Abstract
Lima bean (Phaseolus lunatus L.) is an important crop in traditional Mayan agriculture of the Yucatan Peninsula, Mexico, its Mesoamerican center of diversity. Genetic erosion in this species is currently a threat in this region out of 3 of 21 landraces dominate 71.24% of the cultivated area, and 12 are rare landraces grown only in 6.29%. Using 90 ISSR loci, we estimated the diversity and genetic relationships for 21 landraces to analyzing their risk of genetic erosion, and generate data for their in situ conservation. Total genetic diversity was high (h = 0.29), however it was lower than wild gene pool reported (h = 0.69). The abundant landraces had genetic diversity values lower (h = 0.13, I = 0.17) than the common (h = 0.26, I = 0.33) and rare landraces (h = 0.24, I = 0.27). However, the rare landraces are in a higher risk of genetic erosion due to local extinction. The cluster analysis showed no groups corresponding to morpho-phenological characteristics, geographic origin or traditional classification, which resulted from high inter-landraces gene flow levels. The molecular data confirmed that the domesticated Lima bean pool of the Yucatan Peninsula has a high risk of genetic erosion. If current tendencies in landrace cultivation continue, many will no longer be planted within two to three generations, with a consequent loss of their alleles. Programs urgently need to be established for in situ conservation of Lima bean landraces in this region.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to Rural Advancement Foundation International (RAFI), agriculture worldwide has lost three-quarters of the genetic diversity in major food crops and this erosion continues at an annual rate of 1–2% (Mazhar 1997). Genetic erosion is the loss or reduction of genetic diversity between and within populations of the same species over time (Jarvis et al. 2000), and most often results from agricultural, economic and social changes (FAO 1996). In cultivated species this phenomenon has been evaluated at the landrace level (Tsegaye and Berg 2007; Hammer and Laghetti 2005) as this is the primary available genetic pool for hybridization and genetic improvement programs (Harlan and de Wet 1971). It is believed that the loss of landraces generates erosion at the allelic level (Upadhyay and Sthapit 1998). Genetic erosion is a significant issue in crop domestication areas since them: (a) concentrate the highest genetic diversity; (b) traditional producers conserve ancestral landraces, along with the knowledge and cultural practices that created this diversity; and (c) there exist inter-reproductive wild-weedy-domesticated complexes, favoring wild-domesticated gene flow (Bellón and Taylor 1993; Brush 1991). Mexico forms part of the Mesoamerican center of domestication (Vavilov 1926). The ecological, productive and cultural conditions of traditional agroecosystems in Mexico have helped to conserve a large number of domesticated species. These conditions have also maintained these species as part of a dynamic scenario for development of new crops and species evolution, both of which are processes that favor high levels of variation and genetic contact with wild relatives (Hernández-Xolocotzi 1973).
Lima bean is a common crop among many indigenous groups in the Americas. Its primary genetic pool has wild (P. lunatus var. silvester Baudet) and domesticated (P. lunatus var. lunatus) forms (Baudet 1977). It is divided into two main groups: the Mesoamerican and Andean, and a small group of intermediate genotypes (Gutiérrez-Salgado et al.1995; Fofana et al. 1997; Caicedo et al. 1999; Lioi and Galasso 2002). Three cultigroups (cv-gr) are recognized in the cultivated forms (Baudet 1977): (1) Potato, with small and round seeds; (2) Sieva, with medium sized and kidney-shaped seeds; and (3) Big Lima, with large and flat seeds. The Potato and Sieva cultigroups represent the Mesoamerican gene pool and the Big Lima represents the Andean.
