Abstract
Twelve accessions classified as Pisum sativum subsp. elatius, mostly from West and Central Mediterranean, were analysed for three markers from different cellular genomes: rbcL (plastid genome), coxI (mitochondrial genome) and SCA (nuclear genome). Based on geographical distribution of their allele combinations analysed in this and the earlier study, we suggest a putative history of wild representatives of P. sativum. The ancestor of this species belonged to lineage A (coxI+, rbcL+, SCA f); it appeared in East Mediterranean, then spread westward most probably during one of the Pleistocene coolings when the sea was smaller, so that representatives of lineage A remained in the Eastern Mediterranean and on the islands of Sicily and Menorca. Mutation leading to the loss of the restriction site for PsiI in coxI−, gave rise to lineage C (coxI−, rbcL+, SCA f) which spread widely in the Mediterranean and is now found in France, Greece and Ethiopia. Mutation leading to rbcL− gave rise to lineage D (coxI−, rbcL−, SCA f), now found in Egypt (P. sativum subsp. jomardii) and Spain. Mutational transition of SCA f to SCA s most probably took place in North-Eastern Mediterranean since the resulting lineage B (coxI−, rbcL−, SCA s) now occupies the Tauro-Caucasian area. In Asia Minor and North Israel, line B met the ancestral line A so that both lines coexist there presently. The lineage B gave rise to the cultivated P. sativum subsp. sativum.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In an earlier publication we assessed a collection of pea germplasm for polymorphisms of three dimorphic genetic markers residing in the nuclear, plastid or mitochondrial genome and reported that most of peas had either of two combinations (A or B) of their alleles (Kosterin and Bogdanova 2008). Gene SCA resides in the nuclear genome (linkage group V) and encodes a seed cotyledon albumin SCA which has two electromorphs, fast, SCA f, and slow, SCA s. Gene coxI coding for cytochrome oxydase subunit I belongs to the mitochondrial genome and either contains (cox1+) or lacks (cox1−) a recognition site for restriction endonuclease PsiI. Gene rbcL, encoding ribulose 1,5-bisphosphate carboxylase large subunit, resides in the plastid genome and either contains (rbcL+) or lacks (rbcL−) a recognition site for restriction endonuclease AspLEI due to a synonymous nucleotide substitution. The combination cox1+, rbcL+, SCA f, called combination A, was found in all studied representatives of species Pisum fulvum Sibth. et Smith and Pisum abyssinicum A. Br. and in 12 of 35 (34%) studied accessions of wild representatives of Pisum sativum L. The combination cox1−, rbcL−, SCA s, called combination B, was also found in 12 of the 35 accessions of wild P. sativum. The remaining 11 accessions represented other, rare combinations of alleles. The overwhelming majority of cultivated P. sativum have combination B and few have rare combinations (see Table 1 in Kosterin and Bogdanova 2008). The three markers studied are functionally unrelated, alleles of one of them, rbcL having no functional differences as resulted from a synonymous substitution. These results were interpreted so that: (1) combination A was ancestral for the genus Pisum and was inherited by all the three extant species; (2) the alleles of the combination B arose among wild P. sativum and became fixed in some populations which then radiated out to give rise to about half of modern wild representatives of this species; (3) rare combinations might result from secondary introgression of lineages ‘A’ and ‘B’ within wild P. sativum or some of them might represent subtle evolutionary lineages diverged before fixation of all the three ‘B-alleles’; (4) cultivated P. sativum arose from some wild representatives ‘B-lineage’.
