Abstract
A floristic study of vascular flora of ancient olive groves of Apulia (Italy) was carried out from 2009 to 2011. Research was made in the fields and in the ecological infrastructures of four olive groves, located in protected areas: National Park of Gargano, Park of Dune Costiere, State and Marine Natural Reserve of Torre Guaceto and of Le Cesine. Floristic analysis was carried out by Raunkiaer and Braun-Blanquet. Biological forms and chorological types were named according to Raunkiear. Overall, 408 taxa were identified, of which 332 species, 73 subspecies and 3 varieties, belonging to 275 genera and 74 families. A small segment of 18 taxa, out of 408, was considered of conservation interest. For these taxa the topography of the collecting site, plant community, population density, degree of vulnerability and habitats were recorded, according to the Directive 92/43/EEC. Another segment of 111 taxa, out of a total of 408, was considered important for usage, which for the sake of presentation has been divided in five arbitrary categories: food crops, fodder crops, medicinal, aromatic and officinalis, crop wild relatives and edible wild species. For each of these taxa, an attempt to provide relevant information was made. Only two taxa, i.e., Muscari parviflorum and Aegilops uniaristata are common to both segments. The work was carried out within the competence of the LIFE+ CENT.OLI.MED. (LIFE07 NAT/IT/000450) project, with the aim to gather information for improving conservation and management of olive groves of Apulia, as well as of their related wild life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is widely accepted that one of the most important plants in the history and culture of the Mediterranean people is the olive tree (Olea europaea L. subsp. europaea, synonymous Olea europaea L subsp. sativa (Weston) Arcang.).
Today, olives are produced in 39 countries worldwide on an area of over 8 million hectares and is the most extensively cultivated temperate fruit crop in the world (FAO 2002). Production has increased 44 % since 1992. Olive oil production was 2.4 million of tons in 2002. Over 75 % of the world’s olive oil is produced in just 3 countries: Spain, Italy, and Greece.
In Italy, Apulia is the most important region since it provides 40 % of the italian oil production (Bartolini et al. 2005). These data show the importance of olive trees in the world, as well as in Apulia. In Italy, fossilized remains of the olive tree’s ancestor were found near Livorno, dating from twenty million years ago, although actual cultivation probably did not occur until the fifth century B.C. (Zohary 1973). Olives were first cultivated in the Eastern part of the Mediterranean and moved westwards over the millennia. Beginning in 5000 B.C., olive cultivation spread from Crete to Syria, Palestine, and Israel; commercial networking and application of new knowledge then brought it to Southern Turkey, Cyprus, and Egypt. Until 1500 B.C., Greece was the most heavily cultivated. With the expansion of the Greek colonies, olive culture reached Southern Italy and Northern Africa in the eighth century B.C. Olive trees were planted in the entire Mediterranean basin under the Roman rule (Acerbo 1937; Zohary 1973; Schäfer-Schuchardt 1988).
Ancient olive groves are among of the most important elements of the Apulian landscape, and especially evocative of the coast and hills of the Italian peninsula and of the Mediterranean basin (Perrino et al. 2012).
These Apulian habitats, in addition to their undeniable cultural and landscape values have a major environmental importance, since they can offer a shelter to many plant and animal species, some of which considered of conservation interest. In fact, the presence of many types of plant communities, such as nitrophilous and subnitrophilous communities, spontaneous grasses, field margins (Stellarietea-mediae), annual meadow (Brachypodietalia distachyi), perennial termoxerophilous grasslands (Lygeo-Stipetea), small patches of chasmophytic vegetation (Asplenietea trichomanis), nanophanerofitic and chamaephytic garigues (Cisto-Ericion), evergreen sclerophyllous scrubs (Oleo-Ceratonion) (Perrino et al. 2013), and scattered trees as Ceratonia siliqua, Ficus carica, Laurus nobilis, Prunus dulcis, and sometimes Juglans regia, Morus alba, Prunus domestica, Punica granatum, Pyrus communis, plus many species of Quercus s.l. and Sorbus domestica (Perrino et al. 2011), make the existing agro-ecosystems suitable for hosting several species of amphibians, reptiles, mammals and especially of birds. These agro-ecosystems have called the attention of scientists (Biondi et al. 2007) suggesting to include them in Annex I of the EEC Directive 92/43, as priority habitat “Centuries old olive groves with evergreen Quercus spp. and arborescent Mattoral” (code 6320).
Considering the spreading of olive trees throughout the Mediterranean countries, one can conclude that the olive agro-ecosystems of Apulia are about 2.800 years old (Zohary 1973). Enough old to deserve a study of the vascular flora that has evolved along with and that has contributed to the development of the said agro-ecosystems. So, a botanical study of the vascular flora of ancient olive groves of Apulia with the objective to analyse their biodiversity, with the main emphasis on threatened and of conservation interest, was undertaken in the frame of the above mentioned Life+ project, carrying on a previous work on the same monumental olive trees (Perrino et al. 2011).
Since in the last decades, several scientists (Hammer et al. 1992; Perrino et al. 2004) explored Apulia for collecting plant genetic resources, ancient crop varieties and their wild relatives, a special attention was also paid to plant species used by man, threatened or not by genetic erosion and/or extinction, with the aim to provide more information for improving in situ and ex situ conservation and valorization of plant biodiversity, linked with ancient olive groves.
Materials and methods
The present study was planned and carried out, from 2009 to 2011, in the frame of the LIFE+ CENT.OLI.MED. (LIFE07 NAT/IT000450) Project, for which the protection and sustainable management of biodiversity is of paramount importance for the achievement of the Millennium Development Goals (MDG).
Research work was made in four ancient olive groves, located in different areas of Apulia, where they represent typical environments of the Region.
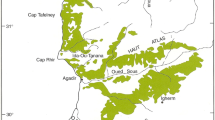
The selected ancient olive groves are located along the cost, in protected areas of Apulia (Southern Italy): National Park of Gargano (Vico del Gargano, province of Foggia), Park of Dune Costiere between Torre Canne e Torre San Leonardo (Fasano, province of Brindisi), State Natural Reserve and Marine Natural Reserve of Torre Guaceto (Carovigno, province of Brindisi), State Natural Reserve of Le Cesine (Vernole, province of Lecce) (Fig. 1). In the Park of Dune Costiere the olive trees grow on the bottom of a ephemeral river; in the site of Natural Reserve Le Cesine, olive trees, extensively farmed, is contiguous to the wetland and to a Pinus halepensis stand, whose reforestation began in the early years of the last century; in the National Park of Gargano the olive trees grow on a steep slope on the seafront; and finally, in the Natural Reserve of Torre Guaceto olive orchards grow on a flatty land. Even if the selected olive groves have common features, such as organic farming management from an ecological point of view each of them shows its own peculiarities. Each grove presents a small number of olive trees per hectare, never less than 48 plants, extensively managed. The low number of tillage does not impede natural dissemination, compatible with a continuous grass cover and the presence of man-made and/or natural infrastructures, such as hedgerows, small dry stone walls, open free areas, trees and shrubs along field edges.
Usually, at least in Apulia, the trend is that ancient olive groves are prevalently managed by organic farming or traditional agriculture, characterized by a low environmental impact, as in the case of the four ancient olive groves chosen in the present survey, while modern olive orchards are managed mainly by industrial agriculture, with a high o very high environmental impact. In the first case, wild plants have more chances to survive and this explains why genetic erosion in ancient olive groves is expected to be very low or much lower than that occurring in modern and/or industrial olive orchards.
The list of species was built up step by step, according to several surveys made for floristic analysis and for an evaluation of the level of biodiversity, before and during each planned action and sampling.
In each olive grove, floristic analysis was carried out at two levels: the field, i.e., strictly the olive orchard, and the ecological infrastructures, as above described. The analysis was worked out by three different methodologies: (A) surveys in the fields were made according to the Raunkiaer method (Cappelletti 1976), as simplified by Vazzana and Raso (1997), i.e., using a metal frame of 0.25 m and performing a number of launches, that varied (from 6 to 10) in function of the uniformity of the vegetation and the chance to detect new species; (B) samplings were performed in the ecological infrastructure according to the method of Braun-Blanquet (1932); (C) visual and oriented surveys, based on the experience of the botanist, were made to detect species that could escape with A and B methodologies. The data collected with the first two methods (A and B) will be used to calculate the Shannon–Weaver’s indices (1949) that will be published in a different paper.
Species were determined according to Pignatti (1982) and Tutin et al. (1964–80). Taxa nomenclature follows Conti et al. (2005) and subsequent integration (Conti et al. 2007), except for the genus Aegilops, Taraxacum and Thymbra capitata, for which Van Slageren (1994), Pignatti (1982) and Morales Valverde (1987), were adopted respectively. The systematics of families and their arrangement was made according to Smith et al. (2006) for the vascular cryptogams megaphylls, to Haston et al. (2007, 2009) for the Angiosperms, and to the criteria proposed by Angiosperm Phylogeny Group (Stevens 2008; APG III 2009) for the boundaries. The biological forms and the chorology were named according to Raunkiear (1934) and their acronyms are reported in Table 1. Taxa are listed in alphabetical order and grouped in families according to Pignatti (1982). Geographic position (U.T.M.—WGS84), site name, distribution data, motivation of conservation interest, general information on plant communities, and the relationships with the habitat of Directive 92/43 EEC (European Commission Dg Environment 2007; Biondi and Blasi 2009) are reported only for taxa of conservation interest (see the ad hoc paragraph: Analysis of taxa of conservation interest). For these species, different acronyms indicating the reasons of conservation interest were used. They are: CR: critically endangered; EN: endangered; VU: vulnerable; LR: lower risk; NT: near threatened; I: endemic; Ad: amphiadriatic, PI: phytogeographic interest; B: International Convention of Berne, 1979; CI: Convention on International Trade in Endangered Species (Cites 1973); DH: Habitat Directive 92/43 EEC; r: rare; (*) common to all of the surveyed olive groves. The threatened categories of species follow Conti et al. (1997), and only for Aegilops uniaristata and Asyneuma limonifolium subsp. limonifolium, the categories follow Perrino and Wagensommer (2012) and Perrino et al. (2012) respectively.
As far as plant species used by man is concerned, different acronyms were used, according to the category they have been assigned: CRO for food crops; FOD for fodder crops; MAP for medicinal and aromatic plants; CWR for crop wild relatives and EWS for edible wild species (see Floristic list of Appendix). In practice, since these plants may have more than one use, the criterion to assign a plant to one category, instead of another, was that of considering its most frequent and/or important use. For plants used by man the most cited sources of information have been Hammer et al. (1992, 1999), Perrino et al. (2004) and Zeven and Zhukovsky (1975).
Results
Acronymes of biological forms and chorological types are listed in Table 1. Percentages of species per family (Table 2) and per chorological spectrum (Table 3) were calculated dividing the number of the detected species by the total number of species found in all olive groves. Since the figures indicating the number of species per family or per chorotype do not express number of plants, but simple presence or absence, they are not suitable for any statistical analysis. That is why similarities or differences among olive groves, between fields and infrastructures are presented and discussed only on the base of presence/absence character. Data such as the number of individuals per sampling/per olive grove, per level and per year, will be performed in a next paper, where the approach will be different from a floristic one.
In whole, 408 taxa were recorded, including 332 species, 73 subspecies and 3 varieties, belonging to 275 genera and 74 families of vascular flora (Table 2). The three most represented families are: Asteraceae (12.7 %) with 52 taxa, followed by Poaceae (11.3 %) and Fabaceae (11.3 %), both with 46 taxa. The other families, each with 20 taxa or less, include the remaining 264 taxonomical entities.
The biological spectrum of life forms is presented separately for the fields and ecological infrastructures (Fig. 2), only for the fields (Fig. 3) and only for the ecological infrastructures (Fig. 4). That of the fields and infrastructures shows that the therophytes (T) are the most abundant (45.1 %), followed by hemicryptophytes (H), with a significative presence (26.7 %), and geophytes (G) and phanerophytes (P), with lower percentages (10.3 and 9.8 % respectively). The remaining life forms, namely chamaephytes (Ch) and nanophanerophytes (NP), are much less present (4.9 and 3.2 % respectively).
The biological spectrum of life forms in the fields (Fig. 3) shows a high presence of therophytes (59.2 %), i.e., of annual forms, taking an advantage on other life forms, i.e., of perennial ones, especially of chamaephytes and phanerophytes, which occur with very low presence (1.2 and 5.2 % respectively).
The biological spectrum in the ecological infrastructures (Fig. 4) shows to be very similar to that of the merged fields and ecological infrastructures, except for a slight increases in perennial forms (56.0 %) and decrease of the annual ones, i.e., only of the therophytes (44.0 %).
The chorological spectrum is presented in three distinct data sets: merged and separately fields and ecological infrastructures (Table 3). It shows that the presence of the Mediterranean types in the fields (59.7 %) is slightly different from that in the ecological infrastructure (61.9 %). In particular, the Stenomediterranean types in the ecological infrastructure are slightly prevalent (23.1 %) to those in the fields (20.1 %), while the Eurimediterranean types in the fields are slightly prevalent (21.8 %) to those in the ecological infrastructures (19.6 %). In any case, both types show percentages always higher than 19.0 %, which means that, on an average, Stenomediterranean (21.8 %) and Eurimediterranean types (20.1 %) represent each about 1/3 of all of the Medietrranean types (61.5 %).
Taking into consideration the other chorotypes, the most prevalent species are those with widely distribution, as shown by the percentage corresponding to fields and ecological infrastructures (14.6 %), a percentage that is higher in the fields (21.0 %) than in the ecological infrastructures (14.2 %). Other chorotypes less present but still worth of consideration in the fields and in the ecological infrastructures are the Paleotemperate (4.7 %) and the Eurasiatic (4.5 %) ones, whose percentages change very little or nothing when looking only at the fields (4.8 and 4.4 % respectively) or only at the ecological infrastructures (4.6 and 4.3 % respectively). The other 8 classified chorotypes (Pontic, Eurosiberian, European s.l., Amphiadriatic, Endemic, Paleo-Subtropical, Subtropical and Neotropical nat.) show percentages variable from 0.0 to 0.4 % in the fields, occurring for Pontic and Neotropical nat. respectively, to a maximum of 4.0 % in ecological infrastructures (as for European s.l.). Individually Pontic, Eurosiberian, European s.l., Amphiadriatic, Endemic, Paleo-Subtropical, Subtropical and Neotropical nat. are present with a very low percentage, but altogether as a group with a value of 19.4 %, they are more present they are more present (19.4 %) than the species with widely distribution, that reach the maximum value of 14.6 %, both in the fields and in the ecological infrastructures. The same group is more present in the ecological infrastructure (19.6 %) than in the fields (16.2 %). The species with widely distribution are less present in the ecological infrastructure (14.2 %) than in the field (21.0 %); On the contrary, other geoelements, especially European entities, range from 1.7 % in the fields to 4.0 % in the ecological infrastructure. The chorotypes reported as “others”, appear to be slightly more present in the ecological infrastructures (4.3 %) than in the fields (3.1 %).
The results show also the presence of 18 critical taxa, of which three are at risk, four are endemic and the others are rare or important for conservation interest, especially at regional and/or national level (Floristic list of Appendix).
Finally, 111 species, out of 408, were recognized as plants used by man (Floristic list of Appendix). According to the most prevalent use they have been assembled in five categories: 29 food crops; 29 fodder crops; 26 medicinal and aromatic plants; 17 crop wild relatives and 10 edible wild species comestibles.
As far as crop wild relatives is concerned, it is important to point out that they must be understood as wild relatives in a very broad sense, at various levels of the well known gene pools described by Harlan and de Wet (1971). More precisely, for some of them, whether there is or not gene exchange between cultivated and wild relatives, it will be the matter of a different study. More details about this point are reported in the paragraph of crop wild relatives.
