Abstract
Nine spring lines of Polish wheat (Triticum polonicum) were compared with Kamut wheat and two common wheat cultivars. Plants were analyzed to determine morphometric parameters (plant height, spike length, spike density, grain weight per spike and single kernel weight) and some chemical properties of grain: content of protein, ash, fat, crude fiber, minerals and mycotoxins. The studied genotypes produced a relatively weak response to infection with Fusarium culmorum which was expressed by a decrease in grain weight per spike and single kernel weight by 13 % and over 6 %, respectively. The grain of T. polonicum was characterized by a significantly higher protein and ash content than the grain of common wheat (by 19.8 and 23.7 %, respectively) and considerably higher concentrations of fat and dietary fiber than Kamut wheat (by 30.2 and 17.4 %, respectively). In comparison with common wheat, the grain of the examined genotypes was significantly more abundant in sulfur, magnesium and potassium as well as zinc, iron, copper and molybdenum. It contained significantly less aluminum and strontium. The inoculation led to a significant increase in fusariotoxin levels in the grain of all studied genotypes, and the average concentrations of the above metabolites were lower in the grain of T. polonicum than in Triticum aestivum. Polish wheat may constitute valuable genetic material for breeding new wheat varieties characterized by a high nutritive value and satisfactory resistance to FHB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Progressive genetic erosion caused by limited genetic diversity of modern crop plants prompts breeders to search for new sources of variation, including in species which are related to the cultivated crops. The tribe Triticeae is economically the most important group in the family Gramineae. It has given rise to cultivated wheat, barley, rye, oats, and a number of important range grasses (Nevo 2011). Common wheat (Triticum aestivum L.) is an allohexaploid composed of genomes A, B and D (Acquaah 2007). The closely related species Triticum spelta L. has a similar genetic structure, and its grain is characterized by a high nutritional value and processing suitability. The above species is also marked by adverse production attributes, in particular low threshing efficiency and high susceptibility to lodging. In the group of tetraploid wheat species, Triticum turgidum L. with genomes A and B is most widely used in the food processing industry. The above species features several subspecies which are characterized by elongated grain with very high threshing efficiency and significant resistance to drought, including Triticum turgidum L., a taxon with the highest processing suitability. In this group there are four taxa, of which durum wheat (Triticum durum Desf.) is of greatest economic importance. Other wheats which attract the interest of producers and breeders include Triticum polonicum L. (Polish wheat) and Triticum turanicum Jakubz. (Oriental wheat). The former was named by Linnaeus (1797), and it incorrectly suggests the taxon’s Polish origin. Kamut® (http://www.kamut.com) belonging to T. turanicum is the most popular wheat cultivar in this taxon, and some authors claim that it is a Polish wheat (Stallknecht et al. 1996). The results of microsatellite genotyping performed by Khlestkina et al. (2006) suggest that Kamut is a hybrid between T. polonicum and T. durum which occurred spontaneously in the Fertile Crescent region. Kamut is mass produced mostly in organic farms in the US, and its grain is used in the production of health foods. Dried and powdered has been changed to supplements (http://www.pureplanet.com/productpage.aspx?itemid=623).
Polish wheat is sporadically grown, and it is of marginal importance on the contemporary grain market. This thermophilous taxon shows high levels of resistance to brown rust, and it is grown in the warm and mild climate of southern Spain, in particular in Andalusia (Rodríguez-Quijano et al. 2003), as well as in Italy, western Ukraine, warm regions of Asia, Algeria and Ethiopia (Eticha et al. 2006; http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?406898). Its spikes, with or without awns, are characterized by very large glumes which reach 4 cm in length (Wang et al. 2002). Grain is not susceptible to shattering, kernels are elongated and plump, and thousand kernel weight reaches 80 g. Despite its considerable resistance to drought and fungal pathogens, the above wheat is susceptible to lodging, and at the full-ripe stage, shanks become brittle, leading to spike shedding and considerable crop loss (Eticha et al. 2006).
Despite differences in the number of chromosomes, the crossing of T. polonicum with T. aestivum and T. spelta and selected species of the genus Aegilops L. can produce fertile and genetically stable hybrids, although hybrid necrosis is sometimes observed (Dorofeev 1987; Mizuno et al. 2010). In view of the desirable attributes of T. polonicum, the International Maize and Wheat Improvement Center (CIMMYT, Mexico) initiated a program for crossing this wheat with selected species of the genus Aegilops, which produced a variety with record-breaking yield potential of up to 18 t ha−1. However, the discussed hybrid has extremely high fertilizer requirements which makes it a very costly and environmentally unsustainable crop (Holden 1998). Despite the above, similarly to Kamut, Polish wheat constitutes valuable material for breeding highly productive wheat varieties characterized by well-filled grain (Rajaram and Dubin 1999). According to Watanabe (2004), T. polonicum IC12196 may be an alternative source of semi-dwarfing genes at the Rht-B1 locus, and it may have potential for the development of durum wheat cultivars.