The analysis of three unrelated markers of different cellular genomes allowed us to propose a preliminary subdivision of wild representatives of P. sativum (except for carriers of rare combinations) into phyletic lineages A and B. Another bipartite subdivision of wild peas had been long existing, with tall forms called P. sativum subsp. elatius (Bieb.) Schmalh. or P. elatius Bieb. versus small forms called P. sativum subsp. syriacum (Boiss. et Noe) Berger or P. humile. This early classification was unnatural as it was based on size which is a general ecological adaptation: tall forms being connected with maquis vegetation while short forms are associated with steppe-like plant communities (Ben-Ze’ev and Zohary 1973). Currently this classification is not widely used and the wide range of wild representatives of P. sativum are usually attributed, following C. Townsend (1968) and Davis (1970), to the same subspecies P. sativum subsp. elatius s. l. (Maxted and Ambrose 2001). Using cytogenetic analysis Ben-Ze’ev and Zohary (1973) discovered that short forms, ‘P. humile’ in their nomenclature, can be divided into ‘northern humile’ (Turkey and Golan Heights), karyologically identical to cultivated peas, and ‘southern humile’ (mainly Israel), differing from them by chromosome rearrangements but close in this respect to tall ‘P. elatius’. Kosterin and Bogdanova (2008) found that Zohary and Ben-Ze’ev’s accessions of ‘southern humile’ had combination A, as well as their accessions of ‘elatius’ while the only studied accession (716 = JI1794) of ‘northern humile’ had combination B, also shared by cultivated peas. In fact, the data by Kosterin and Bogdanova (2008) would also allow subdivision of ‘P. elatius’ into ‘northern elatius’ and ‘southern elatius’, since among the accessions originally identified as ‘elatius’, those belonging to lineage A originated from Israel, Turkey, Sicily, while those belonging to lineage B originated from Crimea, the Caucasus and again Turkey. Hence, we face hints of general geographical dichotomy: lineage A occurs predominantly in the south with lineage B in the north, both occurring in Turkey.
The earlier study by Kosterin and Bogdanova (2008) was based on incomplete coverage of the geographical range of wild peas, with only one accession from West Mediterranean and few from the Apennine and Balkan Peninsulas. In this study, the analysis was extended to include further accessions from western and central Mediterranean available at the world pea germplasm collection of John Innes Centre, Norwich, UK (for its online searchable database see http://data.jic.bbsrc.ac.uk/cgi-bin/germplasm/pisum). This resulted in further insights into the history of wild Pisum sativum and modified the hypothesis on the origin of lineage B.
Materials and methods
Twelve accessions from the John Innes Centre, classified as Pisum elatius, were analysed which originated from Spain, Italy, France, Greece, Ethiopia and Iran (Table 1). One of them, JI3553, results from seeds specially collected near Marceille, France by Michel Papazyan.
Plant growing, DNA extraction, PCR amplification of the plastid rbcL and mitochondrial cox1 genes, endonuclease restriction of the PCR products, isolation and electrophoreses of SCA protein were done as described in (Kosterin and Bogdanova 2008). Sequencing reaction of the PCR-amplified portion of cox1 was performed with the use of BigDye terminators 3.1 and the same primers as for PCR-amplification (5′-tggtaattggtctgttccgattct and 5′-ccactgcttgaagtgattgttacg) at 27 cycles: 45 s denaturation at 96°C, 30 s annealing at 54°C, 4 min elongation at 60°C.
Results
Plant general characteristics
The accessions were first checked for characters diagnostic for wild versus cultivated peas. The main criterion was dehiscing pods (Dpo). Accessions JI2105 (Iran), and JI2115 (Spain, Puerto de Bejar) were large plants with non-dehiscing pods (dpo), non-tuberculate seeds (gty) and lacked any other wild character. For these reasons we excluded them from the analysis of wild peas. All other accessions had dehiscing pods (Dpo) and tuberculate seed testa (Gty).
The six accessions originating from the Mount Athos Peninsula, Chalcidice, Greece differed in many respects: alleles of D (D w in JI1091, JI1093, JI1095; D co in JI1092, d in JI1094), development of pustules on pod walls appearing in greenhouse caused by the gene Np (weak in JI1094, JI1095, very weak in JI1094). There was also difference in histone H1 electrophoretic spectrum: accessions JI1092, JI1093 and JI1096 had a rare allele His6 4 of histone H1 subtype 6 gene, while JI1091, JI1094 and JI1095 had more common variant 63(not shown). All displayed the allele for large hilum (Him). In addition all had an additional subtype of histone H1 which was revealed as a band slightly faster than subtype 3 (not shown).