Discussion
The Poaceae and Fabaceae are slightly more abundant in the field (25.3 %) than in the ecological infrastructures (22.8 %) (Table 2). This relatively small difference (2.5 %) between the two surveyed areas, may be explained by assuming that the flora of ecological infrastructures may be influenced more by the flora growing in the natural neighboring habitats.
Only 27 taxa, 6.6 % of the entire flora, are common to the four olive groves and 59.3 % of them are therophytes. The analysis shows that therophytes (annual species), with a presence of 45.1 %, are significantly dominant within the group of life forms (Fig. 2). This dominance was expected since annual species are prevalent in bioclimatic regions, characterized by hot and dry periods with a very short growing seasons and since therophytes are more competitive than other biological forms in habitats prone to human-induced changes (Raunkiaer 1934).
The comparison between fields (Fig. 3) and ecological infrastructures (Fig. 4) shows that the therophytes are dominant on other biological forms, both in the field (59.2 %) and in the ecological infrastructures (44.0 %), where therophyte have a significant drop-down of 15.2 % (59.2–44.0) in favour of the perennial forms (H, P, G, Ch, NP). In other words, in seminatural environments, i.e., in ecological infrastructures, perennial species take a competitive advantage over some species of therophytes, and altogether increase from 40.8 % (24.5 + 8.2 + 5.2 + 1.7 + 1.2) to 56.0 % (26.8 + 10.3 + 10.3 + 5.1 + 3.5).
With a future perspective to make comparison among olive groves of different areas of the Mediterranean Basin (Southern Italy, Sardinia, Sicily, the Mediterranean coasts of Spain, Southern Balkan Peninsula, Balearic Islands, Crete and Cyprus), it has been suggested to provide a key to simplify the geographical distribution of 32 Mediterranean geoelement, discovered in the following 9 chorological types: Medit.-Mountain, Medit.-Macaronesian range, Medit.-Southern range, Medit.-Northern range, Medit.-Eastern range, Medit.-Western range, Medit.-Atlantic range, Eurimediterranean and Stenomediterranean (Table 4). In particular, the Stenomediterranean types are the Mediterranean types in the strict sense, whose areal does not go over the northern limit of diffusion of the olive trees, while the Eurimediterranean ones are included within the area of grapevine (Vitis vinifera) and therefore they extend to the southern part of Central Europe (Ubaldi 2003). The Mediterranean-Atlantic are Eurimediterranean types, generally mountane ones, whose areal also includes the Atlantic Europe regions. It is possible to distinguish the “Circumediterranean” (distributed around the Mediterranean) types, that can be Steno- or Eurimediterranean ones, and may have predominantly southern distribution (Medit.-Southern), northern distribution (Medit.-Northern), eastern distribution (Medit.-Eastern) or western distribution (Medit.-Western).
The chorological spectrum (Table 3) shows that the Mediterranean stock (see sub-total) is well represented (61.5 %) and that it is notably higher than the one known for Apulian flora (52.0 %) (Marchiori et al. 2000). So, these olive orchards are Mediterranean ecosystems, a character that becomes weaker in the fields, where species with a widely distribution take an advantage, confirming the results of the biological analysis.
The percentages of the Eastern Mediterranean (6.5 %) and Pontic types (0.5 %) for the merged fields and ecological infrastructures, may be seen as a similarity or as a strong correlation with the flora of eastern geographical areas and, in particular, with the Eastern Mediterranean, primary center of origin of olive trees (Acerbo 1937; Zohary 1973; Schäfer-Schuchardt 1988).
Interestingly, a high number of species of conservation interest, 18 out of 408, some seriously endangered, was detected in highly anthropized environments, as it is presented and discussed in the following paragraph.
Analysis of taxa of conservation interest
Aegilops uniaristata [Ad, VU]. GPS: N4641298, E578448; Place: Fasano (Brindisi); Plant community: annual meadow (Brachypodietalia distachyi Rivas-Martínez 1978); altitude: 29 m a.s.; Habitat 92/43/EEC: 6220* (subtype 3) “Pseudo-steppe with grasses and annuals of the Thero-Brachypodietea” (San Miguel 2008). Eastern Mediterranean distribution, known in Croatia, Greece (including the islands), Albania and Italy, while it is doubtful in Turkey (Van Slageren 1994). In Italy it was considered exclusive of Apulia (Groves 1887; Pignatti 1982; Bianco et al. 1989; Marchiori et al. 1993; Caforio and Marchiori 2006; Perrino 2011). Currently it is known for Basilicata Region, while it is doubtful for Calabria (Conti et al. 2005). It is listed as vulnerable (VU) (Perrino and Wagensommer 2012) and as at risk of extinction in the Atlas of Species (Scoppola and Spampinato 2005). The Fasano population counts only three individuals. It seems that the edge of an olive tree grove is one of its favourite habitats (Van Slageren 1994; Perrino 2011; Perrino et al. 2011).
Asyneuma limonifolium subsp. limonifolium [Ad, PI, NT]. GPS: N4641293, E578442; Place: Fasano (Brindisi); Plant community: garrigues; altitude: 31 m a.s.; Habitat 92/43/EEC: not identified. Paleoegeic species of phytogeographic interest, present on both coasts of the Adriatic Sea (Francini Corti 1966). Its distribution includes the north-eastern Mediterranean area (Greuter et al. 1984; Castroviejo et al. 2010). In Italy, it is known in Apulia and eastern Basilicata. The station of Fasano, after those of Monopoli (Bianco and Sarfatti 1961; Cavallaro et al. 2007; Perrino and Signorile 2009) and Polignano a Mare (Perrino and Signorile 2010; Vita and Forte 1990), is the western limit of its distribution. Some authors (Brullo et al. 1994), on the basis of specimens collected in Punta Palascia (Otranto—Lecce), have shown the existence of a karyological corrispondence of these populations with those of Greece and Turkey. The observed population consists of a few individuals. In Apulia, the taxon presents a Near Threatened risk (Perrino et al. 2012).
Barlia robertiana [CI]. GPS: N4641171, E578340; Place: Vico del Gargano (Foggia); Plant community: uncultivated community (Stellarietea mediae R. Tüxen, Lohmeyer et Preising ex Rochow 1951); altitude: 214 m a.s.; Habitat 92/43/EEC: not identified. In Italy, this big orchid is known in the southern territories, while it is lacking in some central and northern regions. Both variants, with purple to greenish shades and whitish tepals, the latter being more rare, were observed.
Crepis brulla [I]. GPS: N4641265, E578408; Place: Vico del Gargano (Foggia); Plant community: uncultivated community (Stellarietea mediae R. Tüxen, Lohmeyer et Preising ex Rochow 1951); altitude: 200 m a.s.; Habitat 92/43/EEC: not identified. Small endemic species exclusive to the southern Italy (Apulia, Basilicata and Calabria) (Conti et al. 2005). Found in several types of vegetation, but always with a few individuals.
Crepis corymbosa [I]. GPS: N4519987, E710627; Place: Fasano (Brindisi); Plant community: annual meadow (Brachypodietalia distachyi Rivas-Martínez 1978); altitude: 34 m a.s.; Habitat 92/43/EEC: 6220* (subtype 3) “Pseudo-steppe with grasses and annuals of the Thero-Brachypodietea” (San Miguel 2008). It is endemic in central and southern Italy, Ionian Islands, Corfu and Kefalonia (Pignatti 1982). It is observed within the annual meadow and, more rarely, within the scrubland communities. This species lacking the fruit (achene) can be easily confused with other species of the same genus.
Cyclamen hederifolium [CI]. GPS: N4641079, E578410; Place: Vico del Gargano (Foggia); Plant community: uncultivated community (Stellarietea mediae R. Tüxen, Lohmeyer et Preising ex Rochow 1951); altitude: 224 m a.s.; Habitat 92/43/EEC: not identified. A species common in all of the Italian Regions, included in the CITES list. Few observed individuals coming from near woods.
Epilobium parviflorum [r]. GPS: N4473077, E782136; Place: Le Cesine (Vernole—Lecce); Plant community: humid grasslands; altitude: 6 m a.s.; Habitat 92/43/EEC: 6420 Mediterranean tall humid herb grasslands of the Molinio-Holoschoenion. It is a wide distribution (Paleotemperate) taxon, rare in Italy. It grows in muddy wet habitat, such as Le Cesine olive grove, where it grows on the vegetation marge, between the wetland and the field.
Erica forskalii [VU]. GPS: N4472988, E782234; Place: Le Cesine (Vernole—Lecce); Plant community: garrigue; altitude: 7 m a.s.; Habitat 92/43/EEC: not identified. Mediterranean eastern species, known in Italy, ex Yugoslavia, Albania, Bulgaria, Greece, Crete, Eastern Aegean Islands, Turkey, Cyprus, Lebanon, Syria, Israel and Jordan (Greuter et al. 1986). In Italy, it is known only in Southern Apulia (Brullo et al. 1986; Conti et al. 2005). Observed in the scrub vegetation, bordering the olive grove.
Gagea granatellii [VU]. GPS: N4520081, E710649; Place: Fasano (Brindisi); Plant community: annual meadow (Brachypodietalia distachyi Rivas-Martínez 1978); altitude: 31 m a.s.; Habitat 92/43/EEC: 6220* (subtype 3) “Pseudo-steppe with grasses and annuals of the Thero-Brachypodietea” (San Miguel 2008). Endemic taxon of western Mediterranean area (Tison 1998). In Italy, it has been found in central and southern regions (Conti et al. 2005, 2007). Its included in the Regional Red List of Apulia and Sicily, with the status of vulnerable (VU), while in Abruzzo, Marche, Molise and Basilicata it is at lower risk (LR) (Conti et al. 1997). Few individuals were found in an annual meadow.
Gagea mauritanica [CR]. GPS: N4520081, E710695; Place: Fasano (Brindisi); Plant community: annual meadow (Brachypodietalia distachyi Rivas-Martínez 1978); altitude: 32 m a.s.; Habitat 92/43/EEC: 6220* (subtype 3) “Pseudo-steppe with grasses and annuals of the Thero-Brachypodietea” (San Miguel 2008). This species is endemic of western Mediterranean (Peruzzi and Tison 2007). In Italy is documented only to Apulia and Sicily Regions (Peruzzi et al. 2009). Only two individuals seen in a vegetation like that of G. Granatelli.
Helianthemum jonium [I]. GPS: N4520146, E710764; Place: Fasano (Brindisi); Plant community: garrigue (Helianthemum jonii-Fumanetum thymifoliae Taffetani et Biondi 1992); altitude: 30 m a.s.; Habitat 92/43/EEC: not identified. Endemic species to Morocco (Ruiz De La Torre 1956) and Central and Southern Italy (Emilia Romagna, Apulia, Basilicata and Molise) (Conti et al. 2005). The population grows into Thymbra capitata (L.) Cav. garrigue. In some cases, isolated individuals are found in scrub vegetation.
Muscari parviflorum [PI, r]. GPS: N4509660, E645633; Place: Torre Guaceto (Carovigno—Brindisi); Plant community: uncultivated community (Stellarietea mediae R. Tüxen, Lohmeyer et Preising ex Rochow 1951); altitude: 27 m a.s.; Habitat 92/43/EEC: not identified. Species of phytogeographical interest, with central and eastern Mediterranean distribution, extended from Spain and northern Africa to western Asia (Tutin et al. 1980). It is rare in many regions of Italy (Conti et al. 2005). The station of Torre Guaceto is the first found for the province of Brindisi, the second in Apulia after that of Salento (Mele et al. 2001). The population of the Torre Guaceto olive grove counts about 100 individuals, but in the territory may be larger.
Ophrys incubacea [CI]. GPS: N4520177, E710787; Place: Fasano (Brindisi); Plant community: uncultivated community (Stellarietea mediae R. Tüxen, Lohmeyer et Preising ex Rochow 1951); altitude: 28 m a.s.; Habitat 92/43/EEC: not identified. It is a relatively common species in grasslands and uncultivated communities, but rare in olive groves.
Orchis palustris Jacq. [CI, EN]. GPS: N4473078, E782160; Place: Le Cesine (Vernole—Lecce); Plant community: humid grasslands; altitude: 6 m a.s.; Habitat 92/43/EEC: 6420 Mediterranean tall humid herb grasslands of the Molinio-Holoschoenion. Euroasiatic species (if it includes O. elegans) or eurimediterranean (if it includes O. palustris s.s.) (Alessandrini and Medagli 2008), which, in Italy, shows a scattered distribution (Conti et al. 2005). The population shows few individuals, that grow in the same habitat of Epilobium parviflorum.
Orchis purpurea [CI]. GPS: N4641017, E578387; Place: Vico del Gargano (Foggia); Plant community: uncultivated community (Stellarietea mediae R. Tüxen, Lohmeyer et Preising ex Rochow 1951) and scrub vegetation (Oleo-Ceratonion siliquae Br.-Bl. 1936 em. Rivas Martínez 1975); altitude: 240 m a.s.; Habitat 92/43/EEC: not identified. Eurasiatic species, found in Europe and in Turkey, unique for its size it may reach a meter of height. Rare in Apulia, it was observed at the margins of the olive groves, road banks and forest oak (Del Fuoco 2003). The flower looks like an old country-side woman (with a hat and wide skirt) and is called “Lady Orchid”.
Satureja cuneifolia [PI]. GPS: N4520145, E710764; Place: Fasano (Brindisi); Plant community: garrigue (Cisto-Ericion Horvatić 1958); altitude: 29 m a.s.; Habitat 92/43/EEC: not identified. Amphiadriatic (Greuter et al. 1986), in Italy found only in Apulia and Basilicata Regions (Conti et al. 2005). The observed population is well preserved.
Scrophularia lucida [Ad, PI]. GPS: N4520019, E710650; Place: Fasano (Brindisi); Plant community: rocky slopes (Campanulion versicoloris Quezel 1964); altitude: 34 m a.s.; Habitat 92/43/EEC: 8210 “Calcareous rocky slopes with chasmophytic vegetation”. It is a casmophytes of amphiadriatic and phytogeographic interest; known in France, Italy, Greece and the Aegean Islands (Crete and Karpathos). In Italy, it is known only in Apulia and Basilicata, while it is uncertain in Piemonte (Conti et al. 2005). It was observed on the drystone walls bordering the valley of the field.
Stipa austroitalica subsp. austroitalica [I, B, DH]. GPS: N4520082, E710696; Place: Fasano (Brindisi); Plant community: perennial grasslands; altitude: 32 m a.s.; Habitat 92/43/EEC: not identified. Endemic species of high conservation interest. The subsp. austroitalica, one of the four subspecies (Moraldo and Ricceri 2003), was found with few isolated individuals.
Analysis of plant species used by man
As previously stated, 111 plant species, out of 408, were recognized as plants that are somewhat used by man (Floristic list of Appendix). For a rational presentation, the 111 species were grouped in five categories: food crops; fodder crops; medicinal and aromatic plants; crop wild relatives; edible wild species. Most of the species should be included in more than one category, simple because they have more than one use, but for practical reasons it was decided to include them only in one category, the one considered the most appropriate. In any case, when a species was recognized important for more than one use, it was pointed out. Only two, out of 111species, i.e., Muscari parviflorum and Aegilops uniaristata, are also mentioned among the group of the 18 species of conservation interest (Floristic list of Appendix). The rest of the species are not threatened by genetic erosion and/or extinction or at least not all of them are threatened in the same way, but since they represent important plants used by man it was decided to point out some of their peculiarities with the aim to emphasize their value and enhance their conservation and valorization. For this purpose, references such as Zeven and Zhukovsky (1975), Hammer et al. (1992, 1999), Perrino et al. (2004) have been the main sources of information.