Kamut is similar to T. durum with regard to nutritive value and processing suitability, and it contains much higher levels of selenium and yellow pigments than T. spelta and Triticum dicoccon Schrank (Piergiovanni et al. 2009). The results of a study carried out by Benedetti et al. (2012) indicate that bread made from whole-grain Kamut khorasan protects rats from oxidative stress more effectively than bread made from whole-grain durum wheat. According to Quinn (1999), products made from Kamut grain are safe for consumers who are allergic to common wheat gluten, but the above observation was not confirmed by other authors (Simonato et al. 2002; Colomba and Gregorini 2012). The quality of Polish wheat grain remains poorly investigated. The existing research focused on the structure of gluten proteins, in particular the content of high molecular weight glutenin subunits (HMWGs), and the mechanism of inheritance for this trait (Caballero et al. 2008; Pan et al. 2007; Sissons and Batey 2003). The grain of T. polonicum contains starch with up to 40 % amylose content (Rodríguez-Quijano et al. 2003). This is a desirable trait because foods where the amylose to amylopectin ratio spans a wide range of values have a higher nutritive value and are more biodegradable. According to Wijaya and Maresa (2012) T. polonicum contains high levels of apigenin di-c-glycosides which are found in greater abundance only in common wheat and spelt. Apigenin di-c-glycosides have anti-inflammatory, antibiotic and anticarcinogenic properties. Marotti et al. (2011) observed that Kamut wheat, similarly to other tetraploid wheat taxa, is a rich source of soluble dietary fiber (SDF) and a good substrate for in vitro cultures of Bifidobacterium pseudocatenulatum and Lactobacillus plantarum. According to the above authors, wheat-based prebiotics can be used in the production of synbiotics.
There is scant data illustrating the response of T. polonicum to infections caused by pathogenic Fusarium fungi which cause Fusarium head blight. Mycotoxins produced by those pathogens lead to various metabolic disorders in humans and animals, and high mycotoxin concentrations in grain cause mycotoxicoses (Richard 2007). Oliver et al. (2008) analyzed the responses of 376 accession lines of T. turgidum tetraploid wheat, Persian wheat (T. carthlicum Nevski), cultivated emmer wheat (T. dicoccon), Polish wheat (T. polonicum), Oriental wheat (T. turanicum) and Poulard wheat (T. turgidum). In the above study, 16 accessions of T. carthlicum and four accessions T. dicoccon showed high or satisfactory levels of resistance to FHB. Durum wheat, the most popular tetraploid wheat which is closely related to Polish wheat, is highly susceptible to spike and grain infections (Stack et al. 2002; Wiwart et al. 2011).
The aim of this study was to determine whether T. polonicum constitutes valuable material for breeding new wheat varieties characterized by a high nutritive value and satisfactory resistance to FHB.
Materials and methods
Field experiment
The field experiment was established in the Experimental Center in Bałcyny near Ostróda (53°36′N; 19°51′E) (Poland) on soils of a good wheat complex. The experimental material comprised nine spring genotypes of Polish wheat (T. polonicum) reproduced locally as a selection of wheat accessions obtained from the National Center for Plant Genetic Resources in Radzików (NCPGR) (Table 1). The studied lines were compared against Kamut® wheat (T. turanicum) grown from seeds obtained from the US, purchased in a supermarket in Austria, and two T. aestivum cultivars, Frontana and Parabola. Frontana is a Brazilian cultivar which is characterized by significant resistance to FHB (Steiner et al. 2004). It was reproduced from seeds supplied by the Institute of Plant Genetics of the Polish Academy of Sciences in Poznań, Poland. Parabola, a cultivar marked by high processing suitability, is recommended by the Research Center for Cultivar Testing in Poland as a reference standard for comparisons with wheat taxa other than T. aestivum and T. durum ( http://www.coboru.pl/English/index_eng.aspx; List of Agriculture Cultivars 2008). Parabola grain was supplied by Nasiona Kobierzyc (Kobierzyce, Poland), a conservation breeding farm. The experiment was performed in a randomized block design with three replications. Plot area was 6 m2. Sowing density was 220 germinating kernels per m2 for T. polonicum and Kamut, and 360 kernels per m2 for common wheat. NPK fertilizer was applied at a pre-sowing rate of 40-25-80 kg ha−1. Seeds were not dressed, and chemical weed control was not applied during the growing season.
Spikes were inoculated by spraying experimental plots (100 mL inoculum per 1 m2) at the flowering stage (BBCH 65 according to Witzenberger et al. 1989) twice, at two-day intervals, with a spore suspension of F. culmorum at the concentration of 500,000 spores per cm3. Wheat plants from untreated plots served as control. The height of wheat plants was measured prior to harvest (30 plants from each plot). At the full ripe stage (BBCH 89), 30 spikes were harvested manually from each plot for morphometric measurements (spike length, spike density, number and weight of grains per spike, single kernel weight).
Analysis of the basic nutrient content of grain
Grain samples were milled using a Cyclotec 1093 sample Mill (FOSS, Denmark).
Crude protein content (N × 5.7) (Hoseney 1994) was determined in two replications, using the Bűchi system (K-424 Digestion Unit and B-324 Distillation Unit, Switzerland). Crude fat was extracted by the Soxhlet method (Bűchi Extraction System B-811, Switzerland) (solvent: diethyl ether (POCh Gliwice, Poland), extractor size 100 mL, 2.5 g analytical samples of air-dried ground grain). Extraction was carried out at the temperature of 60 °C for 4 h, in two replications. After ether evaporation, solvent caps containing crude fat were dried for 2 h at 105 °C in the exsiccator and weighed.
Crude fiber content was determined using the Fibertec 2010 system (FOSS, Denmark) and the Weende method. Ground samples of 2 g were placed in FOSS crucibles with P2 porosity (40–100 μm). The samples were placed in a hot extraction unit, immersed in 1.25 % H2SO4 (POCh Gliwice, Poland) and boiled for 40 min. Sulfuric acid was removed, the samples were rinsed three times with hot demineralized water, placed in a cold extraction unit and rinsed with acetone (POCh Gliwice, Poland). The samples were dried at 105 °C for 3 h, and the amount of fiber was determined in a quantitative analysis.