Plants of the accession JI254 from Ethiopia had purple stems, diffuse spots of fine purple specking on pod walls located above the ovules (a Pur allele), manifestations of D co, Np and Him, pyriform leaflets and very large flowers. They looked strikingly like the tall ‘southern elatius’ from Israel. Accession JI3553 (Marceille) had Him, Np, d and looked as a common ‘northern elatius’. JI2055 (Italy, Monte Alburni) had D co, Np, him and an extremely narrow and long pods covering the seeds so tightly that the walls opened before seed maturation. JI2724 (Spain, Menorca) had D w, Him and was unique in failing to initiate flowering on the main stem in the greenhouse, although it grew very long, and few flowers appeared on basal laterals.
Alleles of coxI, rbcL and SCA
We sequenced (EMBL accession FN435841) the amplified portion of gene coxI from accession VIR320, which has allele coxI+. Recognition site TTATAA for PsiI in this allele results from substitution C → A in position 260 from the start of the upstream primer used (position 3,331 of the pea coxI gene sequence, accession X14409). This nucleotide substitution corresponds to an amino acid substitution Leu → Ile. Besides, in position 995 from the upstream primer used, the sequenced portion of coxI+ contains C versus A (position 4,066 of X14409), which corresponds to the substitution Lys → Gln.
Alleles of the three markers found in the accessions are presented in Table 1. The two none-wild accessions have the conventional combination B of these markers. Of the wild accessions, one, from Menorca, had combination A. All other wild accessions originating from quite distant localities, namely six accessions from Mt. Athos Peninsula, one from Italy, one from Marceille, and one from Ethiopia, all had the same combination coxI−, rbcL+, SCA f which was earlier found in just two accessions (Kosterin and Bogdanova 2008).
Discussion
Wild and cultivated peas
Plants with non-dehiscing pods cannot effectively propagate in nature while seeds from dehiscing pods cannot be effectively harvested. Hence, lack of dehiscing ability is a manifestation of domestication, while dehiscing pods indicate that plant is not cultivated and is either a member of natural plant community or a weed. The dehiscing ability is provided by presence of Dpo. Homozygotes for recessive allele dpo in normal conditions have non-dehiscing pods but may dehisce under very high temperatures and low humidity, hence, natural dispersal of dpo plants is still possible after harvesting but very little is known in this respect. Analysis of seed dispersal revealed Dpo only in wild taxa, P. fulvum, ‘P. humile’ and ‘P. elatius’ (Ambrose and Ellis 2008). Among these taxa, dpo was found in only 2 accessions of ‘P. elatius’, which may not be true elatius but reflect possible introgression with satium forms. So, we may regard Dpo as an indicator of a wild pea. Since pea is not a strict self-pollinator and a degree of natural crossing occur (Loenning 1984; Bogdanova and Berdnikov 2000), some gene flow is expected between cultivated and wild peas. However, among 38 accessions of wild peas studied by Kosterin and Bogdanova (2008) only 2 (both from Italy: VIR3115, Catania and 723, Sardinia) had some traits from cultivated pea. We have some evidences of an opposite gene flow. All 7 accessions from the Mt. Athos Peninsula (6 studied here plus an earlier analysed PI244008) have an additional histone H1 subtype, and 3 of these accessions have allelic variant 4 of histone H1 subtype 6. The same H1 bands were found in some accessions of traditional landraces from Greece but nowhere else (Berdnikov et al. 1993). About a half of wild Pisum sativum have rough tuberculate testa (Gty). Although a small number of cultivated peas exists with non-dehiscing pods (dpo) but tuberculate testa (Gty), Gty provides is an additional character as a rule indicating wild peas. Only seeds of wild P. sativum subsp. elatius exhibit large hilum (Him) found, mostly associated with forms with long peduncles and large flowers.