Food crops
This category counts 29 species and they are so well known that there is no need to present and discuss their uses. However, some of them, such as Avena sativa, Ficus carica, Morus alba, Juglans regia, Citrus aurantium, C. limon, C. sinensis, Capparis spinosa, Opuntia ficus-indica, Foeniculum vulgare, Origanum vulgare subsp. viridulum, Rosmarinus officinalis, Prunus avium subsp. avium, Prunus dulcis, Allium ampeloprasum, Capparis spinosa, Raphanus sativus, Pistacia lentiscus, Pistacia terebinthus subsp. terebinthus, are almost widely cultivated. Whereas, others, such as Amaranthus retroflexus, Muscari comosum, Ceratonia siliqua, Mespilus germanica, Rubus canescens, Rubus ulmifolius, Morus alba, Myrtus communis subsp. communis, Diplotaxis erucoides subsp. erucoides, D. tenuifolia, and Borago officinalis (Floristic list of Appendix) are minor o neglected crops. Often, among world wide crops, as in the case of Citrus crops, one can find local varities, like Citrus limon “Femminello”, C. sinensis “Biondo commune del Gargano” and C. sinensis “Duretta del Gargano”, that are unique in the world and cultivated only on Gargano, which makes them vulnerable, suggesting actions for strengthening their in situ/on farm conservation and valorization. This promiscuity of plant species in the ecosystem of ancient olive groves not only increases biodiversity, including that of relevant pollinators, but it also creates the environmental conditions that enhance disease resistance (Keesing et al. 2010).
Fodder crops
This category is represented by 29 species. Some of them are important fodders also or mainly on a global scale. A part from Sorghum halepense, Piptatherum miliaceum subsp. miliaceum and Piptatherum miliaceum subsp. thomasii, that are Poaceae, all of the other genera, from Lupinus, Melilotus, Sulla, Tetragonolobus, and Trigonella, each present with one species, to Lathyrus with 3 species, Medicago and Vicia with 5 species each and Trifolium with 8 species, are Fabaceae. Once, some of these species were though to be progenitors of cultivated plants, as it is the case of Sorghum halepense that gave rise to a new perennial diploid: S. almum (Zeven and Zhukovsky 1975). Previously, the two species of Piptatherum, above mentioned, were included in Oryzopsis (similar to rice), but further research moved them into Piptatherum, a genus with 30 species (Romaschenko et al. 2011). P. miliaceum (syn. Oryzopsis miliacea subsp. miliacea ) is a Eurasian taxon, now established in several parts of the world. In its native range it grows, often as a common species, primarily in disturbed areas, wadis, and oases, penetrating into the semidesert regions of northern Africa and western Asia. It is used as a fodder in northern Africa (this explains why it was placed in this category). In Maryland (USA), it was found even on a ballast dump (Barkworth 2007) and considered to be a good plant for phytoremediation (García et al. 2004), from soil depollution from heavy metals. Among Fabaceae, the following facts are worth of quoting. Melilotus sulcatus must be mentioned for its coumarine deficiency and resistance to drought. Sulla capitata, has a high capacity of colonizing new soils (gullies, ravines), but also as a medicinal plant. Tetragonolobus purpureus, is cultivated for its edible green seed pods, but also as a fodder (Hammer et al. 1999; Perrino et al. 2004). Trigonella monspeliaca (syn. Medicago monspeliaca), is largely cultivated as a forage in many areas of the world. The three species of Lathyrus (L. cicera, L. ochrus and L. sylvestris subsp. sylvestris) are used as food and feed and in the case of L. sylvestris also as an ornamental plant. None of the five Medicago species are present in the list of the 30 species cited by Zeven and Zhukovsky (1975). Medicago truncatula, due to its very small genome and fast rate of growth, is used as a model for studying the biology of Fabaceae. Vicia sativa, V. sativa subsp. macrocarpa and V. villosa, three of the five species found in the olive groves, are present in the list of the 18 species cited by Zeven and Zhukovsky (1975). V. sativa and V. villosa are also cited by Hammer et al. (1999) and Perrino et al. (2004). V. angustifolia, the wild relative of V. sativa (Mettin and Hanelt 1964; Hanelt and Mettin 1989) was not found in the olive groves. Both V. hybrida and V. lutea, two of the five species found in the olive groves, are not mentioned by Zeven and Zhukovsky (1975). Useful information on their relationships with other species of the genus Vicia are reported also by Maxted (2008). Only two species, Trifolium pratense and Trifolium resupinatum, out of eight species of Trifolium found in the olive groves, are present in the list of the 29 species cited by Zeven and Zhukovsky (1975) and of the nine species cited by Hammer et al. (1999) and Perrino et al. (2004). T. pratense (red clover) is wide spread in Europe, West and Central Asia and North Africa. It was probably first cultivated in the Netherlands, in the beginning of the sixteenth Century (Zeven and Zhukovsky 1975). The wild type has more leaves and new shoots emerge from internode at the butt end, while the cultivated type has less leaves and new shoots emerge from the leaf rosette. The variable wild type is described as var. pratense and the cultivated one as var. sativum (syn. T. sativum) (Zeven and Zhukovsky 1975). The specimens found in the olive groves are closer to the wild type, but they are still under observation. T. resupinatum (syn. T. suaveolens), known as Persian clover, is widespread from the Mediterranean region to Iran, Afghanistan and India (Zeven and Zhukovsky 1975), and it is the most popular among new Australian crops (Australian new crops 2013). Trifolium squarrosum, native of the area and only recently domesticated (Hammer et al. 1990; Perrino et al. 2004) is a threatened species in Bulgaria (Boller et al. 2003). This, as well as other legumes, is often used also as a cover crop or intercrop. From an agricultural point of view, the presence in the olive groves of so many species of legumes ensures the sourcing of organic nitrogen, improved fertility and soil structure, along with improved oil quality.
The Mediterranean basin is the center of origin and/or of diversification of several forage species, but it seems that not all the species have been adequately investigated. The project of olive groves may offer the possibility to develop studies aimed to identify new potential fodders in collaboration with experts in animal feeding.
Medicinal and aromatic plants
Another interesting category o plants, consisting of 26 species, is that of medicinal and aromatic plants. Most of them are well known only by local and traditional people, but almost neglected by the majority of people. A few and selected items and/or specialities for each taxon are reported. Ceterach officinarum is used for making infusions as a diuretic, astringent and enlargement of spleen (Chiej 1984). Pinus halepensis (Aleppo pine), is the most widely distributed pine of the Mediterranean Basin; the resin is used to flavor the Greek wine and for the wicks; the dry powder of the bark is used for diaper rash and to solve respiratory problems (González-Tejero et al. 2008); the essential oil possesses antifungal activity against Aspergillus flavus, A. niger, Fusarium oxysporum, Rhizopus stolonifer and thus can be used as a natural treatment for fungal infections, as well as natural preservative in food (Abi-Ayad et al. 2011); in the Maltese Archipelago is one of several medicinal plants used for inhalation and ointment for catarrh and as diuretic (Attard and Pacioni 2012). Laurus nobilis is used as a digestive, antiseptic, balsamic, carminative, and antitussive; fluid extracts and essence are also used as an infusion and tincture (Chiej 1984). Gladiolus italicus: the decoction of roots and flowers of this plant is used for heart and lung infections (Mosaddegh et al. 2012), while leaves and bulbs are used as galactogogue, aphrodisiac and emmenagogue (Penza 1969). Rosa sempervirens, is a good source of secondary metabolites with high potential antioxidant properties and for producing jam (Ghazghazi et al. 2012). Paliurus spina-christi: decoction from fruits is used internally for heart diseases, diabetes, kidney stones and abdominal pain (Tuzlaci et al. 2010); decoction from fruits and roots is used for headache, bronchitis, urethra inflammation, toothache, as stomachic, for rheumatism, hemorrhoid, kidney ailments, as tonic and antitussive (Koçyiğit and Özhatay 2006); poultices from leaves are used to cure wounds and furuncles (Ugulu 2011); fruits gathered from wild plants in Turkey are exported to USA (Report 2003). Urtica dioica subsp. dioica (Stinging nettle), is a perennial plant that has antioxidant properties and thanks to the stinging hairs is rarely eaten by herbivores, so it provides long-term shelter for insects, such as aphids or caterpillars of many butterflies and moths; insects, in turn, provide food for small birds (Mavi et al. 2004; WHO 2004). Urtica urens (dwarf nettle) is an annual plant, whose seeds are anthelmintic, purgative and anti enuretic; extracts of the plant are the basis of many hair lotions with marked action antiseborroic and slightly revulsive on the scalp; used also for psoriasis, vaginal discharge and for draining the liver; it is also cardiotonic, diuretic, tonic astringent, vasoconstrictor, hemostatic and anti-anemic, though these properties are not always confirmed by the European Medicines Agency (2007). The mating system of both species of Urtica have been investigated, (Lahav-Ginott and Cronk 1993; Woodland et al. 1982) and it seems that natural hybridization may occur between them. Hypericum perforatum is antidepressant, antiviral, and antibacterial; pharmacological activities, including photosensitive reactions, may be due to hypericin and flavonoid constituents (Barnes et al. 2001). Ruta chalepensis is a good herbal remedy for a number of ailments, such as fever and inflammation and even as a digestive (Al-Said et al. 1990). Malva sylvestris subsp. sylvestris, is used against irritations of the mucosa of the mouth and throat, irritative cough, ulcers, and also as a vegetable. Chenopodium hybridum is edible, but with causion since leaves and seeds contain saponins, which are toxic; it is used also as hemostatic, against irregular menstruation, uterine bleeding, hematemesis, epistaxis, hemoptysis, hematuria (China Wikipedia 2013). Buglossoides purpureocaerulea is used for its antioxidant activity and in Southern Italy is appreciated for its beneficial effects on liver diseases (Negro et al. 2013). Solanum nigrum, has fruits that possess a potential CNS-depressant action (Perez et al. 1998); the plant is a cadmium hyperaccumulator, suggesting to be good for phytoremediation and to be a good indicator of fields contaminated by heavy metals (Wei et al. 2005); it is a cosmopolite taxon, underutilized and neglected crop (Edmonds and Chweya 1997); it is one of the 90 species cited by Zeven and Zhukovsky (1975). Calamintha nepeta is recognized for its aromatic, diaphoretic, expectorant, febrifuge and stomachic properties (PFAF 2012). Marrubium vulgare possesses tonic, aromatic, stimulant, expectorant, diaphoretic and diuretic properties. It is helpful for bronchial asthma and non-productive cough. It was formerly much esteemed in various uterine, visceral and hepatic affections and in phthisis (Chopra et al. 1956). The plant possess hypoglycemic (Roman et al. 1992), vasorelaxant (El-Bardai et al. 2003), antihypertensive (El-Bardai et al. 2004), analgesic (De Souza et al. 1998), anti-inflammatory (Sahpaz et al. 2002), antioxidant (Weel et al. 1999) and antioedematogenic activity (Stulzer et al. 2006), and many other biological activities. The genus Salvia with over 900 species is not monophyletic, which means that all members have evolved from different ancestors (Walker et al. 2004). Salvia verbenaca, found in the olive groves, is a hexaploid (2n = 6x = 60) and is used as a medicinal plant, but it was much more used in Roman time (Hammer et al. 1999; Perrino et al. 2004). Satureja montana is known for its antibacterial, antioxidant, antiseptic, astringent, good sore throats and carminative properties, and is recommend for gas and digestive upsets, including colic, diarrhea and indigestion (Bezić et al. 2005). Thymbra capitata is cited for its antilisterial activity (Faleiro et al. 2005). Acanthus spinosus as a medicinal plant may be used against bleeding and diarrhea (Caramia 2005); a phytochemical investigation revealed the presence of useful chemicals in the leaves (Loukis and Philianos 1980); it is used also as an ornamental plant and in the garden with its allelochemicals may control weeds (Putnam 1988); in addition, A. spinosus is in the red data book of Bulgaria (Dimitrova 2011), where, according to the national and international Nature conservation register, the taxon is in the list of those species with a nature preservation status, as reported on Biological Diversity Law (BDL), promulgated in Official Gazette, issue 77/2002 (Radanova 2009). Achillea millefolium is well known for its antioxidant activity and folklore remedies (Candan et al. 2003; Perrino et al. 2004). Calendula officinalis is a medicinal and ornamental plant (Hammer et al. 1999; Perrino et al. 2004). Calendula arvensis is also used as a medicinal plant. Eupatorium cannabinum is a toxic plant, but in very small quantities may be antitumoral, diaphoretic, febrifugal, laxative, tonic, diuretic, depurative and cholagogue (PFAF 2012; Arvis et al. 1990). Matricaria chamomilla has sedative and spasmolytic effects and acaricidal activity (Avallone et al. 2000; Macchioni et al. 2004). Sambucus nigra is used as condiment, aromatic, food, cosmetic, pharmacological and ornamental plant (Hammer et al. 1999).
Since the Mediterranean basin is the center of origin and/or of diversification of several medicinal, aromatic and officinal plants, it is probable that in the future, the development of the project may consider the possibility to investigate this group of plant species in more details in collaboration with national and international laboratories, specialized in biochemistry, pharmacopy, phytotherapy and ethnobotany.
