles was higher than that of the mesocotyl in both lines (Table 7). Taken together, these results suggest that the percent change (day 7 to day 4 ratio) of Put concentration in both coleoptile and mesocotyl, rather than the absolute concentrations, were positively correlated with chilling tolerance of maize seedlings. These results agree with those of rice (Lee et al. 1995).
Spd and Spm inhibited chilling injury by retarding lipid peroxidation and preserving membrane integrity, also, Spd and Spm might interact with membranes either by inhibiting the transbilayer movement of phospholipids, or by stabilizing molecular complexes of thylakoid membranes (Bouchereau et al. 1999). The percent change of Spd in mesocotyl and coleoptile of HuangC were higher than those of Mo17, the ratio of Spd to total PA concentrations showed a similar trend (Table 7). This indicates that the percent change of Spd levels and the ratio of Spd to total PA concentrations in both coleoptile and mesocotyl were negatively correlated with chilling injury parameters, and positively correlated with chilling tolerance of maize seedlings.
Spm concentrations were very low compared to Put and Spd concentrations in all three tissues of both lines, and the ratio of Spm to total PA concentrations was therefore the smallest. This was consistent with the results of Szalai et al. (1997); Németh et al. (2002). Shen et al. (2000) considered the contribution of Spm to the chilling tolerance of cucumber to be much smaller than Spd. The role of Spm in chilling tolerance of maize seedling warrants further study.
Various PAs may play different roles in chilling tolerance. The chilling injury parameters in root were mainly correlated with Spd concentration, in the mesocotyl they were mainly related with Put and Spd concentration and in coleoptile were mainly related with Spd and Spm concentration. Therefore, it is likely that a more reliable approach is to combine the results of a stepwise regression analysis with measured changes of PAs in maize seedling when screening for chill-tolerant in maize. Thus, under chilling stress conditions, the selection of chill-tolerant maize should not only consider PA components and concentrations in different tissues of maize seedlings, but also the percent change of these PA components and the ratio of these PA components to total PA concentrations.
References
Anderson MD, Prasad TK, Stewart CR (1995) Changes in isozyme profiles of catalase, peroxidase, and glutathione reductase during acclimation to chilling in mesocotyls of maize seedlings. Plant Physiol 109:1247–1257
Bouchereau A, Aziz A, Larher F, Martin-Tanguy J (1999) Polyamines and environmental challenges: recent development. Plant Sci 140:103–125. doi:10.1016/S0168-9452(98)00218-0
Dumortier FM, Flores HE, Shekhwat NS, Galston AW (1983) Gradients of polyamines and their biosynthetic enzymes in coleoptiles and roots of corn. Plant Physio1 72:915–918
Elste EF (1982) Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol 33:69–73
Flores HE, Galston WA (1982) Analysis of polyamine in higher plants by high performance liquid chromatography. Plant Physiol 69:701–706
Gao CH, Hu J, Zheng YY, Zhang S (2006) Changes of antioxidant enzyme activities and proline content in maize seedling and their relationship to chilling tolerance. Chin J Appl Ecol 17(6):1045–1050
Hu LP (2001) Windows SAS 6.12 & 8.0 practical statistical analysis course. Military Medical and Science Press, Beijing (in Chinese)
Joseph M, Tomaso D, Jonathan JH, Leon VK (1992) Transport kinetics and metabolism of exogenously applied putrescine in roots of intact maize seedlings. Plant Physiol 98:611–620
Koetje DS, Kononowicz H, Hodges TK (1993) Polyamines metabolism associated with growth and embryogenic potential of rice. J Plant Physiol 141:215–221
Lee TM, Huu SL, Chu C (1995) Absisic acid and putrescine accumulation in chilling-tolerant rice cultivars. Crop Sci 35:502–508
Li HS (2000) Principles and techniques of plant physiological biochemical experiment. Higher Education Press, Beijing (in Chinese)
Liu N, Gao YB, Jia CY (2000) Changes in POD activity, free proline content and cytomembrane permeability of Lolium multiflorum leaves under different levels of osmotic stress. Plant Physiol Commun 36:11–14
Markowski A, Augustyniak G, Janowiak F (1990) Sensitivity of different species of field crops to chilling temperature: 3 ATP content and electrolyte leakage from seedling leaves. Physiol Plant 12:167–173
Nadeau P, Delaney S, Chouinard L (1987) Effects of cold hardening on the regulation of polyamines levels in wheat (Triticum aestivum L.) and alfalfa (Medicago sativa L.). Plant Physiol 84:73–77
Nayyar H, Chander S (2004) Protective effects of polyamines against oxidative stress induced by water and cold stress in chickpea. J Agron Crop Sci 190:355–365. doi:10.1111/j.1439-037X.2004.00106.x
Németh M, Janda T, Horváth E, Páldi E, Szalai G (2002) Exogenous salicylic acid increases polyamines content but may decrease drought tolerance in maize. Plant Sci 162:569–574. doi:10.1016/S0168-9452(01)00593-3
Páldi E, Szalai G, Marton CL, Pál M, Janda T (2002) Role of some N-containing compounds in chilling tolerance of maize. Acta Biol Szeged 46:99–100. In: Proceedings of the 7th Hungarian congress on plant physiology, S2-P11
Sepulveda G, Sanchez-de-Jimenez E (1988) Polyamine distribution among maize embryonic tissues and its relation to seed germination. Biochem Biophys Res Commun 153:881–887. doi:10.1016/S0006-291X(88)81178-1
Shen WY, Nada K, Tachibana S (2000) Involvement of polyamines in the chilling tolerance of cucumber cultivars. Plant Physiol 124:431–439. doi:10.1104/pp.124.1.431
Songstad DD, Duncan DR, Widholm JM (1990) Proline and polyamine involvement in chilling tolerance of maize suspension cultures. J Exp Bot 41:289–294. doi:10.1093/jxb/41.3.289
Sowiński P, Rudzińska-Langwald A, Adamczyk J, Kubica I, Fronk J (2005) Recovery of maize seedling growth, development and photosynthetic efficiency after initial growth at low temperature. J Plant Physiol 162:67–80. doi:10.1016/j.jplph.2004.03.006
Stamp P (1984) Chilling tolerance of young plants demonstrated on the example of maize (Zea mays L.). J Agron Crop Sci 7:1–83
Szalai G, Janada T, Bartók T, Páldi E (1997) Role of light in changes in free amino acid and polyamine content at chilling temperature in maize (Zea mays L.). Physiol Plant 101:434–438. doi:10.1111/j.1399-3054.1997.tb01018.x
Zheng YY, Hu J, Zhang S, Gao CH (2006) The identification of chilling-tolerance in maize inbred lines at germination and seedling growth stages. J Zhejiang Univ (Agric Life Sci) 32:41–45 (in Chinese with an English abstract)
Acknowledgements
The research was supported by National Nature Science Foundation of China (30370911). The authors are grateful to the editor and anonymous reviewers for comments that improved the presentation of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, C., Hu, J., Zhang, S. et al. Association of polyamines in governing the chilling sensitivity of maize genotypes. Plant Growth Regul 57, 31–38 (2009). https://doi.org/10.1007/s10725-008-9315-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-008-9315-2