In Mexico, Lima bean is the fourth main crop for the Maya of the Yucatan Peninsula. It is planted into the traditional Mesoamerican agricultural system known as the “milpa”. It is based on the traditional farming system, where the vegetation is cyclically slashed and burned to plant crops in the area during a period of 2–4 years and then left on fallow for the next 5–15 years when a new cycle can be initiated (Hernández-Xolocotzi 1959). The Yucatan Peninsula has the highest morphological variation of landraces in the country (Ballesteros 1999) and is within the putative domestication area of the Mesoamerican genetic pool (Gutiérrez-Salgado et al. 1995). To date, the genetic diversity of the cultivated gene pool of this region has been studied using morphological and phenological markers (Ballesteros 1999; Martínez-Castillo et al. 2004), but no molecular data exist nor there are any studies associating these data to in situ conservation of this gene pool. Martínez-Castillo et al. (2004), in a sample of 160 Mayan producers from the four main areas of traditional agriculture in the Peninsula, they found that out of 25 landraces planted, three accounted for 71.24% of the cultivated area. Most of the remaining 22 landraces were rare, meaning each accounted for less than 2% of the cultivated area; in many cases they were grown by a single producer. This situation puts this species at serious risk of genetic erosion, which is increased by three factors: (1) environmental factors such as drought and hurricanes have led to loss of seed; (2) intensification of the traditional Mayan agriculture, which displaces cultivation of these landraces; and (3) increasing rural population and socioeconomic changes have led to migration of Mayan producers to tourist centers, with consequent abandonment of agricultural activity and changes in the traditional Mayan diet (Cuanalo and Arias 1997; Ku-Naal 1995; Reyes and Aguilar 1992).
In the Yucatan Peninsula, inter-landrace gene flow and natural introgression of wild alleles may prevent the genetic erosion of Lima bean landraces. Martínez-Castillo et al. (2004) reported the planting of up to seven landraces in a single milpa and existence of a wide variety of hybrid seeds. Using eight microsatellite markers (SSR), Martínez-Castillo (2005) observed very high gene flow levels between landraces in each of the studied agricultural regions of the Peninsula. Martínez-Castillo et al. (2006) reported high genetic diversity levels in the region’s wild pool and Martínez-Castillo et al. (2007) documented wild-domesticated gene flow and weedy forms derived from this flow. Weedy forms have been reported previously (Debouck 1979; Ballesteros 1999). Natural wild-domesticated introgression has played a vital role in the evolution of domesticated species and continues to be an important factor in increasing genetic diversity in modern crops (Arnold 1992; Harlan 1965; Quiros et al. 1992; Slatkin 1987).
The Inter-Simple Sequence Repeats (ISSR) technique allows detection of polymorphism without previous knowledge of DNA sequences. This dominant molecular marker involves polymerase chain reaction (PCR) amplification of DNA using a single primer composed of a microsatellite (SSR) sequence, anchored at the 3′ or 5′ end by two to four arbitrary, often degenerate, nucleotides (Zietkiewicz et al. 1994). Unlike codominant markers such as SSR, the heterozygote cannot be directly distinguished from the dominant homozygote phenotype (band) at individual loci and consequently, the estimation of allele frequencies from dominant markers presents some statistical difficulties (Lynch and Milligan 1994). These difficulties have been resolved using new methods as Bayesian ones (Zhivotovsky 1999) and better estimators for the analysis of dominant markers (Krauss 2000; Lynch and Milligan 1994; Nybom 2004). On the other hand, Kimberling et al. (1996) suggested to use a big number of loci. In relation to this, Kremer et al. (2005) using AFLP dominant markers, show that the monolocus estimation of gene diversity has the potential to vary strongly with variations in the fixation index, but that the multilocus estimate is rather robust to deviations in Hardy–Weinberg equilibrium, due to a mechanistic effect of compensation between negative and positive biases of genetic diversity estimates for different AFLP loci exhibiting contrasting frequencies of the null homozygote.
Since Zietkiewicz et al. (1994) invented the ISSR technique, it have proven to be a rapid, simple and inexpensive way to assess structure and genetic diversity (Culley et al. 2007; González et al. 2005; Payro de la Cruz et al. 2005), to analyze genetic relationships among cultivars (Prevost and Wilkinson 1999; Martins et al. 2003), and to study evolutionary processes (Galván et al. 2003; Zizumbo-Villarreal et al. 2005). In the present study, ISSR markers were used to estimate diversity and the genetic relationships of 21 Lima bean landraces forming part of traditional Mayan agriculture on the Yucatan Peninsula, Mexico, with the objective to analyze their risk of genetic erosion and to generate molecular data applicable to their in situ conservation.