Crop wild relatives
With 17 species is the most intrigued category. Not all of the 17 species are wild relatives in the strict sense, i.e., that they exchange genes with their putative cultivated patterns. Even so, they have been placed in this category because of their very close taxonomical and systematic position, in addition to their similar way of use, as a food, feed, medicinal, etc. Some of them could be placed in the tertiary and/or secondary gene pool, while others, for which gene flow is certain, can be included in the primary gene pool (Harlan and de Wet 1971). An example of taxa for which we have no knowledges about geneflow is that of the 3 wild species of Muscari (M. commutatum, M. neglectum and M. parviflorum) that grow along with the edible and somewhat often cultivated Muscari comosum (L.) Mill. (see crop category). The genus Muscari consists of 35–55 bulbous species, grouped into four subgenera, within a distribution area, extending from the Macaronesian region to the Caucasus, although being mainly found in the Mediterranean basin. These information would suggest taxonomical and genetic investigation to provide further contributions to the so complicated evolution of this group of polyploid species, with different ploidy level, even within the same species (Suárez-Santiago et al. 2007). Analogous considerations may be developed for the 2 species of Aegilops (A. ovata and A.uniaristata), that can somewhat be placed in one of the gene pools of Triticum sp., and the same can be said for Dasypyrum villosum. Avena barbata (2n = 28) is not the wild ancestor of Avena sativa (2n = 42), also because they have a different genome formula (AsAs and AACCBB respectively), but since the genetic origin of A. sativa is not yet fully understood (Zeven and Zhukovsky 1975) and since the two species share the A genome, it would not be so mistaken to consider A. barbata as a species close to A. sativa, also because they have been sharing, for centuries, if not millennia, exactly the same environment. None of the 2 species of Hordeum (H. murinum L. and H. murinum subsp. leporinum), found in the ancient olive groves, have something to do with H. spontaneum (2n = 14), which is thought to be the wild parent of H. vulgare (2n = 14), though one has to say that the origin of the crop is still under discussion (Svitashev et al. 1994). Pyrus spinosa though not present among the many species (more than 30) cited by Zeven and Zhukovsky (1975), along with other wild species, such as P. nivalis (Heywood and Zohary 1995) and P. syriaca is interfertile with the cultivated P. communis (Hammer et al. 1999; Perrino et al. 2004). Linum bienne is a possible wild forebear of the cultivated Linum usitatissimum; the latin name usitatissimum means most useful, pointing to the several traditional uses of the plant and their importance for human (Zhukovsky 1964; Brezhnev and Korovina 1981; Hammer et al. 1999; Perrino et al. 2004). Raphanus raphanistrum L. is a wild leaf vegetable that may have contributed to increase the genetic diversity of Raphanus sativus landraces via introgression (Hammer et al. 1999; Perrino et al. 2004); the primary centre of origin of Raphanus sativus, with 2n = 18 (genome RR), a very polymorphic species, including annual and biennial forms, is suggested to be Japan and the coastal areas of the mainland; if this is so, the crop may have derived from different wild species, including Raphanus raphanistrum, also with 2n = 18 (arrived from Greece or Asia Minor); both, cultivated and wild forms spreaded over the Old World probably introgressing with other wild species and ecotypes (Wein 1964). It is also probable that feral radish evolved from cultivated forms (Campbell and Snow 2009). Sinapis alba occurs as a weed (subsp. dissecta) and as a weed and cultivated (subsp. alba). The wild subsp. alba, found in the olive groves, is mostly used as a medicinal plant, rarely as a vegetable (Hammer et al. 1999; Perrino et al. 2004). Often Sinapis alba is reported as syn. of Brassica alba. Though S. alba (2n = 24) is not the wild relative of B. oleracea (2n = 18) and of B. napus (2n = 38), genes for resistance to the beet cyst nematode (Heterodera schachtii) have been transferred by means of sexual and somatic hybridization from S. alba to B. napus (Lelivelt et al. 1993). Sinapis arvensis subsp. arvensis is an annual plant, whose leaves are edible at the juvenile stage; the seeds, except for birds, are toxic, especially if consumed in large quantities; once the seeds are ground, they produce a kind of mustard. Molecular-based phylogenetic studies have indicated that S. arvensis is the most likely progenitor species giving rise to Brassica nigra (genome B) and that these taxa constitute a separate lineage from that containing B. rapa and B. oleracea (genomes A and C, respectively). This close relationship of S. arvensis and B. nigra is strongly supported by several data sets, from cytological to hybridization, electrophoretic, morphological and shared presence of genome B (Warwick and Black 1991; Warwick et al. 2000). Successful hybridizations of S. arvensis have been achieved with B. napus L. and hybrids B. napus × S. arvensis were successfully backcrossed to B. napus (Plümper 1995). S. arvensis is a useful source of blackleg fungus (Leptosphaeria maculans) resistance for the oilseed rape B. napus (Snowdon et al. 2000). Sinapis can exchang genes even with Raphanus (Bang et al. 1996). Beta vulgaris can be found in crop, wild and weedy forms, all of which are interfertile. Genetic diversity in wild beets appear to be very high in comparison with that observed in the cultivated beets (Desplanque et al. 1999). The wild plants may form sources of resistance to disease, such as Cercospora, yellow mosaic and to increase the variability to select for new high yielding types (Zeven and Zhukovsky 1975). In Italy there are different wild types (Hammer et al. 1999). In Europe “The sugar beet breeding gene pool is considered to be narrow. It mainly lacks sufficient genetic variation for resistance and tolerance to biotic and abiotic stress” and sources to face drought stress, due to climatic changes can be found exploiting excisting wild types of the three gene pools (Pgr Forum 2004). Cichorium intybus L. is a wild biennial or perennial herbaceous plant. Young plants are edible and are much used by local people, all over Apulia. As a medicinal plant it exhibits antihepatotoxic, antimicrobial, antinflammatory, anticoagulant, antioxidant, anticancer and antimalarial activity (Hazra et al. 2002). It is considered the wild type of many cultivated forms of C. intybus var. sativum. The cultivation area of this taxon coincides with that of its wild relative, C. intybus, which may lead to gene flow between wild and cultivated types. For this reason the genetic diversity within and between types was studied and it was found that both wild and cultivated populations are not much differentiated, as expected for a largely allogamous species, but the gene pool of cultivated individuals can nevertheless be distinguished from the gene pool of wild individuals (Van Cutsem et al. 2003). The possibility to exchange genes with other species includes C. endivia, which can be included in the secondary or primary gene pool of C. intybus and vice versa (Hammer et al. 1999; Perrino et al. 2004). Lactuca serriola is probably the wild relative of the cultivated lettuce (L. sativa). Both cultivated and wild Lactuca differ greatly in shoot and root characteristics (Johnson et al. 2000). Usually, wild progenitors of crop plants tend to have root systems that can exploit more unpredictable and stressful soil environments than their cultivated forms (Chapin et al. 1989; Jackson and Koch 1997). For this reason efforts are made to improve the root system of L. sativa landraces by crossing them with the wild L. serriola (Johnson et al. 2000). The present marked variation of lettuce is probably a product of hybridization with L. serriola (Hammer et al. 1999; Perrino et al. 2004), but may have also been induced by some natural mutation (Whitaker 1969).
All or nearly all of the wild species listed in this category will be further investigated in collaboration with genebanks and international organizations. In fact, crop wild relatives (CWR) are a key component for food production and security and for the maintenance of agro-ecosystems. They have been successfully used in plant breeding, but many of them are becoming increasingly threatened. The establishment of genetic reserves is one of the approaches for CWR conservation that has been assessed in Europe under the AEGRO project (AGRI GENRES 057 “An Integrated European In Situ Management Work Plan: Implementing Genetic Reserves and On Farm Concepts”) co-funded by the European Commission, DG AGRI within the framework of council regulation 870/2004.
Edible wild species
The 10 species included in this category are wild plants gathered for human feeding. Often, they are recognized as wild leafy vegetables (Maggioni and Spellman 2001). They are: Asparagus acutifolius, Portulaca oleracea subsp. oleracea, Carduus pycnocephalus subsp. pycnocephalus, Reichardia picroides, Sonchus asper, Sonchus oleraceus, Sonchus tenerrimus, Taraxacum officinale, Urospermum picroides and Smyrnium olusatrum. For each species, some relevant peculiarities that indicate their use as food are provided. Asparagus acutifolius is a native, perennial plant widely distributed throughout the Mediterranean areas, whose flowers are classified as dioecious and are mainly bee-pollinated; in general it does not reproduce by self-pollination and grows in bushy and semi-dry places, sunny or semi-shade, mainly on limestone (Sica et al. 2005); it is becoming an interesting niche crop for marginal areas in Europe (Benincasa et al. 2007); the shoots are eaten boiled and dressed with oil and vinegar, in an omelet, in oil, risotto, soups, stews and soups; the decoction of the shoots is taken orally as a diuretic and generic anti-inflammatory; breeders have crossed A. acutifolius with the well known cultivated species A. officinalis, as well as with other wild species of Asparagus (González Castañón and Falavigna 2008). Portulaca oleracea subsp. oleracea is a cosmopolitan weed whose place of origin is doubtful or unknown (Walters 1920) or possibly it has originated in western Asia (Hammer 1986; Hammer et al. 1999); it seems to be a polyploid complex, with four ploidy levels: diploid (2n = 18), tetraploids, hexaploid and pentaploid (Danin et al. 1978); it is considered the wild form of the cultivated P. oleracea subsp. sativa, originated in the Old World (Danin et al. 1978; Hammer 1986); both subspecies are edible and have similar comestible characteristics, usable both for humans and for animals (Bosi et al. 2009); according to different classical authors, it was one of the leafy vegetables (from both spontaneous and cultivated plants) eaten in Italy during the first century A.D. (Pitrat and Foury 2003); Varrone praised its dietary virtues (Arcidiacono and Pavone 1994). Carduus pycnocephalus subsp. pycnocephalus: at least in Calabria is among several weeds eaten by man (Conforti et al. 2008); the leaves, despinulated, are used in the kitchen; the fleshy root is used boiled or steamed and is excellent as a side dish for meat and fish, and is used as a substitute roasted coffee; the dried flowers are used for the coloration of aliments; it is also thought to have a great potential as an antitumoral plant, because of the high phenolic and favonoid contents in its plant extracts (Conforti et al. 2008); though some Carduus species are known to accumulate nitrates in toxic quantities, C. pycnocephalus has apparently not been incriminated as a toxic weed (Goeden 1974); the genus Carduus is native to the Eastern Hemisphere, where its distribution extends over Europe, central Asia, and East Africa; Flora Europaea recognizes 48 species (Franco 1921). Reichardia picroides is widely used as a wild vegetable in Italy (Hammer et al. 1999), but also in other areas of the Mediterranean, in some of which the roots are also consumed. Recently, cultivation has been attempted (Ficarra 2013). Sonchus asper is native to Europe, West Asia, and North Africa. The plant has been introduced to North America, South America, East Asia, South Africa, Australia, and New Zealand (Hyatt 2006); it is considered by the University of Alaska Anchorage (UAA) an invasive species with a rank of 46, from 0 to 100, where 0 represents a plant that poses no threat and 100 a plant that poses a major threat to native ecosystems; all Sonchus species are listed as noxious weeds in Ontario; it reproduces only by seeds (DiTomaso and Healy 2007), with each plant producing 20,000 to 26,000 seeds (Hutchinson et al. 1984). Seeds can survive between 2 and 8 years in the soil in field conditions; it is a host for several nematode and aphid species and supports several major plant viruses (Hutchinson et al. 1984); the plant is edible and may be grazed by herbivores (Lewin 1948); all these information would suggest to consider S. asper an indesirable weed, but not in its native area (including Italy), where there is a lack of studies on the relationship with other species of the ecosystem, especially from the point of view of any benefits that may arise avoiding the plants to reach flowering and spread, which is possible if they are subjected to grazing or harvesting as vegetables when they are in the rosette stage, a common practice for plants that grow in the olive groves. Sonchus oleraceus, though considered a pest (weed) in more than 55 countries, is more edible than S. asper and therefore appreciated in the popular Italian cooking (Ficarra 2013); in different parts of the world is also appreciated as a medicinal plant with many different properties: anticancer, anti-inflammatory, cathartic, digestive, diuretic, vermicide, and many others (PFAF 2012). Sonchus tenerrimus is edible and used as a medicinal plant: “Pliny the Elder, naturalist of ancient Rome in the first century A.D. argued that a plate of crespigno fed Theseus, the mythical greek hero, before he went to face the Minotaur. In the Middle Ages it was grown in the gardens of the simple within the monasteries, to use as a diuretic and depurative”. Taraxacum officinale is used both in the kitchen and the popular pharmacopoeia; the therapy of leaves or roots is called “tarassacoterapia”; it is a plant of great interest in beekeeping, which provides both pollen and nectar (Rutherford and Deacon 1972; Cyr and Bewley 1990). Urospermum picroides has the basal leaves and buds that are used cooked, before flowering and preferably mixed with other plants, either as a vegetable or in soups and stuffings; it is a bitter plant (Acta Plantarum 2013). Smyrnium olusatrum has leaves and young shoots that can be eaten raw in salads or cooked in soups, stews and others; the plant comes into growth in the autumn and the leaves are often available throughout the winter, with a strong celery-like flavor; leafy seedlings can be used as a parsley substitute; the spicy seeds are used as a pepper substitute (Ficarra 2013; PFAF 2012). After this presentation of comestible plant species, one has, however, to stress the point that the number of comestible species that grow in the olive groves is higher than 10. Infact, are comestible species also some of those included in the category of crops, as for example the following eight species: Muscari comosum, Rubus canescens, Rubus ulmifolius, Myrtus communis subsp. communis, Capparis spinosa, Diplotaxis erucoides subsp. erucoides, Diplotaxis tenuifolia and Amaranthus retroflexus. The same reasoning can be applied to at least three species included in the category of medicinal, aromatic and officinal plants, i.e., Urtica dioica subsp. dioica, U. urens and Solanum nigrum, or to at least six species included in that of the crop wild relatives, i.e., Raphanus raphanistrum, Sinapis alba, Sinapis arvensis subsp. arvensis, Beta vulgaris, Cichorium intybus and Lactuca serriola, and, finally, to at least four species included in that of fodders, i.e., Lathyrus cicera, Lathyrus ochrus, Lathyrus sylvestris subsp. sylvestris, and Tetragonolobus purpureus. The sum of all these edible species, included in other categories, is equal to 21. In this way, the total number of comestible species is then not 10, but 31. On the other hand, the 10 species included in the category of comestibles could have also been included in the category of medicinal, aromatic and officinal plants and even of other categories. But as explained earlier it was decided to list them only in one category, the one that reflects better their prevalent use on a small or global scale.
A part from specific peculiarities, the all species, grouped under the voice of plants used by man must be considered as an important reservoir of plant genetic resources to be saved both in situ and ex situ. The reasons are that, for years or centuries, these species have been changing genes or pieces of DNA with other species of the community, through different natural mechanisms, and therefore their genomes are carrying gene sequencing nearly ready for meeting climiting or environmental changes.
Conclusions
The vulnerability of the agro-ecosystems of ancient olive groves was already pointed out by Perrino et al. (2011). The results of the present study provide further information for improving conservation and management of plant biodiversity and especially of plant species at risk and/or having conservation interest, that are able to survive in ancient olive groves. Thus, they are important not only for their beautiful landscape, olive production, efficient carbon dioxide sequestration and for their ability to face climatic changes, but also for conserving entire agro-ecosystems, in which several endangered plant, animal and microbial species may survive and provide speciation, adaptation and evolution. In fact, in whole, 408 taxa, belonging to 275 genera and 74 families of vascular flora were found to grow in ancient olive groves. The three most represented families are: Asteraceae (12.7 %) with 52 taxa, followed by Poaceae (11.3 %) and Fabaceae (11.3 %), both with 46 taxa. The other families, each with 20 taxa or less, include the remaining 264 taxa. Ancient olive groves provide environments suitable to conserve endangered plant species and/or of conservation interest. In fact, 18 taxa, out of 408, considered to be critical, were found to grow in olive groves and not likely to see in modern and industrialized olive orchards. For some of these species, conservation actions have been undertaken for plants growing in natural habitats of National Park of “Alta Murgia” and Natural Regional Park “Terra delle Gravine”. In these two protected areas of Apulia, specimens of Aegilops uniaristata, Asyneuma limonifolium, Helianthemum jonium, Satureja cuneifolia, Scrophularia lucida and Stipa austroitalica subsp. austroitalica were collected and stored, for ex situ conservation, at the Germplasm Bank of the Botanical Garden Museum, University of Bari (BG-MOBB). Actions for in situ conservation, in the same protected areas, have been taken only for Stipa austroitalica subsp. austroitalica.
The results have shown that the spectrum of plant species in the fields and infrastructures, is different from that in the fields and that in the infrastructures. That of the fields and infrastructures shows that the therophytes, are more abundant (45.1 %), that perennial species: hemicryptophytes (26.7 %), geophytes (10.3 %), phanerophytes (9.8 %), chamaephytes (4.9 %) and nanophanerophytes (3.2 %). That of the fields shows an increase of the therophytes (59.2 %), against a decrease of other life forms (perennial species), especially of chamaephytes (1.2 %) and phanerophytes (5.2 %). Finally, that of ecological infrastructures shows to be very similar to that of the merged fields and ecological infrastructures, except for a slight increases in perennial life forms (56.0 %) and a decrease of the therophytes (44.0 %). In seminatural environments, i.e., in ecological infrastructures, perennial forms take a competitive advantage over some therophytes, showing an increase from the fields (40.8 %) to the ecological infrastructures (56.0 %).
The percentages of the Eastern Mediterranean (6.5 %) and Pontic types (0.5 %) for the merged fields and ecological infrastructures, though show low values they seem to suggest similarities or strong correlations with the flora of eastern geographical areas and, in particular, with the Eastern Mediterranean, primary center of origin of olive trees.
From an agricultural point of view, the co-evolution of at least 111 plant species, out of the 408, belonging to plants used by man, it is of great importance both for in situ conservation and valorization of plant genetic resources related to the agroecosystems of ancient olive trees.