The range of wild representatives of P. sativum extends from Iran and Turkmenistan through Anterior Asia, North Africa and South Europe to Spain, Portugal and Morocco (Makasheva 1979; Maxted and Ambrose 2001). There is no report that this range includes Ethiopia. Accession JI254 is undoubtedly a wild P. s. subsp. elatius, and is recorded as “Jimma market place”. Until such plants are proved growing wild in Ethiopia, the true provenance of this material will remain doubtful. Presently we lack wild pea germplasm from the east of the Caspian Sea (JI2105 from Iran appeared to be not a wild pea), from Maghreb and further samples from Iberian Peninsula southern France and Italy are desirable. Nevertheless, the addition of six accessions from Greece, one from Menorca and one from Ethiopia clarified geographical distribution of different wild peas.
Combinations of alleles of coxI, rbcL and SCA
Kosterin and Bogdanova (2008) introduced the following designations: combination A for coxI+, rbcL+, SCA f and combination B for coxI−, rbcL−, SCA s . Nine of ten wild pea accessions analysed here had the combination coxI−, rbcL+, SCA f. We make a further step and designate this as combination C. We should make a qualification that in (Kosterin and Bogdanova 2008) this combination was found in two accessions, PI344008 (the Mt. Athos Peninsula, Greece) and PI343993 (Turkey), see Table 1 (Kosterin and Bogdanova 2008: 739–740). The text on p. 746 “combination SCA f, rbcL+, cox1− not found at all” was a mistake, since the two mentioned accessions were erroneously ascribed the combination cox1–, rbcL+, SCA s on p. 747. In total we now have 11 accessions with combination C: one from Ethiopia, one from Turkey, one from Italy, seven from Greece and one from France and it is noteworthy that all accessions from Greece and the only one from France possess this combination. We now designate the combination coxI−, rbcL−, SCA f as combination D which was found in three accessions of wild peas VIR7327, VIR7328 (Turkey), PI344537 (Italy) and five accessions of cultivated peas VIR3424, VIR3429, VIR3439 (Egypt), VIR7335 (Tadjikistan), VIR3171 (with the provenance specified as “Madrid, Botanical garden”), (Kosterin and Bogdanova 2008).
It is evident that the four designated combinations could originate from one another by single mutation events in the order A → C→D → B: the first transition is due to the loss of PsiI restriction site in coxI, the second is due to the loss of the AspLEI restriction site in rbcL, and the third is due to the substitution converting the fast SCA variant into the slow one. Therefore we may suppose that combinations C and D represent subtle pea lineages (‘lineages C and D’) having diverged between fixation of these mutations rather than ‘recombinants’ resulting from introgression of ‘lineages A and B’. The other designated four possible allele combinations do not fit that supposed sequence of mutational events. Three of these four combinations were found in one accession each, and combination cox1−, rbcL+, SCA s in two accessions (WL805 and VIR1975) (the correct data from Table 1 in Kosterin and Bogdanova 2008; for an error in the text see above). These four combinations are indeed rare (6 of 108 accessions studied in total) and quite possibly result from gene introgression in sympatric populations representing the ‘regular’ combinations A, B, C, D. Jing et al. (2007) stressed the role which introgression and recombination played in evolution of wild peas. The reticulate evolution, however, mostly concerned the very base of the genus phylogeny; and the main here accepted taxonomic groups were well resolved by Jing et al. 2007. Only a few accessions showed signs of recent introgression, none of which were involved in our analysis.
Application of the logic by Kosterin and Bogdanova (2008) that the lineages studied deserve subspecies status would demand a subspecies name for lineage C (the name P. s. subsp. jomardii was ascribed for lineage D in the cited paper). But here we abstain from further proliferation of subspecies within P. sativum.