Materials and methods
Plant material and DNA extraction
Plant material and data of relative abundance were those used in Martínez-Castillo et al. (2004). These authors made a study about the intraspecific diversity and morpho-phenological variation in P. lunatus of the Yucatan Peninsula, Mexico. They collected seeds of domesticated populations from the four main areas of traditional agriculture in the Yucatan Peninsula: southeast of the Yucatan State (SEYUC); southern Yucatan State (SYUC); the central-eastern of Quintana Roo State (CEQROO); and the northeast of Campeche State (NECAMP) (Fig. 1). Using morpho-phenological and ethnobotanical data, they found 25 landraces with Potato, Sieva and intermediate forms between these groups, and characterized their abundance relative based on the percentage of cultivated area and the number of producers using each landrace.
For this study, a total of 21 landraces recognized for Martínez-Castillo et al. (2004) were chosen. Seeds of these landraces was obtained from the own collection of the first author. For each landrace a number of accessions ranging from 1 to 5 was analyzed (Table 1). Five seeds from each landrace were planted in a greenhouse at the Centro de Investigación Científica de Yucatán (CICY), in Merida, Yucatan, Mexico. When possible, the seeds were taken from accessions collected in the four agricultural regions considered for Martínez-Castillo et al. (2004) to provide greater genetic representativity. Using the CTAB method (Doyle and Doyle 1987), genomic DNA was extracted from trifoliate leaves and then DNA of the five plants of the same landrace was pooled to create a genetic pool for each landrace.
ISSR analysis
Martínez-Castillo (2005) made an exploratory analysis about the genetic relationships among Lima bean landraces from Yucatan Peninsula, Mexico, using ISSR markers and found a big amount of polymorphic loci. Previously, he made the same analysis using SSR markers and found that of 25 loci tested 24 were monomorphic. Considering these results, we decided to use ISSR markers instead SSR ones.
Each ISSR band was considered as an independent locus and polymorphic bands were scored as absent (0) or present (1) for all samples. Only clearly reproducible bands were scored and differences in band intensity were not considered. ISSR technique was done according to González et al. (2005). Four primers were used: (GACA)3 RG, YR (GACA)3, (GACAG)3 AG and (CACAG)3 RG (Table 2). Each 20 μl amplification reaction consisted of 10 mM Tris–HCl (pH 9.0), 50 mM KCl, 2 mM MgCl2, 200 μM of each deoxyribonucleotide phosphate, 1 μM of primer, 1 unit of Taq polymerase (Promega, Madison, WI), and 20 ng of template DNA. Amplification was performed in a GeneAmp PCR System 9700 (Applied Biosystems, Foster City, USA), under the following conditions: 4 min at 94°C for the initial step, followed by 35 cycles of 2 min at 94°C, 1 min at 42 or 54°C (depending of primer), and 2 min, and followed by a final step of 5 min at 72°C for final extension. Four microliter of formamide, containing 0.45% of bromophenol blue and 0.25% of xylene-cianol were added to each product of PCR. Four microliter of the reaction mixture were loaded on 320 by 380 by 0.4 mm of 6% nondenaturing 30:1 bis-acrylamide gels containing 3 M urea and TBE 1× buffer (100 mM Tris–borate, pH 8.0, 2 mM EDTA) (Zietkiewicz et al. 1994). A 123-bp molecular marker standard was included in each gel. Electrophoresis was carried out at 300 V (SQ3 Sequence Hoeffer) and the products of the amplification were visualized with the technique of silver staining using the Promega Q4132 kit and following the instructions of the supplier.