It is difficult to say which species are really threatened and which are not because of lack of monitoring data. This is why the European Community must continue to support monitoring studies, started with LIFE+ CENT.OLI.MED project. In any case, it is out of question that species of conservation interest and the habitats of Directive 92/43 EEC must be preserved. We can assume that farmers who have chosen to preserve their ancient olive groves with agricultural practices with low environmental impact are also interested to protected wild species. However, there are three historical-cultural problems that should not be underestimated. The first one is that we do not know what will be the attitude of the new generation of farmers. The second one is that we do not know if in a reasonable time we will be able to raise awareness for species of conservation interest and for those used by man (food and not food), which have been found in the ancient olive groves. The third problem is that farmers who do not have interest in the protection of biodiversity and that often react negatively to in situ conservation actions, are or may be an obstacle to conservative farming practices. Obviously, the solution to these problems is of political nature.
Although the results show a scientific advancement, they suggest to extend the present study to other olive groves of Apulia, to other regions of Italy and of the Mediterranean basin, with the aim to have a more comprehensive picture of the vascular flora, including plants used by man, to further improve our knowledge on olive groves agro-ecosystems. Bearing in mind that an improvement in planning and management of the olive groves would help to meet both the economical and social aspects of olive production and the conservation of the related ecosystems. In turn, it would meet the philosophy of the “LIFE+ CENT.OLI.MED” Project and that of one of the goals of the Millennium Development Goals (MDG): “ensure environmental sustainability”.
References
Abi-Ayad M, Abi-Ayad FZ, Lazzouni HA, Rebiahi SA, Ziani-Cherif C, Bessiere J (2011) Chemical composition and antifungal activity of Aleppo pine essential oil. J Med Plants Res 5(22):5433–5436
Acerbo G (1937) La marcia storica dell’olivo nel Mediterraneo. Atti Società per il Progresso delle Scienze, riunione XXV (Vol. 1) Fasc. 2:1–22
Acta Plantarum (2013) http://www.actaplantarum.org/floraitaliae/viewtopic.php?t=24448. Accessed 20 Mar 2012
Alessandrini A, Medagli P (2008) Orchis palutris Jacq. Informatore Botanico Italiano 40(suppl. 1):93–95
Al-Said MS, Tariq M, Al-Yahya MA, Rafatullah S, Ginnawi OT, Ageel AM (1990) Studies on Ruta chalepensis, an ancient medicinal herb still used in traditional medicine. J Ethnopharmacol 28:305–312
Apg III (2009) An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants, Apg III. Bot J Linn Soc 161:105–121
Arcidiacono S, Pavone P (1994) Erbe spontanee commestibili del territorio etneo. Bollettino Accademia Gioenia Scienze Naturali 27(346):461–588
Arvis PY, Bichet N, Fleurentin J, Pruvost M, Mortier F, Gouy D, Pelt JM (1990) Anti-hepatotoxic action of Eupatorium cannabinum against carbon tetrachloride and d-galactosamine tested in vitro on rat and human hepatocytes in culture. In: Fleurentin J, Cabalion P, Mazars J, Dos Santos J, Younos C (eds) Proceedings, ethnopharmacology: sources, methods, objectives, pp 374–375
Attard E, Pacioni P (2012) The phytochemical and in vitro pharmacological testing of maltese medicinal plants (5):93–112. In: Rasooli I (ed) Bioactive compounds in phytomedicine, Intech, p 218
Australia New Crops (2013) http://www.newcrops.uq.edu.au/listing/species_pages_T/Trifolium_resupinatum.htm. Accessed 20 Jan 2013
Avallone R, Zanoli P, Puia G, Kleinschnitz M, Schreier P, Baraldi M (2000) Pharmacological profile of apigenin, a flavonoid isolated from Matricaria chamomilla. Biochem Pharmacol 59(11):1387–1394
Bang SW, Kaneko Y, Matsuzawa Y (1996) Production of intergeneric hybrids between Raphanus and Sinapis and the cytogenetics of their progenies. Breed Sci 46:45–51
Barkworth ME (2007) Key to tribes: Piptatherum P. Beauv.: 144–151. In: Barkworth ME, Capels KM, Long S, Anderton LK, Piep MB (eds) Flora of North America North of Mexico, vol 24. Oxford University Press, New York
Barnes J, Anderson LA, Phillipson DJ (2001) St John’s wort (Hypericum perforatum L.): a review of its chemistry, pharmacology and clinical properties. J Pharm Pharmacol 53:583–600
Bartolini G, Prevost G, Messesi C, Carignani G (2005) Olive germplasm: cultivars and world-wide collections. Seed and Plant Genetic Resources Service, FAO
Benincasa P, Tei F, Rosati A (2007) Plant density and genotype effects on wild Asparagus (Asparagus acutifolius L.) spear yield and quality. HortScience 42(5):1163–1166
Bezić NB, Scočibušić M, Dunkić V (2005) Phytochemical composition and antimicrobial activity of Satureja montana L. and Satureja cuneifolia Ten. essential oils. Acta Botanica Croatica 64(2):313–322
Bianco P, Sarfatti G (1961) Stazioni di roccia a Monte S. Nicola (Monopoli, Puglia) con osservazioni sull’areale di Campanula versicolor Sib. et Sm., Carum multiflorum Boiss. e Scrophularia lucida L., Nuovo. Giornale Botanico Italiano 68(1–2):21–35
Bianco P, Medagli P, D’emerico S (1989) Nuovi dati distributivi e osservazioni morfologiche su Aegilops uniaristata Vis. (Gramineae), entità mediterraneo-orientale riaccertata per la flora italiana. Webbia 43(1):19–24
Biondi E, Blasi C (ed.) (2009) Manuale Italiano di interpretazione degli habitat della direttiva 92/43 EEC. http://vnr.unipg.it/habitat/index.jsp. Accessed 18 Apr 2012
Biondi E, Biscotti N, Casavecchia S, Marrese M (2007) “Oliveti secolari”: habitat nuovo proposto per l’inserimento nell’Allegato I della Direttiva (92/43 CEE). Fitosociologia 44 (2)(suppl. 1):213–218
Boller B, Willner E, Maggioni L, Lipman E, compilers (2003) Report of a Working Group on Forages, Eighth meeting 10–12 Apr 2003, Linz, Austria. International Plant Genetic Resources Institute
Bosi G, Guarrera PM, Rinaldi R, Bandini Mazzanti M (2009) Ethnobotany of purslane (Portulaca oleracea L.) in Italy and morphobiometric analyses of seeds from archaeological sites in the Emilia Romagna Region (Northern Italy). In: Morel JP, Mercuri AM (ed) Plants and culture: seeds of the cultural heritage of Europe, pp 129–139
Braun-Blanquet J (1932) Plant sociology. McGraw Hill, London
Brezhnev DD, Korovina ON (1981) Wild relatives of cultivated plants of the flora of the USSR. Kolos 308–309
Brullo S, Minissale P, Spampinato G, Signorello P (1986) Studio fitosociologico delle garighe ad Erica manipuliflora del Salento (Puglia meridionale). Archivio Botanico Italiano 62:201–214
Brullo S, Guglielmo A, Pavone P, Terrasi MC (1994) Numeri cromosomici per la Flora Italiana: 1334. Informatore Botanico Italiano 26:211–213
Caforio F, Marchiori S (2006) Nuove segnalazioni e specie rare per la flora infestante le colture della Puglia. Informatore Botanico Italiano 38(1):37–40
Campbell LG, Snow AA (2009) Can feral weeds evolve from cultivated radish (Raphanus sativus, Brassicaceae)? Am J Bot 96(2):498–506
Candan F, Unlu M, Tepe B, Daferera D, Polissiou M, Sökmen A, Akpulat AH (2003) Antioxidant and antimicrobial activity of the essential oil and methanol extracts of Achillea millefolium subsp. millefolium Afan. (Asteraceae). J Ethnopharmacol 87(2–3):215–220
Cappelletti C (1976) Trattato di botanica. Utet, Torino
Caramia G (2005) I Presidi Farmacoterapici nei Secoli—Evoluzione Storica, X Incontro Nazionale “Insieme per Crescere”, Grado 26–28 Ottobre 2005, p 48
Castroviejo S, Aldasoro JJ, Alarcón M (with contributions from Hand R) (2010) Campanulaceae. In: Euro + Med Plantbase, the information resource for Euro-Mediterranean plant diversity. http://www.emplantbase.org/home.html. Accessed 21 June 2012
Cavallaro V, Angiulli F, Forte L, Macchia F (2007) Indagine floristica di Lama Belvedere (Monopoli-Bari). Informatore Botanico Italiano 39(1):204
Chapin FS, Groves RH, Evans LT (1989) Physiological determinants of growth-rate in response to phosphorus supply in wild and cultivated Hordeum species. Oecologia 79(1):96–105
Chiej R (1984) Encyclopaedia of medicinal plants. MacDonald, London, p 447
Chopra RN, Nayer SL, Chopra IC (1956) Glossary of Indian medicinal plants. CSIR, V (ed) New Delhi 12:157
China Wikipedia (2013) Chinese herbal medicine. http://www.wiki86.com/view/278253.htm. Accessed 2 Mar 2013
Conforti F, Ioele G, Statti GA, Marrelli M, Ragno G, Menichini F (2008) Antiproliferative activity against human tumor cell lines and toxicity test on Mediterranean dietary plants. Food Chem Toxicol 46:3325–3332
Conti F, Manzi A, Pedrotti F (1997) Liste Rosse Regionali delle Piante d’Italia, World Wildlife Fund (WWF) Italia, Società Botanica Italiana (SBI), Centro Interdipartimentale Audiovisivi e Stampa, Università di Camerino, p 139
Conti F, Abbate G, Alessandrini G, Blasi C (eds) (2005) An annotated checklist of the Italian vascular flora. Palombi Editori, Roma
Conti F, Alessandrini G, Bacchetta G, Banfi E, Barberis G, Bartolucci F, Bernardo L, Bouvet D, Bovio M, Del Guacchio E, Frattini S, Galasso G, Gallo L, Gangale C, Gottschlich G, Grünanger P, Gubellini L, Iiriti G, Lucarini D, Marchetti D, Moraldo B, Peruzzi L, Poldini L, Prosser F, Raffaelli M, Santangelo A, Scassellati E, Scortegagna S, Selvi F, Soldano A, Tinti D, Ubaldi D, Uzunov D, Vidali M (2007) Integrazione della checklist della flora vascolare italiana. Natura Vicentina 10:5–74
Convention on International Trade in Endangered Species of Wild Fauna and Flora—Cites (1973) Signed in Washington 3 March 1973. http://www.cites.org/. Accessed 5 Apr 2012
Convention on the Conservation of European Wildlife and Natural Habitats (1979) Adopted in Berne 19 September 1979. http://conventions.coe.int/Treaty/en/Treaties/Word/104.doc. Accessed 5 Apr 2012
Cyr DR, Bewley JD (1990) Proteins in the roots of the perennial weeds chicory (Cichorium intybus L.) and dandelion (Taraxacum officinale Weber) are associated with overwintering. Planta 182(3):370–374
Danin A, Baker I, Baker HG (1978) Geography and taxonomy of the Portulaca oleracea L. Polyploid Complex. Israel J Bot 27:177–211
De Souza MM, de Jesus RAP, Cachinel Filho V, Schlemper V (1998) Analgesic profile of hydroalcoholic extract obtained from Marrubium vulgare. Phytomedicine 5:103–107
Del Fuoco C (2003) Orchidee del Gargano. Edizioni del Parco, Foggia
Desplanque B, Boudry P, Broomberg K, Saumitou-Laprade P, Cuguen J, Van Dijk H (1999) Genetic diversity and gene flow between wild, cultivated and weedy forms of Beta vulgaris L. (Chenopodiaceae), assessed by RFLP and microsatellite markers. Theor Appl Genet 98:1194–1201
Dimitrova D (2011) Acanthus spinosus L. In: Peev D (ed) Red Data Book of the Republic of Bulgaria, vol 1 plants and fungi 365
DiTomaso JM, Healy EA (2007) Weeds of California and other western states, vol 1. University of California Agriculture and Natural Resources, Oakland, p 834
Edmonds JM, Chweya JA (1997) Promoting the conservation and use of under-utilized and neglected crops: black nightshades (Solanum nigrum L.) and related species. International Plant Genetic Resources Institute, Rome, p 90
El-Bardai S, Morel N, Wibo M, Fabre N, Llabres G, Lyoussi B, Quetin-Leclercq L (2003) The vasorelaxant activity of Marrubenol and Marrubiin from Marrubium vulgare. Planta Med 69(1):75–77
El-Bardai S, Lyoussi B, Wibo M, Morel N (2004) Comparative Study of the antihypertensive activity of Marrubium vulgare and of the dihydropyridine calcium antagonist amlodipine in spontaneously hypertensive rat. Clin Exp Hypertens 26(6):465–474
European Commission Dg Environment (2007) Interpretation manual of European Union habitats (version EUR27). European Commission DG Environment, Brussels
European Medicines Agency (2007) Assessment report on Urtica dioica L., Urtica urens L., folium, Committee on Herbal Medicinal Products
Faleiro L, Miguel G, Gomes S, Costa L, Venâncio F, Teixeira A, Figueiredo AC, Barroso JG, Pedro LG (2005) Antibacterial and antioxidant activities of essential oils isolated from Thymbra capitata L. (Cav.) and Origanum vulgare L. J Agric Food Chem 53(21):8162–8168
Fao (2002) Classification, origin, diffusion and history of the olive. FAO book, Rome
Ficarra P (2013) Piante spontanee in cucina e altri sentieri di etnobotanica. http://www.piantespontaneeincucina.info/. Accessed 27 Jan 2013
Francini Corti E (1966) Aspetti della vegetazione pugliese e contingente paleoegeico meridionale della Puglia. Annali Accademia Italiana di Scienze Forestali 15:137–193
Franco JMAA (1921) Carduus: 220–232. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (ed) (1976) Flora Europaea, vol 4 Plantaginaceae to Compositae (and Rubiaceae). Cambridge University Press, Cambridge
García G, Faz Á, Cunha M (2004) Performance of Piptatherum miliaceum (Smilo grass) in edaphic Pb and Zn phytoremediation over a short growth period. Int Biodeterioration Biodegradation 54(2–3):245–250
Ghazghazi H, Miguel MG, Hasnaoui B, Sebei H, Figueiredo AC, Pedro LG, Barroso JG (2012) Leaf essential oil, leaf methanolic extract and rose hips carotenoids from Rosa sempervirens L. growing in North of Tunisia and their antioxidant activities. J Med Plants Res 6(4):574–579
Goeden RD (1974) Comparative survey of the phytophagous insect fauna of Italian thistle (Carduus pycnocephalus) in southern California and southern Europe relative to biological weed control. Environ Entomol 3:464–474
González Castañón ML, Falavigna A (2008) Asparagus germplasm and interspecific hybridization. Proc Abstr XI Int Asparagus Symp Acta Horticolturae 776:319–326
González-Tejero MR, Casares-Porcel M, Sánchez-Rojas CP, Ramiro-Gutiérrez JM, Molero-Mesa J, Pieroni A, Giusti ME, Censorii E, de Pasquale C, Della A, Paraskeva-Hadijchambi D, Hadjichambis A, Houmani Z, El-Demerdash M, El-Zayat M, Hmamouchi M, ElJohrig S (2008) Medicinal plants in the Mediterranean area: synthesis of the results of the project Rubia. J Ethnopharmacol 116(2):341–357
Greuter W, Burdet HM, Long G (eds) (1984) Med-checklist, a critical inventory of vascular plants of the circum-mediterranean countries, 1: Pteridophyta 2, Gymnospermae, Dicotyledones (Acanthaceae–Cneoraceae). Conservatoire et Jardin botanique de la Ville de Genève, Genève
Greuter W, Burdet HM, Long G (1986) Med-Checklist, A critical inventory of vascular plants of the circum-mediterranean countries, 3: Dicotyledones (Convolvulaceae-Labiatae). Conservatoire et Jardin botanique de la Ville de Genève, Genève
Groves E (1887) Flora della costa meridionale della Terra d’Otranto. Giornale Botanico Italiano 19:110–219