Determination of elements in grain
The mineral content of grain was determined using the method described by Suchowilska et al. (2012). An Anton Paar High Pressure Asher (HPA) equipped with seven 30 mL quartz vessels was used. Wheat samples of 500 mg ± 5 mg (whole grain) were weighed into the quartz vessels. After the addition of 2 mL HNO3, the vessels were sealed with Teflon tape and quartz glass disks, they were placed in a digestion unit and pressurized with nitrogen at 100 bar. The vessels were heated up to 110 °C in 15 min, after which the temperature was rapidly increased to 260 °C and maintained for 95 min. After cooling to approximately 30 °C, clear digestion solutions were obtained. The solutions were transferred to pre-weighed 100 mL bottles and diluted with ultra-pure water to ~75 mL. After the addition of 1 mL internal standard (IS) solution containing Ge and Tl at 1 mg L−1 each in 0.5 % HNO3 (v/v), the samples were filled up with ultra-pure water to 100 g ± 0.1 g. To determine the content of macroelements (P, S, Mg and K), a 1:250 dilution of the samples was additionally prepared by diluting 200 μL of the sample solution spiked with 0.5 mL of the above IS solution with 0.5 % HNO3 (v/v) to 50 g. All sample solutions were stored at 6 °C until ICP-SFMS analysis.
ICP-SFMS measurements were performed using double-focusing ICP-sector field MS Finnigan ELEMENT 2 (Software 2.42, Thermo Electron Corporation, Bremen, Germany) equipped with a CETAC ASX-520 autosampler (CETAC Technologies, Omaha, NE, USA). The instrument was equipped with a cyclonic spray chamber (Jacketed Cinnabar Cyclonic, 20 mL, from Glass Expansion, West Melbourne, Australia) and a micro-flow nebulizer made of PFA (MicroFlow Nebuliser PFA-ST from Elemental Scientific Inc., Omaha, NE, USA) connected to a 700 μL min−1 self-aspiration capillary (0.5 mm inner diameter) (both from AHF Analysentechnik, Tübingen, Germany). Argon (Ar 4.6, 99.996 % from Messer Austria GmbH) cool gas flow was 16 L min−1, auxiliary (plasma) gas and sample (nebulizer) gas flows were optimized daily before each measurement series to obtain the maximum signal intensity, the former typically between 0.75 and 0.90 L min−1, the latter in the range of 0.85–0.95 L min−1. RF power was between 1,185 W and 1,195 W. The following isotopes were measured in low-resolution mode, Rs = 300, 10 % valley definition (LRM): 11B, 85Rb, 88Sr, 97Mo and 208Pb. 23Na, 24Mg, 27Al, 31P, 32S, 44Ca, 55Mn, 56Fe, 63Cu, 66Zn, 138Ba and 111Cd were determined in medium-resolution mode of Rs = 4,000 (MRM), whereas 39K was measured in high-resolution mode of Rs = 10,000 (HRM). In order to compensate for drifts in signal intensity during measurement sequences, 72Ge and 205Tl were used as internal standards. Generally, 25 scans with 0.02 s per peak were measured. A quantitative analysis of the samples was performed by external calibration. For that purpose, multi-element standard solutions in 0.5 % HNO3 (v/v) were prepared at four concentration levels for all elements.
Determination of mycotoxins in grain
Mycotoxin concentrations in grain were determined according to the method described by Suchowilska et al. (2010).
Mycotoxin standards were supplied by Biopure Referenzsubstanzen GmbH (Tulln, Austria) and Sigma (Vienna Austria), and were dissolved in acetonitrile/water 1:1, v/v. Enniatin standard mixtures were provided by Marika Jestoi of EVIRA (Helsinki, Finland).
Grain samples of 10 g were ground to fine powder in a laboratory mill (Cullati MFC, Switzerland). 0.5 g of ground sub-samples was extracted with 2 mL of the solvent mixture (acetonitrile/water/acetic acid 79:20:1, v/v/v). Acetonitrile (LC gradient grade) was supplied by J. T. Baker (Deventer, The Netherlands) and glacial acetic acid (p.a.) by Sigma–Aldrich (Vienna, Austria). Water was purified successively by reverse osmosis using the Milli-Q plus system from Millipore (Molsheim, France). The samples were extracted for 90 min using a GFL 3017 rotary shaker (GFL, Burgwedel, Germany) and centrifuged for 2 min at 3,000 rpm (radius 15 cm) in a GS-6 centrifuge (Beckman Coulter Inc., Fullerton, CA). After centrifugation, the extracts were transferred to glass vials using Pasteur pipettes. 350 μL aliquots were diluted with the same volume of the dilution mixture (acetonitrile/water/acetic acid 20:79:1, v/v/v) and were directly injected into the LC–MS/MS instrument. For the determination of DON concentrations higher than 500 μg L−1 (corresponding to 4 mg kg−1 in samples), the raw extract was diluted 1 + 49 (v + v).
Chromatographic separation was performed at 25 °C on an 1,100 Series HPLC System (Agilent, Waldbronn, Germany) equipped with a C18 4 × 3-mm-i.d. security guard cartridge and a Gemini® C18 column, 150 × 4.6-mm i.d., 5-μm particle size (Phenomenex, Torrance, CA, USA). Detection and quantification was performed in Selected Reaction Monitoring (SRM) mode using the QTrap 4000 LC–MS/MS System (Applied Biosystems, Foster City, CA) equipped with a TurboIonSpray electrospray ionization (ESI) source.