Data analysis
Genetic diversity
The 21 landraces were listed on the basis of the percentage of cultivated area that a sample of 160 farmers used for each landrace (i.e. number of accessions). This produced three groups (Fig. 2, Table 1): (a) abundant landraces, consisting of three landraces, each grown on more than 16% of the cultivated area and planted by 10–33 producers in the four agricultural zones; (b) common landraces, including six landraces, each grown on 3–5% of the area and by 5–14 producers (this group included Chak-petch and Balche, with low percentages but planted by nine and five producers, respectively); and (c) rare landraces, consisting of 12 landraces, each planted on less than 2% of the area and grown by 1–4 producers.
Groups of Lima bean landraces used in this study. Line A (abundant landraces): Mulición, Sac, Putsica-sutsuy; line B (common landraces): Bacalar, Nuk, Chak-saac, Mejen, Chak-petch, Balche; line C (rare landraces): Box-petch, Balam-pach, Tsisibal, Kan, Chak-mejen, Madzakitam; line D (rare landraces): Pool-santo, Tabaco, Box-uolis, Chak-uolis, Chak-chí, Chocolate. Landraces are named from left to right. Culti-groups: P (cv-gr Potato), S (cv-gr Sieva), I (intermediate forms between Potato and Sieva)
With the objective of compare the Lima bean genetic diversity present in the Yucatan Peninsula with the values reported by other studies and with those observed within each landrace group, genetic diversity was estimated at two levels: (a) total domesticated gene pool, and (b) landraces groups. To avoid common problems associated with the analysis of dominant data (Culley and Wolfe 2001; Lynch and Milligan 1994), analyses did not involve Hardy–Weinberg equilibrium (HWE). It was considered due to domesticated populations of P. lunatus from the Yucatan Peninsula are known to deviate from Hardy–Weinberg equilibrium with co-dominant microsatellite markers (Martínez-Castillo 2005). It was assumed that there was no co-migration of alleles from different loci, alleles shared by two individuals descend from a common ancestor and each locus consisted of only two alleles that segregate in Mendelian inheritance. The parameters used were: (1) polymorphic loci percentage (% P), calculated directly from the data; (2) the Shannon-Weaver diversity index (I) (Shannon and Weaver 1949) obtained with the POPGENE ver. 1.31 program (Yeh and Boyle 1999); (3) Nei genetic diversity (h) considering the Taylor expansion (Lynch and Milligan 1994) and obtained with the TFPGA program (Miller 1997). Although in this study was considered that the data are not in Hardy–Weinberg equilibrium, based in the results reported by Kremer et al. (2005) we decided to evaluate average heterozygosity (H) using the Bayesian approach proposed by Zhivotovsky (1999). This estimator was obtained with AFLP-SURV version 1.0 program (Vekemans 2002). Paired Student t tests were done to compare I, h and H values between pairs of landrace groups (α = 0.05) using the Statistica ver. 6.0 program (Statsoft, Tel Aviv, Israel).
Genetic relationships
Genetic relationships among landraces were determined based on the Nei and Li similarity coefficient (Nei and Li 1979). This is a good estimator to use with dominant markers due to it does not use band absences or assume Hardy–Weinberg equilibrium. Similarity coefficients between pairs of landraces were calculated using MVSP ver 3.1 program (Kovach Computing Services, Pentraeth, Isle of Anglesey, UK). Genetic relationships were represented by building a dendrogram using the UPGMA method (unweighted pair group method with arithmetic mean) with the NEIGHBOR and CONSENSE programs of the Phylip package ver. 3.6 (Felsenstein 2005). Topology robustness was evaluated by selecting the random 1,000 replication option with replacement over loci (Felsenstein 1985). Dendrogram was visualized using TREEVIEW program (Page 1996). The UPGMA results were compared by generating genetic distances from the binary data of the 21 landraces using the Gower General Similarity coefficient (Gower 1966). With these data, a Principal Coordinates Analysis (PCoA) (Gower 1966) was done and the first and second component values graphed using the MVSP ver 3.1 program (Kovach Computing Services, Pentraeth, Isle of Anglesey, UK).
Results and discussion
Genetic diversity
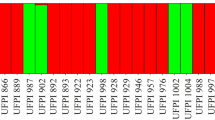
A total of 90 loci were analyzed of which 71 were polymorphic and 19 monomorphic. Primer 32 had the largest number of loci (33), the highest level of polymorphism (Table 2) and the highest number of specific loci for some of the studied landraces (Fig. 3).