Geographic distribution of combinations A, B, C and D of alleles of coxI, rbcL and SCA
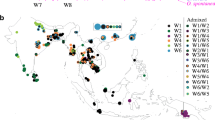
An updated set of accessions of wild Pisum sativum, including some from West Mediterranean, allowed us to map the geographic distribution of accessions with different allele combinations of the markers studied (Fig. 1). Accessions with uncertain provenance or not representing wild peas are excluded. Accessions representing P. s. subsp. jomardii, are indicated as one dot in Egypt. Although not wild, they might represent an independently domesticated local form. Provenance of accessions of two other species, P. fulvum (wild) and P. abyssinicum (cultivated, domesticated but independently from sativum) are also shown as smaller circles. On the map, we see areas occupied by only one combination: combination A in Israel (excluding Golan Heights), combination B in the Crimea and Caucasus, combination C in Greece. Southern France and Ethiopia are represented by single accessions, both of combination C. Turkey is an area where combinations A and B overlap. In Italy we observed three accessions with combinations A (Sardinia), C (Mt. Alburni) and D (Sicily).
A map of provenance of accessions of wild peas Pisum fulvum and Pisum sativum subsp. elatius and locally cultivated Pisum abyssinicum and Pisum sativum subsp. jomardii, with indication of alleles of the three markers studied. Large circles: P. sativum; small circles: P. fulvum and P. abyssinicum; left upper sector, filled: Cox1+, empty: cox1−; right upper sector, filled: rbcL+, empty: rbcL−; lower sector, filled: SCAf, empty: SCA s. Insert shows provenance of accessions in Israel. Numerals are the numbers of accessions of the same type geographical position of which cannot be resolved in the map scale
This distribution does not support a supposition by Kosterin and Bogdanova (2008), based on scarcity of transitory forms in their accession set, that three mutations transforming combination A into B were rapidly fixed in some small population. A revised hypothetical scenario for the evolution of wild P. sativum is suggested where combination A is the ancestral state of the genus and was inherited by the early P. sativum which most probably first appeared in the eastern Mediterranean, judging from the present area of the lineage A in Israel. Here P. sativum grows sympatrically with P. fulvum, which also has combination A. P. abyssinicum, another species with exclusively combination A, occurs in Yemen and N Ethiopia, that is also in East Mediterranean sensu lato. From this area, lineage A spread within wild P. sativum to the west over the Mediterranean. The two accessions with combination A found on Sardinia and Menorca may represent island refugia of that early spread. Obtaining and analysing peas from other islands where P. sativum subsp. elatius have been recorded: Malta, Corsica, Crete, Cyprus, East Aegean islands (http://www.ildis.org/LegumeWeb?sciname=Pisum+elatius), would be highly desirable to test this hypothesis. During oscillations of the sea level the islands were repeatedly isolated and merged again. We may suppose that the westward spread of line A occurred during some of the Pleistocene climate coolings, when the sea occupied less area. In a part of this westward wave of pea dispersal, a loss of restriction site in coxI was fixed, giving rise to lineage C which spread widely over Central and West Mediterranean and even NE Africa, now occurring in France, Italy, Greece and Ethiopia (although the latter point needs confirmation). The loss of restriction site in rbcL, marked the origin of lineage D, whose representatives were found in Egypt, Sicily, Turkey and ‘Madrid Botanical Garden’ (VIR3171, doubtful in representing a local wild flora). The origin and manner of spread of lineage D cannot be retrieved from our data but it should have given rise to lineage B by change in the SCA elctrophoretic mobility. This final mutation most probably took place somewhere near the Black Sea since wild peas of lineage B presently exclusively occupy the areas north of this sea. Asia Minor was an area affected by two opposite spreading waves of peas: that of lineage A from the south and that of lineage B from the north. We cannot make conjectures as to their timing but most probably lineage A was first to spread there, just because it is more ancient. Overall we observe a clear pattern: the oldest and youngest lineages occupy the areas east of the Mediterranean Sea, the oldest in the south, the youngest in the north, both in centre, while the two intermediate lineages C and D predominate in West and Central Mediterranean. It was probably in the West and/or Central Mediterranean where transitions between lineages A and B took place and left intermediate descendants (C and D). Augmentation of wild pea germplasm from European and African Mediterranean and Ethiopia is a high priority for a better understanding of the history of wild Pisum sativum.