For quantification, external calibration was performed using multi-analyte standards prepared and diluted in a 1:1 mixture of extraction and dilution solvent. The results were adjusted for apparent recoveries, which were determined by analyzing a blank wheat sample that had been spiked at one concentration level in triplicate. The relevant values of analytes in the investigated Triticum samples were generally in the range of 100 ± 10 %, with the exception of DON-3-glucoside (D3G, 47 %), nivalenol (NIV, 82 %), alternariol (AOH, 69 %), alternariol monomethylether (AME, 69 %) and β–zearalenol (β–ZOL, 76 %).
Statistical analysis
The results of all analyses were processed statistically using STATISTICA software (Statsoft Inc. 2008). The significance of differences between means was estimated by analysis of variance, and mean values were compared by the Student–Newman–Keuls (SNK) test at p < 0.01. The data were subjected to a principal component analysis (PCA) performed for three sets of traits i.e. for plant height, spike length and spike density, for the content of crude protein, fat, ash and dietary fiber, and for microelement concentrations in grain.
Results
Weather conditions
The weather conditions in the experimental year supported the growth and development of T. polonicum, while they were less conducive to the development of toxin-producing Fusarium species which cause Fusarium head blight. With the exception of April, the temperatures reported during the study exceeded the multi-year average for 1961–2005. In May, when wheat plants reached the BBCH 21-29 stage, the average temperature was consistent with the long-term average (12.2 °C), and in between stages BBCH 51 (beginning of heading) and BBCH 71 (watery ripe), air temperatures insignificantly exceeded the multi-year average (by 0.9–1.0 °C). In 2008, in all months of the growing season, precipitation levels were below the multi-year average, in particularly at the turn of June and July when plants entered stage BBCH 51 (total precipitation in June and July reached 27 and 46 mm, respectively, in comparison with the multi-year average of 68 and 80 mm for the respective months).
Results of biometric measurements
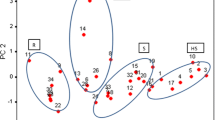
The average height of wheat plants of all T. polonicum lines was 109.8 cm. This result differed considerably from common wheat (88.8 cm), and from Kamut wheat (89.3 cm) (Table 2). Plant height did not exceed 100 cm only in two lines (P-4 and P-6), indicating that the studied genotypes of Polish wheat are susceptible to lodging. The average length and density of T. polonicum spikes did not differ significantly from the values observed in common wheat, yet the differences between lines were considerable (RSD 20.6 and 15.1 %, respectively). The shortest spikes were observed in Kamut plants (5.98 cm), and the most dense spikes were reported in the line P-4 (22.7 spikelets per 10 cm of rachis). The results of PCA performed for the above three traits indicate that the P-4 line was most similar to both T. aestivum cultivars and Kamut wheat (Fig. 1).
Grain weight per spike is the key yield-forming trait of cereal crops. In control plants which were not subjected to spike inoculation, the highest grain weight per spike was reported in ‘Frontana’ (2.17 g) and Kamut wheat (1.90 g) (Table 3). The analyzed trait was characterized by similar and relatively high values (1.35–1.78 g) in all T. polonicum lines which exceeded the results noted in the high-yielding ‘Parabola’ (1.31 g). Spike inoculation with F. culmorum led to a highly significant drop in grain weight per spike which reached 13.1 % on average in all examined lines. Lines P-5 and P-3 showed the weakest response to inoculation (0.4 and 1.5 %, respectively), and the resulting decrease in grain weight per spike was even lower than that noted in the FHB-resistant cv. Frontana. The highest grain plumpness was observed in Kamut wheat (kernel weight of 73.6 mg), and the analyzed trait varied considerably across the studied genotypes of Polish wheat (relative standard deviation was equal to 65 %). Although spike inoculation reduced the weight of T. polonicum kernels by an average of 4.8 %, inoculated spikes of five T. polonicum genotypes produced plumper grain that non-inoculated spikes, and the difference ranged from 0.7 % (P-5) to 20.1 % (P-7). The above suggests that unlike in Kamut wheat and common wheat, the inoculation of T. polonicum spikes leads mainly to a drop in the number of grains per spike.
Basic nutrient content of grain
The control grain of T. polonicum was characterized by significantly higher average concentrations of protein (16.61 %) and ash (2.14 %) than common wheat (13.87 and 1.73 %, respectively) (Table 4). The average dietary fiber content of T. aestivum was 17 % higher and crude fat content was 7 % higher in comparison with the studied tetraploid wheat genotypes. PCA results illustrate the characteristic nutrient profiles of the examined genotypes (Fig. 2). Kamut wheat showed significant similarity to P-6, P-1, P-7 and P-8 lines. An analysis of factor loading values for PC1 and PC2 indicates that Kamut wheat had lower ash and fat content and higher protein content than T. polonicum. Both PCs explained 83.05 % total variance in the analyzed traits. Inoculation had no significant influence on the content of crude fat, fiber and ash in the grain of tetraploid wheat, but it contributed to a considerable increase in protein concentrations (by an average of 6 % in comparison with control). A significant increase in ash levels and a minor increase in the content of the remaining nutrients was noted in common wheat.