Hammer K (1986) Portulacaceae. In: Schultze-Motel J (ed) Rudolf Mansfelds Verzeichnis landwirtschaftlicher und gärtnerischer Kulturpflanzen (ohne Zierpflanzen). Akademie-Verlag, Berlin, pp 130–134
Hammer K, Knüpffer H, Perrino P (1990) A checklist of the south Italian cultivated plants. Die Kulturpflanze 38(3):191–310
Hammer K, Knüpffer H, Laghetti G, Perrino P (1992) Seeds from the past, a catalogue of the crop germplasm of South Italy and Sicily. CNR—Germplasm Institute, Bari, p 174
Hammer K, Knüpffer H, Laghetti G, Perrino P (1999) Seeds from the past, a catalogue of crop germplasm in Central and North Italy. CNR—Germplasm Institute, Bari, p 254
Hanelt P, Mettin D (1989) Biosystematics of the genus Vicia L. (Leguminosae). Annu Rev Ecol Syst 20:199–223
Harlan JR, de Wet JMJ (1971) Toward a rational classification of cultivated plants. Taxon 20(4):509–517
Haston E, Richardson JE, Stevens PE, Chase MW, Harris DJ (2007) A linear sequence of Angiosperm Phylogeny Group II families. Taxon 56:7–12
Haston E, Richardson JE, Stevens PE, Chase MW, Harris DJ (2009) The Linear Angiosperm Phylogeny Group (LAPG) III: a linear sequence of the families in APG III. Bot J Linn Soc 161:128–131
Hazra B, Sarkar R, Bhattacharyya S, Roy P (2002) Tumour inhibitory activity of chicory root extract against Ehrlich ascites carcinoma in mice. Fitoterapia 73:730–733
Heywood VH, Zohary D (1995) A catalogue of the wild relatives of cultivated plants native to Europe. Flora Mediterranea 5:375–415
Hutchinson I, Colosi J, Lewin R (1984) The biology of Canadian weeds 63, Sonchus asper (L.) Hill and S. oleraceus L. Can J Plant Sci 64(3):731–744
Hyatt P (2006) Sonchus asper (L.) Hill. In: Flora of North America Editorial Committee (ed) (1993), Flora of North America North of Mexico, New York and Oxford, vol 19, p 275
Jackson LE, Koch GW (1997) The ecophysiology of crops and their wild relatives. In: Jackson LE (ed) Ecology in agriculture. Academic Press, San Diego, pp 3–37
Johnson WC, Jackson LE, Ochoa O, van Wijk R, Peleman J, Clair DA, Michelmore RW (2000) Lettuce, a shallow-rooted crop, and Lactuca serriola, its wild progenitor, differ at QTL determining root architecture and deep soil water exploitation. Theor Appl Genet 101:1066–1073
Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, Hot RD, Hudson P, Jolles A, Jones KE, Mitchell CE, Myers SS, Bogich T, Ostfeld RS (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468:647–652
Koçyiğit M, Özhatay N (2006) Wild plants used as medicinal purpose in Yalova (Northwest Turkey). Turkish J Pharm Sci 3(2):91–103
Lahav-Ginott S, Cronk QCB (1993) The mating system of Elatostema (Urticaceae) in relation to morphology: a comparative study. Plant Syst Evol 186(3–4):135–145
Lelivelt CLC, Leunissen EHM, Frederiks HJ, Helsper JPFG, Krens FA (1993) Transfer of resistance to the beet cyst nematode (Heterodera schachtii Schm.) from Sinapis alba L. (white mustard) to the Brassica napus L. gene pool by means of sexual and somatic hybridization. Theor Appl Genet 85(6–7):688–696
Lewin R (1948) The biological flora of the British Isles Sonchus L. J Ecol 36(1):203–223
Loukis A, Philianos S (1980) Phytochemical investigation of Acanthus spinosus L. Fitoterapia 51(3):141–142
Macchioni F, Perrucc S, Cecchi F, Cioni PL, Morelli I, Pampiglione S (2004) Acaricidal activity of aqueous extracts of camomile flowers, Matricaria chamomilla, against the mite Psoroptes cuniculi. Med Vet Entomol 18(2):205–207
Maggioni L, Spellman O (compilers) (2001) Report of a Network Coordinating Group on Vegetables, ad hoc meeting, 26–27 May 2000, Vila Real, Portugal. International Plant Genetic Resources Institute, Rome, Italy
Marchiori S, Medagli P, Sabato S, Ruggiero L (1993) Remarques chorologiques sur quelques taxa nouveaux ou rares dans le Salento (Pouilles, Italie). Informatore Botanico Italiano 25(1):37–45
Marchiori S, Medagli P, Mele C, Scandura S, Albano A (2000) Caratteristiche della flora vascolare pugliese. Cahiers Options méditerranénnes 53:67–75
Mavi A, Terzi Z, Özgen U, Yildirim A, Coşkun M (2004) Antioxidant Properties of Some Medicinal Plants: Prangos ferulacea (Apiaceae), Sedum sempervivoides (Crassulaceae), Malva neglecta (Malvaceae), Cruciata taurica (Rubiaceae), Rosa pimpinellifolia (Rosaceae), Galium verum subsp. verum (Rubiaceae), Urtica dioica (Urticaceae). Biol Pharm Bull 27(5):702–705
Maxted N (2008) A phenetic investigation of Vicia L. subgenus Vicia (Leguminosae, Vicieae). Bot J Linn Soc 111(2):155–182
Mele C, Medagli P, Marchiori S (2001) Notula 1042. Informatore Botanico Italiano 33(2):424
Mettin D, Hanelt P (1964) Cytosystematische Undersuchungen in der Artengruppe von Vicia sativa L. I. Die Kulturpflanze 12:163–225
Moraldo B, Ricceri C (2003) Alcune novità tassonomico-nomenclaturali sul genere Stipa L. (Poaceae) in Italia. Webbia 58(1):103–111
Mosaddegh M, Naghibi F, Moazzeni H, Pirani A, Esmaeili S (2012) Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. J Ethnopharmacol 141(1):80–95
Negro C, De Bellis L, Miceli A (2013) Antioxidant activity of Buglossoides purpureocaerulea (L.) I.M. Johnst. Extract. Nat Prod Res 27(4–5):509–512
Penza C (1969) Flora Maltija Mediċinali. Progress Press Co. Ltd., Malta
Perez RMG, Perez JAL, Garcia LMD, Sossa HM (1998) Neuropharmacological activity of Solanum nigrum fruit. J Ethnopharmacol 62(1):43–48
Perrino EV (2011) New data on Aegilops uniaristata Vis. in Italy. Natura Croatica 20(1):117–123
Perrino EV, Signorile G (2009) Costa di Monopoli (Puglia): check-list della flora vascolare. Informatore Botanico Italiano 41(2):263–279
Perrino EV, Signorile G (2010) Dati preliminari sulla flora vascolare del litorale di Polignano a Mare (Puglia). In: Proceedings of abstracts 105 congress of the Italian Botanical Society, Reggio Calabria 17–19 Sept 2010
Perrino EV, Wagensommer RP (2012) Schede per una Lista Rossa della Flora vascolare e crittogamica Italiana: Aegilops uniaristata Vis. Informatore Botanico Italiano 44(1):201–203
Perrino P, Laghetti G, Knupffer H, Hammer K (2004) Semi del passato—Catalogo del germoplasma agrario della Sicilia e dell’Italia meridionale, p 210
Perrino EV, Calabrese G, Ladisa G, Viti R, Mimiola G (2011) Primi dati sulla biodiversità della flora vascolare di oliveti secolari in Puglia. Informatore Botanico Italiano 43(1):39–64
Perrino EV, Wagensommer RP, Medagli P (2012) Schede per una Lista Rossa della Flora vascolare e crittogamica Italiana: Asyneuma limonifolium (L.) Janch. subsp. limonifolium. Informatore Botanico Italiano 44(2):414–416
Perrino EV, Ladisa G, Tartaglini N, Veronico G, Calabrese G (2013) Vegetazione degli oliveti monumentali in Puglia: dati preliminari (Vegetation of monumental olive orchards of Apulia: preliminary data). In: Proceedings of abstracts IX national congress of biodiversity, CIHEAM-IAMB Valenzano (Bari) 6–7 Sept 2012
Peruzzi L, Tison JM (2007) Typification of six critical Mediterranean Gagea Salisb. (Liliaceae) taxa. Candollea 62(2):173–188
Peruzzi L, Scuderi L, Raimondo FM (2009) Distribution of the genus Gagea (Liliaceae) in Sicily. Flora Mediterranea 19:25–47
PFAF—Plants For A Future (2012) A resource and information centre for edible and otherwise useful plants. http://www.pfaf.org/user/AboutUs.aspx. Accessed 23 Jan 2013
Pgr Forum (2004) European crop wild relative diversity—assessment and conservation forum, crop wild relative 1–20. http://www.pgrforum.org. Accessed 23 Jan 2013
Pignatti S (1982) Flora d’Italia. Edagricole, Bologna
Pitrat M, Foury C (2003) Histoires de legumes: des origines a l’oree du 21 siecle. Institut national de la recherche agronomique, Paris, p 410
Putnam AR (1988) Allelochemicals from plants as herbicides. Weed Technol 2(4):510–518
Radanova S (2009) Variety of medicinal plants in a cultigenic ecosystem. Biotechnol Biotechnol Equip 23(2):389–392
Raunkiear C (1934) Life forms of plants and statistical plant geography. Oxford University Press, Oxford
Report (2003) Special products and areas, prepared by Save under contract no. 114-C-00-02-00086-00 for the United States Agency for International Development Caucasus, pp 1–19
Roman RR, Aharcon AF, Lara LA, Flores SJL (1992) Hypoglycemic effect of plants used in Mexico as antidiabetics. Arch Med Res 23(1):59–64
Romaschenko K, Peterson PM, Soreng RJ, Futorna O, Susanna A (2011) Phylogenetics of Piptatherum s.l. (Poaceae: Stipeae): evidence for a new genus, Piptatheropsis, and resurrection of Patis. Taxon 60(6):1703–1716
Ruiz De La Torre J (1956) La vegetatión natural del norte de Marruecos y la elección de especies para su repoblación forestal. Centro Investigaciones Esperiencias Forestales, Larache
Rutherford PP, Deacon AC (1972) β-Fructofuranosidases from Roots of Dandelion (Taraxacum officinale Weber). Biochem J 126:569–573
Sahpaz S, Garbacki N, Tits M, Bailleul F (2002) Isolation and pharmacological activity of phenylpropanoid esters from Marrubium vulgare. J Ethnopharmacol 79(3):389–392
San Miguel A (2008) Management of Natura 2000 habitats. 6220 *Pseudo-steppe with grasses and annuals of the Thero-Brachypodietea, European Commission
Schäfer-Schuchardt H (1988) L’oliva la grande storia di un piccolo frutto, Regione Puglia, Assessorato all’Agricoltura. Cooperativa Grafica Italiana, Bari
Scoppola A, Spampinato G (2005) Atlante delle specie a rischio di estinzione, Versione 1.0, CD-Rom enclosed to the volume. In: Scoppola A, Blasi C (eds) Stato delle conoscenze sulla flora vascolare d’Italia. Palombi Editori, Roma
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Sica M, Gamba G, Montieri S, Gaudio L, Aceto S (2005) ISSR markers show differentiation among Italian populations of Asparagus acutifolius L. BMC Genet 6:17
Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG (2006) A classification for extant ferns. Taxon 55(3):705–731
Snowdon RJ, Winter H, Diestel A, Sacristán MD (2000) Development and characterisation of Brassica napus–Sinapis arvensis addition lines exhibiting resistance to Leptosphaeria maculans. Theor Appl Genet 101(7):1008–1014
Stevens PF (2008) Angiosperm Phylogeny Website, Version 9, June 2008 [and more or less continuously update since]. http://www.mobot.org/MOBOT/research/APweb/. Accessed 20 Oct 2012
Stulzer HK, Tagliari MP, Zampirolo JA, Cechinel-Filho V, Schlemper V (2006) Antioedematogenic effect of marrubiin obtained from Marrubium vulgare. J Ethnopharmacol 108(3):379–384
Suárez-Santiago NV, Salinas JM, Romero-Garcia TA, Carrido-Ramos AM, del la Herrán R, Ruiz-Rejón C, Ruiz-Rejón M, Branca G (2007) Poliploidy, the major speciation mechanism in Muscari subgenus Botryanthus in the Iberian Peninsula. Taxon 56(4):1171–1184
Svitashev S, Bryngelsson T, Vershinin A, Pedersen C, Säll T, von Bothmer R (1994) Phylogenetic analysis of the genus Hordeum using repetitive DNA sequences. Theor Appl Genet 89(7–8):801–810
Tison JM (1998) Gagea granatellii (Parl.) Parl. en France. Le Monde des Plantes 462:1–6
Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA (ed) (1964–80) Flora Europaea, 1–5, University Press, Cambridge
Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (1980) Flora Europaea, vol 5. Alismataceae to Orchidaceae (Monocotyledones). Cambridge University Press, Cambridge
Tuzlaci E, Alparslan İşbilen DF, Bulut G (2010) Turkish folk medicinal plants, VIII: Lalapaşa (Edirne). Marmara Pharm J 14:47–52
Ubaldi D (2003) Flora, fitocenosi e ambiente (Elementi di Geobotanica e Fitosociologia. Clueb, Bologna
Ugulu I (2011) Traditional ethnobotanical knowledge about medicinal plants used for external therapies in Alasehir, Turkey. Int J Med Aromat Plants 1(2):101–106
Van Cutsem P, du Jardin P, Boutte C, Beauwens T, Jacqmin S, Vekemans X (2003) Distinction between cultivated and wild chicory gene pools using AFLP markers. Theor Appl Genet 107(4):713–718
Van Slageren MW (1994) Wild wheats: a monograph of Aegilops L. and Amblyopyrum (Jaub. et Spach) Eig (Poaceae), Wagningen Agricoltural University. Int Center Agric Res Dry Areas 9(7):1–513
Vazzana C, Raso E (1997) Una metodologia europea per la progettazione e realizzazione di un agroecosistema a basso o nullo impatto ambientale, S.I.T.E. Notizie. Bollettino Società Italiana di Ecologia 17:51–54
Vita F, Forte L (1990) Un lembo di vegetazione da tutelare la lama di macchia lunga. Umanesimo della Pietra Verde 5:34–38
Walker JB, Sytsma KJ, Treutlein J, Wink M (2004) Salvia (Lamiaceae) is not monophyletic: implications for the systematics, radiation, and ecological specializations of Salvia and tribe Mentheae. Am J Bot 91(7):1115–1125
Walters SM (1920) Portulacaceae: 114. In: Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA (ed) (1964) Flora Europaea, vol 1, Lycopodiaceae to Platanaceae. Cambridge University Press, Cambridge
Warwick SI, Black LD (1991) Molecular systematics of Brassica and allied genera (Subtribe Brassicinae, Brassiceae) chloroplast genome and cytodeme congruence. Theor Appl Genet 82(1):81–92
Warwick SI, Beckie HJ, Thomas AG, McDonald T (2000) The biology of Canadian weeds. 8. Sinapis arvensis L. (updated). Can J Plant Sci 80(4):939–961
Weel KCG, Venskutonis PR, Pukalskas A, Gruzdiene D, Linssen JPH (1999) Antioxidant activity of horehound (Marrubium vulgare) grown in Lithuania. Fett/Lipid 101(10):395–400
Wei SH, Zhou QX, Wang X (2005) Cadmium-hyperaccumulator Solanum nigrum L. and its accumulating characteristics. Environ Sci 26:167–171
Wein K (1964) Die Geschichte des Rettichs und des Radieschens. Kulturpflanze 12:33–74
Whitaker TW (1969) Salads for everyone—a look at the lettuce plant. Econ Bot 23(3):261–264
Woodland DW, Basset JI, Crompton C, Forget S (1982) Biosystematics of the perennial North American taxa of Urtica. I. Chromosome number, hybridization and palynology. Syst Bot 7(3):269–281
World Health Organization (WHO) (2004) Monographs on selected medicinal plants, vol 2. p 358
Zeven AC, Zhukovsky PM (1975) Dictionary of cultivated plants and their centres of diversity, excluding ornamentals, forest trees and lower olants. Centre for Agricultural Publishing and Documentation, Wageningen, p 219
Zhukovsky PM (1964) Cultivated plants and their relatives, 2nd edn. Leningrad, Kolos, p 791
Zohary D (1973) Geobotanical foundations of the Middle East. Gustav Fischer Verlag, Stuttgart
Acknowledgments
The authors whish to thank the Mediterranean Agronomic Institute at Valenzano (Bari), Italy, as the coordinating beneficiary of the LIFE+ CENT.OLI.MED. (LIFE07 NAT/IT/000450) project, that made the present study possible. They wish also to thank the owners of the investigated olive orchards for providing access to their farms and facilities. Thanks are due to the Botanical Garden Museum of the University of Bari, Italy, for the availability of the herbarium and library. Finally, authors whish to thank Prof. Piero Medagli for valuable comments and suggestions for the manuscript.