A number of phylogenetic studies of peas via different molecular approaches have been reported but they are non-critical in accepting original taxonomical attribution of the accessions studied (especially with the names ‘P. humile’ and ‘P. elatius’) and did not involve analysis of their provenance. The set of accessions used in Vershinin et al. (2003) and Jing et al. (2007) contains a number of wild peas with uncertain provenance; including JI2105 referred to as ‘P. elatius’ but lacking characters of a wild pea. Only five accessions of this set (JI224, JI261, JI1094, JI1796, JI2105) are in common with our study (Kosterin and Bogdanova 2008 and this paper). All the three cellular genomes bear signs of natural history of pea forms. A joint phylogenetic and geographic analysis based on many sequences from all cellular genomes and involving as many accessions of wild peas as possible is necessary for reliably reconstructing the history of wild P. sativum.
References
Ambrose MJ, Ellis THM (2008) Ballistic seed dispersal and associate seed shadow in wild Pisum germplasm. Pisum Genet 40:5–10
Ben-Ze’ev N, Zohary D (1973) Species relationship in the genus Pisum L. Israel J Bot 22:73–91
Berdnikov VA, Bogdanova VS, Rozov SM, Kosterin OE (1993) The geographic patterns of histone H1 allelic frequencies formed in the course of pea (Pisum sativum L.) cultivation. Heredity 71:199–209
Bogdanova VS, Berdnikov VA (2000) A study of potential ability for cross-pollination in pea originating from different parts of the world. Pisum Genet 32:16–17
Davis H (1970) Flora of Turkey and the East Aegean Islands, vol 3. Edinburgh
Jing R, Johnson R, Seres A, Kiss G, Ambrose MJ, Knox MR, Ellis THN, Flawell AJ (2007) Gene-based sequence diversity analysis of wild pea (Pisum). Genetics 177:2263–2275
Kosterin O, Bogdanova V (2008) Relationship of wild and cultivated forms of Pisum L. as inferred from an analysis of three markers, of the plastid, mitochondrial and nuclear genomes. Genet Res Crop Evol 55:735–755
Loenning W-E (1984) Cross fertilization in peas under different ecological conditions. Pisum Newsl 16:38-40
Makasheva RK (1979) Kul’turnaya flora SSSR [Cultivated Flora of the USSR] vol. 4, Leningrad, p 1324 (in Russian)
Maxted N, Ambrose M (2001) Peas (Pisum L.). In: Maxted N, Bennett SJ (eds) Plant genetic resources of legumes in the Mediterranean, vol 10. Kluwer Academic Publishers, The Netherlands, pp 181–190
Townsend C (1968) Contribution to the flora of Iraq. V. Notes on Leguminosales. Kew Bull. Roy Bot Gard 21(3):435–458
Vershinin AV, Allnutt TR, Knox MR, Ambrose MJ, Ellis THN (2003) Transposable elements reveal the impact of introgression rather than transposition, in Pisum diversity, evolution and domestication. Mol Biol Evol 20:2067–2075
Acknowledgments
This work was supported by Russian Foundation for Fundamental Research, grant 07-04-00111-a and the State Contract No. 02.512.11.2254 by Russian Agency of Science and Innovations. Financial support for the maintenance of the JI Pisum collection from Defra is also gratefully acknowledged. Sequencing was performed at DNA Sequencing Center, ICG-ICBFM SD RAS. We are grateful to Michel Papazyan for kindly collecting seeds of wild pea.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kosterin, O.E., Zaytseva, O.O., Bogdanova, V.S. et al. New data on three molecular markers from different cellular genomes in Mediterranean accessions reveal new insights into phylogeography of Pisum sativum L. subsp. elatius (Bieb.) Schmalh.. Genet Resour Crop Evol 57, 733–739 (2010). https://doi.org/10.1007/s10722-009-9511-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-009-9511-6