Macro- and microelements content of grain
Higher ash levels in the grain of T. polonicum than T. aestivum can be generally attributed to high concentrations of four macroelements: phosphorus, magnesium, sulfur and potassium (Table 5). The content of the first three elements differed significantly between Polish wheat and common wheat, and the noted difference in sulfur levels was as high as 22 %. The grain of T. aestivum contained 34 % more calcium in comparison with T. polonicum. Polish wheat was more abundant in zinc (by 49 %), iron (by 38 %), molybdenum (by 98 %), copper (by 10 %) and boron (by 8 %) than common wheat, and the differences in the concentrations of the first three elements were statistically significant (Table 6). On average, the studied lines of T. polonicum contained less potentially harmful elements such as aluminum (by 54 %), strontium (by 52 %), manganese (by 28 %), sodium (by 4 %) and rubidium (by 6 %) than T. aestivum. The inoculation of T. polonicum spikes increased calcium (by 39 %), magnesium (by 17 %) and potassium (by 12 %) levels, and it decreased sulfur and phosphorus concentrations (by 12 and 1 %, respectively) (Table 5). The elemental composition of Polish wheat was similar to both common wheat cultivars, but it differed from Kamut wheat. Inoculation increased the concentrations of most microelements in the studied grain (strontium—by 300 %, aluminum—by 174 % and molybdenum—by 7 %) (Table 6). Control grain contained more rubidium (by 27 %), boron (by 14 %) and iron (by 2 %). A similar trend was observed in both common wheat cultivars. PCA results point to completely different micronutrient profiles in the grain of control spikes, inoculated spikes, common wheat and tetraploid wheats (Fig. 3).
Mycotoxin concentrations in grain
The presence of 12 metabolites was observed in the grain of control spikes (Table 7). In comparison with common wheat, the grain of Polish wheat genotypes contained 1.7 times less DON, 5.2 times less DON-3-glucoside and 14 % less tentoxin. Selected lines (excluding P-9) and Kamut wheat contained high levels of NIV (from 34.8 in line P-5 to 828.2 μg kg−1 in Kamut), whereas no traces of the above toxin were noted in common wheat. Spike inoculation completely altered the toxin profile of wheat grain (Table 8). DON concentrations in T. polonicum were approximately 10 % lower in comparison with common wheat. More importantly, the grain of P-5 and P-8 lines contained significantly less DON than the FHB-resistant ‘Frontana’ (1.8 and 1.7 times less, respectively). A similar trend was reported in the concentrations of DON-3-glucoside. The smallest drop in grain weight after inoculation in the P-5 line (0.4 %), indicate that this genotype possesses mechanisms which may condition resistance to FHB. With the exception of Parabola, inoculation considerably increased NIV levels in grain. Infected grain also contained MON, a metabolite produced by Fusarium avenaceum (Fr.) Sacc. and Fusarium tricinctum (Corda) Sacc. Inoculation increased furthermore the concentrations of cyclodepsipeptides which are synthesized by the above Fusarium species. Common wheat grain contained the lowest levels of ZEA and ZEA-sulfate on average, and the above toxins were also observed in very low concentrations in the P-9 line. Type A trichothecenes (T-2, HT-2, 15-MAS) were noted in the grain of six T. polonicum genotypes, and they were most abundant in the P-1 line.
Discussion
This study discusses the results of morphometric measurements and chemical analyses of the grain of nine T. polonicum lines, Kamut wheat and two common wheat cultivars whose spikes were artificially inoculated with F. culmorum. The obtained results are difficult to interpret due to the scarcity of published information about Polish wheat and a general absence of studies investigating the morphometric features and processing suitability of T. polonicum. Kang et al. (2012) observed that Polish wheat could be the source of a recessive dwarfing gene, Rht-dp, which is an allele of the Rht-B1b gene. The above authors had previously produced hexaploid hybrids between a dwarf form of T. polonicum and Aegilops tauschii (Kang et al. 2008), which indicates that this source of the dwarfing gene can be applied in the breeding of common wheat and durum wheat. Polish wheat is generally characterized by long and frail culms which are susceptible to lodging. Our findings confirm this observation. In a study by Angioloni and Colar (2011), the protein, fat and ash content of whole grain flour made of Kamut wheat (17.8, 1.36 and 1.53 %, respectively) correspond with the results of this experiment. Despite high kernel weight (37.8–73.6 mg), the grain of the analyzed tetraploid wheat genotypes contained significantly more protein and ash than common wheat. Macro- and microelements are found mainly in the external regions of the kernel, namely the seed coat and the aleurone layer, which form the bran fraction after milling. Common wheat bran contains approximately 50 % of the ash found in the kernel, and 40 % is localized in the germ (Piironen et al. 2009). Plump grains have a smaller area to volume ratio than fine grains, therefore, the noted results could indicate that minerals are differently distributed in kernel of tetraploid wheat than in kernel of T. aestivum. Macroelement levels in the grain of the studied T. polonicum lines were highly similar to T. dicoccon which also contains more phosphorus, sulfur and magnesium and less calcium than common wheat (Suchowilska et al. 2012; Piergiovanni et al. 1997, 2009). The studied Polish wheat genotypes were also characterized by higher Zn, Fe, Cu, Mn and Mo concentrations, which contributed to their nutritional value and greater health benefits. Aluminum levels, which are correlated with the risk of Alzheimer’s disease (Rogers and Simon 1999), are twice lower in T. polonicum in comparison with common wheat. The global spread of micronutrient malnutrition, also known as “hidden hunger”, contributes to the popularity of agronomic biofortification of wheat, in particular zinc supplementation (Zou et al. 2012), as well as genetic biofortification which creates completely new challenges for breeders (Ruel and Buis 1998; Cakmak 2010). The last 160 years witnessed an unprecedented increase in wheat yield which was accompanied by an equally rapid decrease in the microelement content of grain (Fan et al. 2008). Breeders search for new sources which deliver high levels of minerals. The results of our study indicate that T. polonicum could potentially be such a source.