Author information
Authors and Affiliations
Corresponding author
Appendix: Floristic List
Appendix: Floristic List
Species | Acronyms | |||||
|---|---|---|---|---|---|---|
LF (3) | CT (3) | CIN (1) | PUM (2) | |||
Polypodiidae | ||||||
Dennstaedtiaceae | ||||||
Pteridium aquilinum (L.) Kuhn subsp. aquilinum | G | C | ||||
Pteridaceae | ||||||
Adiantum capillus-veneris L. | G | Pn | ||||
Aspleniaceae | ||||||
Ceterach officinarum Willd. | H | Ese | MAP | |||
Pinidae | ||||||
Pinaceae | ||||||
Pinus halepensis Miller | P | Ms | MAP | |||
Magnoliidae | ||||||
Lauraceae | ||||||
Laurus nobilis L. | P | Ms | MAP | |||
Araceae | ||||||
Arisarum vulgare Targ.-Tozz. | G | Ms | ||||
Arum italicum Miller subsp. italicum | G | Ms | ||||
Dioscoraceae | ||||||
Tamus communis L. | G | Me | ||||
Colchicaceae | ||||||
Colchicum cupanii Guss. | G | Ms | ||||
Smilacaceae | ||||||
Smilax aspera L. | NP | Tps | ||||
Liliaceae | ||||||
Gagea granatellii (Parl.) Parl. | G | Msd | VU | |||
Gagea mauritanica Durieu | G | Mssw | CR | |||
Orchidaceae | ||||||
Barlia robertiana (Loisel.) Greuter | G | Ms | CI | |||
Ophrys incubacea Bianca | G | Ms | CI | |||
Orchis palustris Jacq. | G | Me | CI-EN | |||
Orchis purpurea Huds. | G | Ea | CI | |||
Iridaceae | ||||||
Gladiolus italicus Mill. | G | Me | MAP | |||
Hermodactylus tuberosus (L.) Mill. | G | Msn | ||||
Romulea columnae Sebast. et Mauri | G | Ms | ||||
Xanthorrhoeaceae | ||||||
Asphodelus fistulosus L. | H | Tpsp | ||||
Asphodelus ramosus L. subsp. ramosus | G | Ms | ||||
Amaryllidaceae | ||||||
Allium ampeloprasum L. | G | Me | CRO | |||
Allium roseum L.* | G | Ms | ||||
Allium subhirsutum L. | G | Ms | ||||
Allium trifoliatum Cirillo | G | Mse | ||||
Asparagaceae | ||||||
Asparagus acutifolius L.* | NP | Ms | EWS | |||
Charybdis pancration (Steinh.) Speta | G | Msm | ||||
Loncomelos narbonensis (Torm. in L.) Raf. | G | Me | ||||
Muscari commutatum Guss. | G | Mece | CWR | |||
Muscari comosum (L.) Mill. | G | Me | CRO | |||
Muscari neglectum Guss. ex Ten. | G | Me | CWR | |||
Muscari parviflorum Desf. | G | Mece | PI-r | CWR | ||
Ornithogalum comosum L. | G | Mm | ||||
Ornithogalum gussonei Ten. | G | Ms | ||||
Ornithogalum umbellatum L. | G | Me | ||||
Ruscus aculeatus L. | Ch | Me | ||||
Juncaceae | ||||||
Juncus articulatus L. | G | Cb | ||||
Juncus hybridus Brot. | T | Ma | ||||
Cyperaceae | ||||||
Isolepis cernua (Vahl) Roem. et Schult. | T | Cs | ||||
Poaceae | ||||||
Achnatherum bromoides (L.) P. Beauv. | H | Ms | ||||
Aegilops ovata Auct. | T | Mst | CWR | |||
Aegilops uniaristata Vis. | T | Ad | Ad-VU | CWR | ||
Agrostis stolonifera L. | H | Cb | ||||
Aira caryophyllea L. subsp. caryophyllea | T | Tps | ||||
Arundo donax L. | G | Cs | ||||
Arundo plinii Turra | G | Ms | ||||
Avena barbata Pott ex Link* | T | Me | CWR | |||
Avena sativa L. | T | – | CRO | |||
Brachypodium retusum (Pers.) P. Beauv. | H | Msw | ||||
Brachypodium sylvaticum (Huds.) P. Beauv. | H | Tmp | ||||
Briza maxima L.* | T | Tps | ||||
Briza minor L. | T | Cs | ||||
Bromus diandrus Roth | T | Tpsp | ||||
Bromus hordeaceus L. | T | Cs | ||||
Bromus madritensis L. | T | Me | ||||
Catapodium rigidum (L.) C.E. Hubb.* | T | Me | ||||
Cynodon dactylon (L.) Pers. | G | C | ||||
Cynosurus echinatus L. | T | Me | ||||
Dactylis glomerata L. subsp. glomerata | H | Tmp | ||||
Dactylis glomerata L. subsp. hispanica (Roth) Nyman | H | Ms | ||||
Dasypyrum villosum (L.) P. Candargy | T | Met | CWR | |||
Digitaria sanguinalis (L.) Scop. | T | Cs | ||||
Hordeum murinum L. | T | Cb | CWR | |||
Hordeum murinum L. subsp. leporinum (Link) Arcang. | T | Cb | CWR | |||
Hyparrhenia hirta (L.) Stapf subsp. hirta | H | Tpp | ||||
Lagurus ovatus L. subsp. ovatus | T | Ms | ||||
Lolium perenne L. | H | Cb | ||||
Lolium rigidum Gaudin | T | Tpsp | ||||
Parapholis incurva (L.) C.E. Hubb. | T | Ma | ||||
Phalaris minor Retz. | T | Tpsp | ||||
Phalaris paradoxa L. | T | Ms | ||||
Phleum pratense L. | H | Cb | ||||
Piptatherum miliaceum (L.) Coss. subsp. miliaceum | H | Mst | FOD | |||
Piptatherum miliaceum (L.) Coss. subsp. thomasii (Duby) Freitag | H | Ms | FOD | |||
Poa annua L. | T | C | ||||
Poa bulbosa L. | H | Tmp | ||||
Polypogon monspeliensis (L.) Desf. | T | Tps | ||||
Rostraria cristata (L.) Tzvelev | T | Cs | ||||
Setaria viridis (L.) Beauv. | T | Cs | ||||
Sorghum halepense (L.) Pers. | G | Ctr | FOD | |||
Stipa austroitalica Martinovský subsp. austroitalica | H | I | B-DH-I | |||
Stipa capensis Thunb. | T | Ms | ||||
Trachynia distachya (L.) Link | T | Ms | ||||
Vulpia ciliata Dumort. | T | Me | ||||
Vulpia ligustica (All.) Link | T | Ms | ||||
Papaveraceae | ||||||
Fumaria capreolata L. subsp. capreolata | T | Me | ||||
Fumaria officinalis L. | H | Mem | ||||
Fumaria parviflora Lam. | T | Mt | ||||
Papaver hybridum L. | T | Mt | ||||
Papaver rhoeas L. subsp. rhoeas* | T | Mes | ||||
Ranunculaceae | ||||||
Anemone hortensis L. subsp. hortensis | G | Mn | ||||
Clematis cirrhosa L. | P | Mst | ||||
Clematis vitalba L. | P | Eca | ||||
Delphinium halteratum Sm. subsp. halteratum | T | Ms | ||||
Nigella arvensis L. | T | Me | ||||
Nigella damascena L. | T | Me | ||||
Ranunculus bullatus L. | H | Ms | ||||
Ranunculus ficaria L. | G/H | Ea | ||||
Ranunculus millefoliatus Vahl | H | Mm | ||||
Ranunculus neapolitanus Ten. | H | Mmne | ||||
Ranunculus sardous Crantz | T | Me | ||||
Crassulaceae | ||||||
Sedum rubens L. | T | Meas | ||||
Zygophyllaceae | ||||||
Tribulus terrestris L. | T | C | ||||
Fabaceae | ||||||
Acacia cyanophylla Lindley | P | Aus | ||||
Anagyris foetida L. | P | Mss | ||||
Anthyllis vulneraria L. subsp. maura (Beck) Maire | H | Mssw | ||||
Astragalus hamosus L. | T | Mt | ||||
Bituminaria bituminosa (L.) C.H. Stirt. | H | Me | ||||
Calicotome villosa (Poir.) Link | P | Ms | ||||
Ceratonia siliqua L. | P | Mss | CRO | |||
Coronilla scorpioides (L.) W.D.J. Koch | T | Me | ||||
Dorycnium hirsutum (L.) Ser. | Ch | Me | ||||
Emerus major Mill. subsp. emeroides (Boiss. et Spruner) Soldano et F. Conti | NP | – | ||||
Hippocrepis ciliata Willd. | T | Ms | ||||
Lathyrus cicera L. | T | Me | FOD | |||
Lathyrus ochrus (L.) DC. | T | Ms | FOD | |||
Lathyrus sylvestris L. subsp. sylvestris | H | E | FOD | |||
Lotus corniculatus L. | H | C | ||||
Lotus edulis L. | T | Ms | ||||
Lotus ornithopodioides L. | T | Ms | ||||
Lupinus cosentinii Guss. | T | Mw | FOD | |||
Medicago arabica (L.) Huds. | T | Mw | FOD | |||
Medicago minima L. | T | Tscm | FOD | |||
Medicago orbicularis (L.) Bartal. | T | Me | FOD | |||
Medicago polymorpha L. | T | Me | FOD | |||
Medicago truncatula Gaertn. | T | Ms | FOD | |||
Melilotus sulcatus Desf.* | T | Msd | FOD | |||
Onobrychis aequidentata (Sm.) D’Urv. | T | Mse | ||||
Onobrychis caput-galli (L.) Lam. | T | Ms | ||||
Ononis reclinata L. | T | Mts | ||||
Ononis viscosa (L.) subsp. breviflora (DC.) Nyman | T | Mw | ||||
Scorpiurus muricatus L. | T | Me | ||||
Spartium junceum L. | P | Me | ||||
Sulla capitata (Desf.) B.H. Choi et H. Ohashi | T | Msw | FOD | |||
Tetragonolobus purpureus Moench | T | Ms | FOD | |||
Trifolium campestre Schreb. | T | Mmpw | FOD | |||
Trifolium lappaceum L. | T | Me | FOD | |||
Trifolium pratense L. | T | Cs | FOD | |||
Trifolium resupinatum L. | T | Tmp | FOD | |||
Trifolium scabrum L. subsp. scabrum | T | Me | FOD | |||
Trifolium squarrosum L. | T | Me | FOD | |||
Trifolium stellatum L. | T | Me | FOD | |||
Trifolium tomentosum L | T | Tmp | FOD | |||
Trigonella monspeliaca L. | T | Me | FOD | |||
Vicia hybrida L. | T | Me | FOD | |||
Vicia lutea L. | T | Me | FOD | |||
Vicia sativa L. | T | Cs | FOD | |||
Vicia sativa L. subsp. macrocarpa (Moris) Arcang. | T | Cs | FOD | |||
Vicia villosa Roth | T | Me | FOD | |||
Rosaceae | ||||||
Geum urbanum L. | H | Cb | ||||
Mespilus germanica L. | P | Esp | CRO | |||
Prunus avium L. subsp. avium | P | P | CRO | |||
Prunus dulcis Miller D.A. Webb | P | Msd | CRO | |||
Pyrus spinosa Forssk. | P | Ms | CWR | |||
Rosa sempervirens L. | NP | Ms | MAP | |||
Rubus canescens DC. | NP | Men | CRO | |||
Rubus ulmifolius Schott* | NP | Me | CRO | |||
Sanguisorba minor Scop. | H | Tmp | ||||
Rhamnaceae | ||||||
Paliurus spina-christi Miller | P | Ese | MAP | |||
Rhamnus alaternus L. subsp. alaternus | P | Me | ||||
Moraceae | ||||||
Ficus carica L. | P | Mt | CRO | |||
Morus alba L. | P | Ase | CRO | |||
Urticaceae | ||||||
Mercurialis annua L. | T | Tmp | ||||
Parietaria judaica L. | H | Mem | ||||
Urtica dioica L. subsp. dioica | H | Cs | MAP | |||
Urtica urens L. | T | Cs | MAP | |||
Fagaceae | ||||||
Quercus cerris L. | P | Mn | ||||
Quercus ilex L. subsp. ilex | P | Ms | ||||
Quercus pubescens Willd. subsp. pubescens | P | Esep | ||||
Juglandaceae | ||||||
Juglans regia L. | P | Assw | CRO | |||
Betulaceae | ||||||
Ostrya carpinifolia Scop. | P | P | ||||
Oxalidaceae | ||||||
Oxalis pes-caprae L.* | G | Afs | ||||
Euphorbiaceae | ||||||
Chamaesyce maculata (L.) Small | T | An | ||||
Euphorbia characias L. | NP | Ms | ||||
Euphorbia exigua L. subsp. exigua | T | Me | ||||
Euphorbia helioscopia L. subsp. helioscopia* | T | C | ||||
Euphorbia peplus L. | T | Esb | ||||
Euphorbia segetalis L. | T | Mw | ||||
Euphorbia terracina L. | T | Ms | ||||
Phyllanthaceae | ||||||
Andrachne telephioides L. | Ch | Me | ||||
Violaceae | ||||||
Viola reichenbachiana Jordan ex Boreau | H | Esb | ||||
Linaceae | ||||||
Linum bienne Mill. | H | Me | CWR | |||
Linum strictum L. | T | Ms | ||||
Hypericaceae | ||||||
Hypericum perforatum L. | H | Tmp | MAP | |||
Hypericum triquetrifolium Turra | H | Mse | ||||
Geraniaceae | ||||||
Erodium cicutarium (L.) L’Hér | T/H | Cs | ||||
Erodium malacoides (L.) L’Hér. subsp. malacoides | T | Ms | ||||
Geranium dissectum L. | T | Cs | ||||
Geranium molle L.* | H | Cs | ||||
Geranium purpureum Vill. | T | Me | ||||
Geranium rotundifolium L. | T | Tmp | ||||
Onagraceae | ||||||
Epilobium parviflorum Schreb. | H | Tmp | r | |||
Myrtaceae | ||||||
Myrtus communis L. subsp. communis | P | Ms | CRO | |||
Anacardiaceae | ||||||
Pistacia lentiscus L. | P | Mss | CRO | |||
Pistacia terebinthus L. subsp. terebinthus | P | Me | CRO | |||
Rutaceae | ||||||
Citrus aurantium L. | P | Cn | CRO | |||
Citrus limon (L.) Burm. f. var. femminello | P | Hi | CRO | |||
Citrus sinensis (L.) Osbeck “var. biondo commune del Gargano” | P | Cn | CRO | |||
Citrus sinensis (L.) Osbeck “var. duretta del Gargano” | P | Cn | CRO | |||
Ruta chalepensis L. | Ch | Mss | MAP | |||
Malvaceae | ||||||
Althaea hirsuta L. | T | Me | ||||
Malva cretica Cav. | T | Ms | ||||
Malva sylvestris L. subsp. sylvestris | H | Esb | MAP | |||
Thymelaeaceae | ||||||
Daphne gnidium L. | P | Msm | ||||
Cistaceae | ||||||
Cistus creticus L. | NP | Mec | ||||