As expected, inoculation decreased the weight of grains per spike in all analyzed genotypes, and it completely altered qualitative and quantitative mycotoxin profiles of the studied grain. The observed DON-3-glucoside (D3G) levels are particularly interesting. High concentrations of this metabolite in grain may be the effect of resistance to DON, one of the components of complex resistance to FHB (Lemmens et al. 2005). Plant resistance to DON may result from their ability to inhibit the synthesis of this toxin or to detoxify it. D3G undergoes a number of transformations, but those changes have not yet been fully researched. When masked in glycoside form, DON is still dangerous for consumers because D3G hydrolysis in the gastrointestinal tract of animals and humans may lead to the release of DON (Berthiller et al. 2005). The narrowest DON/D3G ratio was observed in the grain of ‘Frontana’ (1.92), and the widest—in the P-1 line and Kamut wheat (7.91 and 6.99, respectively). In infected grain of T. polonicum, the DON/D3G ratio reached 6.32 on average. DON concentrations are generally lower in the studied lines of Polish wheat than in T. aestivum, therefore the noted results testify to a satisfactory nutritive value of T. polonicum grain. In an experiment investigating the responses of a very large group of T. turgidum genotypes, Oliver et al. (2008) observed that the response of 11 accession lines of T. polonicum to Fusarium graminearum Schwabe infections was weaker than that of the susceptible common wheat cv. Russ, but significantly stronger than that noted in the resistant cultivar Sumai 3. In our experiment, inoculation with F. culmorum increased NIV concentrations nearly four-fold. Interestingly, the applied isolate was a DON-producing chemotype, and the above was determined before the experiment. F. culmorum produces two chemotypes: the NIV chemotype, which includes isolates producing nivalenol and fusarenone X, and the DON chemotype, which includes isolates producing DON and acetyl derivatives of this toxin (Wagacha and Muthomi 2005). Cyclodepsipeptide concentrations increased more than three-fold, and we also observed the presence of MON, T-2 toxin and 15-MAS, metabolites which are not produced by F. culmorum but are released by other species that cause FHB, including Fusarium sporotrichioides Sherbakoff, Fusarium verticillioides (Saccardo) Nirenberg, Fusarium poae (Peck) Wollenweber, F. avenaceum, and F. tricinctum (Bottalico 1998; Kokkonen et al. 2010). The above could imply that inoculation with a single pathogen species contributes to spike colonization by other toxin-producing species. It seems that naturally occurring isolates of the above species are characterized by weak pathogenicity, and they are unable to cause a primary infection.
Grain from inoculated spikes of T. polonicum genotypes was characterized by a higher protein and ash content in comparison with control. Spanic et al. (2012) investigated the response of 24 common wheat cultivars to inoculation with F. culmorum to observe that the protein content of inoculated grain was 9 % higher on average than that of control grain (14.2 vs. 12.9 %). According to Siuda et al. (2010), wheat grain infected in a medium degree contained 15 % more protein on average than healthy kernels. The effect of Fusarium infections on mineral concentrations in wheat grain has not been documented in literature, therefore, the results of our experiment cannot be compared against a reliable standard. An increase in ash levels and the concentrations of eight out of the 11 analyzed microelements could result from an relative increase in the weight of the fruit-seed coat and the aleurone layer in the kernel. The above could be attributed to significant damage of the starch endosperm and changes in the structure of kernel tissues induced by amylolytic and proteolytic enzymes which are produced by the pathogen (Matthäus et al. 2004; Jackowiak et al. 2005).
In the group of the examined genotypes of T. polonicum, the most positive results were reported for the line P-5. This line responded to inoculation with the smallest drop in grain weight, and it also accumulated the lowest amounts of DON and 3-Ac DON (46 % less than the resistant ‘Frontana’). The concentrations of type A trichothecenes (HT-2, T-2 and 15-MAS) did not exceed LOD values. The remaining physical characteristics of the P-5 line were also satisfactory, indicating that this genotype can be successfully used to breed plants of high nutritive value and increased resistance to FHB.
The variation within the nine lines analyzed in the study represents only part of the diversity within T. polonicum. Nevertheless, the obtained results suggest that T. polonicum could be a valuable gene source for breeding new wheat cultivars characterized by a high nutritive value and increased resistance to pathogens causing FHB.
Abbreviations
- DON:
-
Deoxynivalenol
- D3G:
-
DON-3-Glucoside
- NIV:
-
Nivalenol
- ZEA:
-
Zearalenone
- AME:
-
Alternariol monomethyl ether
- α-ZOL:
-
Alpha-zearalenol
- β-ZOL:
-
Beta-zearalenol
- AOH:
-
Alternariol
- MON:
-
Moniliformin
- 15-MAS:
-
15-Monoacetoxyscirpenol
References
Acquaah G (ed) (2007). Breeding wheat. In: Principles of plant genetics and breeding. Blackwell Publishing, USA, pp 471–484
Angioloni A, Collar C (2011) Nutritional and functional added value of oat, Kamut®, spelt, rye and buckwheat versus common wheat in breadmaking. J Agric Food Chem 91:1283–1292
Benedetti S, Primiterra M, Tagliamonte MC, Carnevali A, Gianotti A, Bordoni A, Canestrari F (2012) Counteraction of oxidative damage in the rat liver by an ancient grain (Kamut brand khorasan wheat). Nutrition 28(4):436–441
Berthiller F, Dall’Asta C, Schumacher R, Lemmens M, Adam G, Krska R (2005) Masked mycotoxins: determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography–tandem mass spectrometry. J Agric Food Chem 53:3421–3425
Bottalico A (1998) Fusarium diseases of cereals: species complex and related mycotoxin profiles in Europe. J Plant Path 80(2):85–103
Caballero L, Martín LM, Alvarez JB (2008) Variation of high molecular weight glutenin subunits in two neglected tetraploid wheat subspecies. Czech J Genet Plant Breed 44(4):140–146
Cakmak I (2010) Biofortification of cereals with zinc and iron through fertilization strategy. In: 19th world congress of soil science, soil solutions for a changing world, Brisbane, 1–6 August 2010, pp 4–6
Colomba MS, Gregorini A (2012) Are ancient durum wheats less toxic to celiac patients? A study of α-Gliadin from Graziella Ra and Kamut. Sci World J 2012(837416). doi:10.1100/2012/837416
Dorofeev VF (1987) Pšenica polonicum (T. polonicum L.). In: Dorofeev VF (ed) Pšenicy mira: Vidovoj sostav, dostiženiâ selekcii, sovremennye problemy i ishodnyj material, 2nd edn. Leningrad, Kolos, pp 48–50
Eticha F, Belay G, Bekele E (2006) Species diversity in wheat landrace populations from two regions of Ethiopia. Genet Resour Crop Evol 53:387–393
Fan MS, Zhao FJ, Fairweather-Tait SJ, Poulton PR, Dunham SJ, McGrath SP (2008) Evidence of decreasing mineral density in wheat grain over the last 160 years. J Trace Elem Med Bio 22(4):315–324
Hammer K, Filatenko AA, Pistrick K (2011) Taxonomic remarks on Triticum L. and × Triticosecale Wittm. Genet Resour Crop Evol 58:3–10
Holden C (1998) Wonder wheat. Science 280(5363):527
Hoseney RC (1994) Principles of cereal science and technology. American Association of Cereal Chemists (ed) Inc., St Paul, MN, pp 65–79, 87–93
Jackowiak H, Packa D, Wiwart M, Perkowski J (2005) Scanning electron microscopy of Fusarium damaged kernels of spring wheat. Int J Food Microbiol 98(2):113–123
Kang H-Y, Lin L-J, Song Z-J, Yuan J-Y, Zhong M-Y, Zhang H-Q, Fan X, Sha L-N, Wang Y, Xu L–L, Zeng J, Zhou Y-H (2012) Identification, fine mapping and characterization of Rht-dp, a recessive wheat dwarfing (reduced height) gene derived from Triticum polonicum. Genes Genom. doi:10.1007/s13258-012-0022-z
Kang H-Y, Wang Y, Yuan H-J, Jiang J, Zhou Y-H (2008) A new synthetized 6x-wheats, derived from dwarfing Polish wheat (Triticum polonicum L.) and Aegilops tauschii Cosson. Int J Agr Res 3(4):252–260
Khlestkina EK, Röder MS, Grausgruber H, Börner A (2006) A DNA fingerprinting-based taxonomic allocation of Kamut wheat. Plant Genet Res Characterization Util 4(3):172–180
Kokkonen M, Ojala L, Parikka P, Jestoi M (2010) Mycotoxin production of selected Fusarium species at different culture conditions. Int J Food Microbiol 143:17–25
Lemmens M, Scholz U, Berthiller F, Dall’Asta C, Koutnik A, Schuhmacher R, Adam G, Buerstmayr H, Mesterházy Á, Krska R, Ruckenbauer P (2005) The ability to detoxify the mycotoxins deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol Plant Microbe Interact 18:1318–1324
Linnaeus C (1797) Species plantarum: exhibentes plantas rite cognitas, ad genera relatas, cum differentiis specificis, nominibus trivialibus, synonymis selectis, locis natalibus, secundum systema sexuale digestas. Impensis G. C. Nauk, Berolini, p 478
List of Agriculture Cultivars (2008) Research center for cultivar testing. Słupia Wielka, Poland
Marotti I, Bregola V, Aloisio I, Di Gioia D, Bosi S, Di Silvestro R, Quinn R, Dinelli G (2011) Prebiotic effect of soluble fibres from modern and old durum-type wheat varieties on Lactobacillus and Bifidobacterium strains. J Sci Food Agric 92:2133–2140
Matthäus K, Dänicke S, Vahjen W, Simon O, Wang J, Valenta H, Meyer K, Strumpf A, Ziesenib H, Flachowsky G (2004) Progression of mycotoxin and nutrient concentrations in wheat after inoculation with Fusarium culmorum. Arch Anim Nutr 58(1):19–35
Mizuno N, Hosogi N, Park P, Takumi S (2010) Hypersensitive response-like reaction is associated with hybrid necrosis in interspecific crosses between tetraploid wheat and Aegilops tauschii Coss. PLoS ONE 5(6):e11326
Nevo E (2011) Triticum. In: Kole C (ed) Wild crop relatives: genomic and breeding resources. Cereals. Springer, Heidelberg, pp 407–456
Oliver RE, Cai X, Friesen TL, Halley S, Stack RW, Xu SS (2008) Evaluation of Fusarium head blight resistance in tetraploid wheat (Triticum turgidum L.). Crop Sci 48:213–222