Cistus monspeliensis L. | NP | Ms | ||||
Cistus salviifolius L. | NP | Ms | ||||
Fumana laevipes (L.) Spach | Ch | Ms | ||||
Fumana thymifolia (L.) Spach ex Webb | Ch | Ms | ||||
Helianthemum jonium Lacaita | Ch | I | I | |||
Helianthemum salicifolium (L.) Mill. | T | Me | ||||
Resedaceae | ||||||
Reseda alba L. | T | Ms | ||||
Capparaceae | ||||||
Capparis spinosa L. | Np | Ea | CRO | |||
Brassicaceae | ||||||
Biscutella didyma L. subsp. apula Nyman | T | Mts | ||||
Capsella bursa-pastoris (L.) Medik. subsp. bursa-pastoris | H | C | ||||
Cardamine hirsuta L. | T | C | ||||
Diplotaxis erucoides (L.) DC. subsp. erucoides | T | Msw | CRO | |||
Diplotaxis tenuifolia (L.) DC. | H | Masb | CRO | |||
Erophila verna (L.) DC. | T | Cb | ||||
Lepidium draba (L.) Desv. subsp. draba | G/H | Mt | ||||
Moricandia arvensis (L.) DC. | T | Ms | ||||
Raphanus raphanistrum L. | T | Cb | CWR | |||
Raphanus sativus L. | T | - | CRO | |||
Rapistrum rugosum (L.) Arcang. | T | Me | ||||
Sinapis alba L. | T | Mes | CWR | |||
Sinapis arvensis L. subsp. arvensis | T | Ms | CWR | |||
Sisymbrium irio L. | T | Tm | ||||
Thlaspi arvense L. | T | Asw | ||||
Santalaceae | ||||||
Osyris alba L. | NP | Me | ||||
Polygonaceae | ||||||
Rumex acetosa L. subsp. acetosa | H | Cb | ||||
Rumex buchephalophorus L. subsp. buchephalophorus | T | Mmc | ||||
Rumex crispus L. | H | Cs | ||||
Rumex pulcher L. | H/T | Me | ||||
Caryophyllaceae | ||||||
Arenaria serpyllifolia L. subsp. serpyllifolia | T | Cs | ||||
Cerastium glomeratum Thuill.* | T | Cs | ||||
Minuartia verna (L.) Hiern subsp. attica (Boiss. et Spruner) Graebn. | Ch | Me | ||||
Petrorhagia dubia (Raf.) G. Lopez et Romo | G | Msd | ||||
Petrorhagia prolifera (L.) P.W. Ball. & Heywood | T | Me | ||||
Petrorhagia saxifraga (L.) Link subsp. gasparrinii (Guss.) Greuter et Burdet | H | Me | ||||
Sagina apetala Ard. subsp. apetala | H | Me | ||||
Silene conica L. | H | Tmp | ||||
Silene italica (L.) Pers. | H | Me | ||||
Silene latifolia Poiret | T/H | Ms | ||||
Silene nocturna L. | T | Mmms | ||||
Silene vulgaris (Moench) Garcke | H | - | ||||
Stellaria media (L.) Vill. subsp. media | T | C | ||||
Amaranthaceae | ||||||
Amaranthus retroflexus L. | T | C | CRO | |||
Beta vulgaris L. | H | Me | CWR | |||
Chenopodium hybridum L. | T | Cb | MAP | |||
Portulacaceae | ||||||
Portulaca oleracea L. subsp. oleracea | T | Cs | EWS | |||
Cactaceae | ||||||
Opuntia ficus-indica (L.) Miller | P | Nen | CRO | |||
Ericaceae | ||||||
Erica forskalii Vitm. | Ch/NP | Mes | VU | |||
Primulaceae | ||||||
Anagallis arvensis L.* | T | Me | ||||
Cyclamen hederifolium Aiton | G | Msn | CI | |||
Samolus valerandi L. | H | Cs | ||||
Rubiaceae | ||||||
Asperula aristata L. | H/Ch | Mm | ||||
Galium aparine L. | T | Ea | ||||
Galium lucidum All. | H | Me | ||||
Galium palustre L. subsp. elongatum (C. Presl.) Lange | H | Me | ||||
Galium spurium L. | T | Ea | ||||
Galium verrucosum Huds. | T | Ms | ||||
Galium verum L. | H | Ea | ||||
Rubia peregrina L.* | P | Msm | ||||
Sherardia arvensis L.* | T | Cs | ||||
Theligonum cynocrambe L. | T | Ms | ||||
Valantia muralis L. | T | Ms | ||||
Gentianaceae | ||||||
Blackstonia perfoliata (L.) Huds. subsp. perfoliata | T | Me | ||||
Centaurium erythraea Rafn | H | Tmp | ||||
Centaurium pulchellum (Sw.) Druce subsp. pulchellum | T | Tmp | ||||
Apocynaceae | ||||||
Cynanchum acutum L. subsp. acutum | P | Tpsp | ||||
Boraginaceae | ||||||
Alkanna tinctoria (L.) Tausch subsp. tinctoria | H | Ms | ||||
Borago officinalis L. | T | Me | CRO | |||
Buglossoides arvensis (L.) I. M. Johnst. | T | Me | ||||
Buglossoides purpureocaerulea (L.) I.M. Johnst. | H | Esp | MAP | |||
Cerinthe major L.* | T | Ms | ||||
Cynoglossum creticum Mill. | H | Me | ||||
Echium parviflorum Moench | T | Ms | ||||
Echium plantagineum L. | T/H | Me | ||||
Heliotropium europaeum L. | T | Met | ||||
Myosotis arvensis (L.) Hill subsp. arvensis | T | Easw | ||||
Phacelia tanacetifolia Benth. | T | An | ||||
Convolvulaceae | ||||||
Calystegia sepium (L.) R. Br. subsp. sepium | H | Tmp | ||||
Calystegia sylvatica (Kit.) Griseb. | H | Ese | ||||
Convolvulus althaeoides L. | H | Ms | ||||
Convolvulus arvensis L. | G | C | ||||
Convolvulus cantabrica L. | H | Me | ||||
Convolvulus elegantissimus Mill. | H | Mse | ||||
Cuscuta epithymum L. | T | Eat | ||||
Solanaceae | ||||||
Solanum nigrum L. | T | C | MAP | |||
Oleaceae | ||||||
Fraxinus ornus L. subsp. ornus | P | Menp | ||||
Olea europaea L.* | P | Ms | ||||
Phillyrea latifolia L. | P | Ms | ||||
Plantaginaceae | ||||||
Kickxia spuria (L.) Dumort. | T | Ea | ||||
Linaria reflexa (L.) Desf. | T | Msdw | ||||
Linaria vulgaris Mill. subsp. vulgaris | H | Ea | ||||
Misopates orontium (L.) Raf. subsp. orontium | T | Tmp | ||||
Plantago afra L. | T | Ms | ||||
Plantago bellardii All. | T | Msd | ||||
Plantago lagopus L. | T | Ms | ||||
Plantago lanceolata L. | H | Ea | ||||
Plantago major L. | H | Cs | ||||
Plantago serraria L. | H | Ms | ||||
Veronica hederifolia L.* | T | Ea | ||||
Veronica polita Fries* | T | Cs | ||||
Scrophulariaceae | ||||||
Scrophularia lucida L. | H/Ch | Mm | Ad-PI | |||
Scrophularia peregrina L. | T | Ms | ||||
Verbascum pulverulentum Vill. | H | Ecs | ||||
Verbascum sinuatum L. | H | Me | ||||
Lamiaceae | ||||||
Acinos alpinus (L.) Moench | Ch | Oes | ||||
Ajuga chamaepitys (L.) Schreber | T | Me | ||||
Calamintha nepeta (L.) Savi | H | Oes | MAP | |||
Clinopodium vulgare L. | H | Cb | ||||
Lamium amplexicaule L. | T | Tmp | ||||
Lycopus europaeus L. | H | Cb | ||||
Marrubium vulgare L. | H | Cs | MAP | |||
Micromeria graeca (L.) Benth. ex Rchb. subsp. graeca | Ch | Ms | ||||
Origanum vulgare L. subsp. viridulum (Martin-Donos) Nyman | H | Ea | CRO | |||
Prasium majus L. | Ch | Ms | ||||
Rosmarinus officinalis L. | NP | Ms | CRO | |||
Salvia verbenaca L. | H | Msa | MAP | |||
Satureja cuneifolia Ten. | Ch | Msn | PI | |||
Satureja montana L. | Ch | Omw | MAP | |||
Sideritis romana L. subsp. romana | T | Ms | ||||
Stachys germanica L. subsp. salviifolia (Ten.) Gams. | H | Mne | ||||
Teucrium capitatum L. subsp. capitatum | Ch | Ms | ||||
Teucrium flavum L. | Ch | Ms | ||||
Teucrium scordium L. | H | Eca | ||||
Thymbra capitata (L.) Cav. | Ch | Mse | MAP | |||
Orobanchaceae | ||||||
Bartsia trixago L. | T | Me | ||||
Parentucellia latifolia (L.) Caruel | T | Me | ||||
Parentucellia viscosa (L.) Caruel | T | Mea | ||||
Acanthaceae | ||||||
Acanthus spinosus L. | H | Mse | MAP | |||
Verbenaceae | ||||||
Verbena officinalis L. | H | C | ||||
Campanulaceae | ||||||
Asyneuma limonifolium (L.) Janch. subsp. limonifolium | H | Ad | Ad-PI-NT | |||
Legousia hybrida (L.) Delarbre | T | Ma | ||||
Legousia speculum-veneris (L.) Chaix | T | Me | ||||
Asteraceae | ||||||
Achillea millefolium L. | H | Esb | MAP | |||
Anthemis arvensis L.* | T/H | Cs | ||||
Bellis annua L. subsp. annua | T | Msm | ||||
Bellis sylvestris Cirillo | H | Ms | ||||
Calendula arvensis (Vaill.) L.* | T | Me | MAP | |||
Calendula officinalis L. | T/H | - | MAP | |||
Carduus pycnocephalus L. subsp. pycnocephalus | H | Mt | MAP | |||
Carlina corymbosa L. | H | Ms | ||||
Centaurea nicaeensis All. | H | Mssw | ||||
Chondrilla juncea L. | H | Me | ||||
Cichorium intybus L. | H | Tmp | CWR | |||
Cirsium arvense (L.) Scop. | G | Ea | ||||
Cota tinctoria (L.) J. Gay | H/Ch | Ecp | ||||
Crepis apula (Fiori) Babc. | T | I | I | |||
Crepis corymbosa Ten. | T | I | I | |||
Crepis leontodontoides All. | H | Mmw | ||||
Crepis vesicaria L. | T/H | Masb | ||||
Crupina crupinastrum (Moris) Vis. | T | Ms | ||||
Dittrichia viscosa (L.) Greuter | H | Me | ||||
Erigeron canadensis L. | T | Avv. | ||||
Eupatorium cannabinum L. | H | Tmp | MAP | |||
Galactites elegans (All.) Soldano | H | Ms | ||||
Glebionis coronaria (L.) Spach | T | Ms | ||||
Glebionis segetum (L.) Fourr. | T | Me | ||||
Helichrysum italicum (Roth) G. Don | Ch | Es | ||||
Hyoseris scabra L. | T | Ms | ||||
Hypochaeris achyrophorus L. | T | Ms | ||||
Inula conyzae (Griess.) Meikle | H | Easw | ||||
Klasea flavescens (L.) Holub | H | Msdw | ||||
Lactuca serriola L. | H/T | Mess | EWS | |||
Leontodon crispus Vill. subsp. crispus | H | Es | ||||
Leontodon hispidus L. | H | Eca | ||||
Leontodon tuberosus L. | H | Ms | ||||
Matricaria chamomilla L. | T | Cs | MAP | |||
Onopordum illyricum L. | H | Ms | ||||
Pallenis spinosa (L.) Cass. subsp. spinosa | H | Me | ||||
Picris hieracioides L.* | H | Esb | ||||
Pulicaria dysenterica (L.) Bernh. | H | Me | ||||
Reichardia picroides (L.) Roth* | H | Ms | MAP | |||
Rhagadiolus stellatus (L.) Gaertn. | T | Me | ||||
Senecio leucanthemifolius Poir. subsp. leucanthemifolius | T | Ms | ||||
Senecio vulgaris L. | T | C | ||||
Sonchus asper (L.) Hill | T | Ea | EWS | |||
Sonchus oleraceus L.* | T | Ea | EWS | |||
Sonchus tenerrimus L. | T | Ms | EWS | |||
Symphyotrichum squamatum (Spreng.) G.L. Nesom | T/H | Nen | ||||
Taraxacum officinale Weber | H | Cb | EWS | |||
Tragopogon porrifolius L. | H | Me | ||||
Tripolium pannonicum (Jacq.) Dobrocz. | H | Ea | ||||
Urospermum dalechampii (L.) F. W. Schmidt | H | Me | ||||
Urospermum picroides (L.) Scop. ex F.W. Schmidt | T | Me | EWS | |||
Xanthium spinosum L. | T | Ams | ||||
Adoxaceae | ||||||
Sambucus nigra L. | P | Eca | MAP | |||
Viburnum tinus L. subsp. tinus | P | Ms | ||||
Caprifoliaceae | ||||||
Centranthus calcitrapae (L.) Dufr. subsp. calcitrapae | Ch | Ms | ||||
Centranthus ruber (L.) DC. subsp. ruber | Ch | Ms | ||||
Dipsacus fullonum L. | H | Me | ||||
Knautia integrifolia (L.) Bertol. subsp. integrifolia* | T | Me | ||||
Lonicera implexa Aiton subsp. implexa | P | Ms | ||||
Scabiosa columbaria L. | H | Ea | ||||
Sixalis atropurpurea (L.) Greuter et Burdet subsp. grandiflora (Scop.) Sold. et Conti | H | Ms | ||||
Valerianella muricata (Stev. ex M. Bieb.) J.W. Loudon | T | Ms | ||||
Araliaceae | ||||||
Hedera helix L. | P | Meas | ||||
Apiaceae | ||||||
Ammoides pusilla (Brot.) Breistr. | T | Ms | ||||
Daucus carota L. subsp. carota* | H | Cs | ||||
Eryngium campestre L. | H | Me | ||||
Foeniculum vulgare Miller | H | Msd | CRO | |||
Scandix pecten-veneris L.* | T | Cs | ||||
Smyrnium olusatrum L. | H | Ma | EWS | |||
Tordylium apulum L. | T | Ms | ||||
Tordylium officinale L. | T | Mne | ||||
Torilis arvensis (Huds.) Link | T | Cs | ||||
Sub-total | ||||||
Category | CRO | FOD | MAP | CWR | EWS | Total |
Number of taxa | 29 | 29 | 26 | 17 | 10 | 111 |
Rights and permissions
About this article
Cite this article
Perrino, E.V., Ladisa, G. & Calabrese, G. Flora and plant genetic resources of ancient olive groves of Apulia (Southern Italy). Genet Resour Crop Evol 61, 23–53 (2014). https://doi.org/10.1007/s10722-013-0013-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-013-0013-1