Pan D, Hong L, Wei L, Peng-Fei Q, Yu-Ming W, Zheng YL (2007) Genetic diversity of storage protein in Triticum polonicum L. J Plant Sci 2(4):416–424
Piergiovanni AR, Simeone R, Pasqualone A (2009) Composition of whole and refined meals of Kamut under southern Italian conditions. Chem Eng Trans 17:891–896
Piergiovanni AR, Rizzi R, Pannacciulli E, Della Gatta C (1997) Mineral composition in hulled wheat grains: a comparison between emmer (Triticum dicoccon Schrank) and spelt (T. spelta L.) accessions. Int J Food Sci Nutr 4(6):381–386
Piironen V, Lampi A, Ekholm P, Salmenkallio-Marttila M, Liukkonen K (2009) Micronutrients and phytochemcials in wheat grain. In: Khan PK, Shewry P (eds) Wheat: chemistry and technology, 4th edn. AACC International, St Paul, pp 179–222
Quinn RM (1999) Kamut®: ancient grain, new cereal. In: Janick J (ed) Perspectives on new crops and new uses. ASHS Press, Alexandria, pp 182–183
Rajaram S, Dubin HJ (1999) Can yield potential of wheat be increased? In: CIMMYT. 1999. The tenth regional wheat workshop for Eastern, Central and Southern Africa. Addis Ababa, CIMMYT, pp 1–7
Richard JL (2007) Some major mycotoxins and their mycotoxicoses: an overview. Int J Food Microbiol 119:3–10
Rodríguez-Quijano M, Lucas R, Carrillo JM (2003) Waxy proteins and amylose content in tetraploid wheats Triticum dicoccum Schübl., Triticum durum L. and Triticum polonicum L. Euphytica 134:97–101
Rogers MAM, Simon DG (1999) Aluminium intake and risk of Alzheimer’s disease. Age Ageing 28(2):205–209
Ruel MT, Buis HE (1998) Plant breeding: a long-term strategy for the control of zinc deficiency in vulnerable populations. Am J Clin Nutr 68(suppl):488–494
Simonato B, Pasini G, Giannattasio M, Curioni A (2002) Allergenic potential of Kamut® wheat. Allergy 57:653–654
Sissons MJ, Batey IL (2003) Protein and starch properties of some tetraploid wheats. Cereal Chem 80(4):468–475
Siuda R, Grabowski A, Lenc L, Ralcewicz M, Spychaj-Fabisiak E (2010) Influence of the degree of fusariosis on technological traits of wheat grain. Int J Food Sci Technol 45(2):2596–2604
Spanic V, Drezner G, Horvat D (2012) Changes of agronomic and quality traits in Fusarium-inoculated wheat genotypes. Croat J Food Technol Biotechnol Nutr 7(1–2):85–89
Stack RW, Elias EM, Mitchell Fetch J, Miller JD, Joppa LR (2002) Fusarium head blight reaction of Langdon durum-Triticum dicoccoides chromosome substitution lines. Crop Sci 42:637–642
Stallknecht GF, Gilbertson KM, Ranney JE (1996) Alternative wheat cereals as food grains: Einkorn, emmer, spelt, kamut, and triticale. In: Janick J (ed) Progress in new crops. ASHS Press, Alexandria, pp 156–170
Steiner B, Lemmens M, Griesser M, Scholz U, Schondelmaier J, Buerstmayr H (2004) Molecular mapping of resistance to Fusarium head blight in the spring wheat cultivar Frontana. Theor Appl Genet 109(1):215–224
Suchowilska E, Wiwart M, Kandler W, Krska R (2012) A comparison of macro- and microelement concentrations in the whole grain of four Triticum species. Plant Soil Env 58(3):141–147
Suchowilska E, Kandler W, Sulyok M, Wiwart M, Krska R (2010) Mycotoxin profiles in the grain of Triticum monococcum, Triticum dicoccum and Triticum spelta after head infection with Fusarium culmorum. J Sci Food Agric 90:556–565
Wagacha JM, Muthomi JW (2005) Fusarium culmorum: infection process, mechanisms of mycotoxin production and their role in pathogenesis in wheat. Crop Prot 26(7):877–885
Wang H-J, Huang X-Q, Röder MS, Börner A (2002) Genetic mapping of loci determining long glumes in the genus Triticum. Euphytica 123:287–293
Watanabe N (2004) Triticum polonicum IC12196: a possible alternative source of GA3-insensitive semi-dwarfism. Cereal Res Commun 32:429–434
Wijaya GY, Maresa DJ (2012) Apigenin di-C-glycosides (ACG) content and composition in grains of bread wheat (Triticum aestivum) and related species. J Cereal Sci. doi:10.1016/j.jcs.2012.06.007
Witzenberger A, van den Hack H, Boom T (1989) Erläuterungen zum BBCH-Dezimal-code für die Entwicklungsstadien des Getreides—mit Abbildungen. Ges Pfl 41:384–388
Wiwart M, Perkowski J, Budzyński W, Suchowilska E, Buśko M, Matysiak A (2011) Concentrations of Ergosterol and Trichothecenes in the Grains of Three Triticum species. Czech J Food Sci 29(4):430–440
Zou CQ, Zhang YQ, Rashid A, Ram H, Savasli E, Arisoy RZ, Ortiz-Monasterio I, Simunji S, Wang ZH, Sohu V, Hassan M, Kaya Y, Onder O, Lungu O, Yaqub Mujahid M, Joshi AK, Zelenskiy Y, Zhang FS, Cakmak I (2012) Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil. doi:10.1007/s11104-012-1369-2
Web sites
http://www.kamut.com. Accessed 24 Oct 2012.
http://www.pureplanet.com/productpage.aspx?itemid=623. Accessed 24 Oct 2012.
http://www.ars-grin.gov/cgi-bin/npgs/html/taxon.pl?406898. Accessed 24 Oct 2012.
Acknowledgments
This work was supported by the Polish Ministry of Science and Higher Education, Project No. 0882/B/P01/2009/37.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wiwart, M., Suchowilska, E., Kandler, W. et al. Can Polish wheat (Triticum polonicum L.) be an interesting gene source for breeding wheat cultivars with increased resistance to Fusarium head blight?. Genet Resour Crop Evol 60, 2359–2373 (2013). https://doi.org/10.1007/s10722-013-0004-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-013-0004-2