ISSR profiles for the primer 32 of the Lima bean landraces. Lanes: 100 bp ladder (1), Mulición (2), Sac (3), Putsica-sutsuy (4), Bacalar (5), Mejen (6), Chak-saac (7), Nuk (8), Box-petch (9), Box-petch (10), Chak-petch (11), Kan (12), Madzakitam (13), Pool-santo (14), Chak-mejen (15), Box-uolis (16), Chocolate (17), Balche (18), Tabaco (19), Balam-pach (20), Chak-chí (21), Tsisibal (22). Bands remarked in red are specific loci for that landraces
At the total domesticated gene pool level, genetic diversity was high, and the three estimators showed similar values (I = 0.33, h = 0.28, H = 0.31) (Table 3). These results supported the ones reported by Kremer et al. (2005) showing that H Bayesian could be a good genetic diversity estimator to dominant markers when the molecular analyses use a big number of loci.
Using alloenzymes, Maquet et al. (1997) reported an h = 0.26 for the P. lunatus base collection of the Germplasm Bank of the International Center for Tropical Agriculture (CIAT-Colombia). These authors stated that this is a significant level and higher than reported for other plants that, like P. lunatus, are mixed-mating or short-lived perennial species (h = 0.12) (Hamrick et al. 1991). Using RAPD markers, Nienhuis et al. (1995) found a lower genetic diversity for domesticated Mesoamerican material (h = 0.11) than that found in the present study. This difference may be due to the low representativity of the plant material used by Nienhuis et al. (1995). Castiñeiras et al. (2007), using AFLP molecular markers, analyzed the genetic diversity of Potato-Sieva landraces planted in the Cuban home gardens. They reported a genetic diversity (h = 0.119) similar to the one reported by Nienhuis et al. (1995). Compared with these studies, our results could be reflecting the high genetic diversity maintained by Mayan farmers in the milpa of the Yucatan Peninsula, Mexico. However, the studies cited here were made using different methodologies to collect the plant material (size of samples, sampling methods, different origin of samples -field or genebank-), and cautions should be taken.
Compared to the wild gene pool, the domesticated Lima bean gene pool had lower genetic diversity values. Using eight SSR loci, Martínez-Castillo et al. (2006) reported an h = 0.69 for wild Lima beans, which is almost three times higher than observed here for the domesticated gene pool (h = 0.28). A number of factors may explain these differences: (a) a founder effect occurring during the domestication process, which has been reported for P. lunatus (Gutiérrez-Salgado et al. 1995) and other cultivated species (Ladizinsky 1985); (b) a genetic erosion effect in the domesticated gene pool due to changes associated with intensification of traditional agriculture during recent decades, as it has been reported for common bean (P. vulgaris L.) in central Mexico (Payro de la Cruz et al. 2005; Zizumbo-Villarreal et al. 2005); and (c) the type of data generated by the different markers used in this study (ISSR-dominant markers) vs. Martínez-Castillo et al. (2006) study (SSR-codominant markers).
At the landrace groups level, the common landraces had the highest genetic diversity values (except for % P), although the differences between the three groups were not statistically significant (Table 3). The rare landraces group had genetic diversity values (h and I) slightly lower than the common landraces group, but higher for % P (Table 3). It is probably due to the fact that nine of the 12 rare landraces were represented only by a single accession (Table 1), whereas all the common landraces were represented by at least five accessions. The rare landraces’ minimal abundance is the main factor that most increases the risk of genetic erosion since it can lead to their local extinction. During a germplasm collection in 2007, a farmer from SEYUC reported that he had lost his seed of Pool-santo and Chak-chí landraces in the 2006 agricultural cycle due to a lack of rain. In another case, a farmer from CEQROO stopped planting the Chocolate and Tabaco landraces in 2005 because he became sick that year and did not cultivate his milpa. Unfortunately, when we made the germplasm collection, this farmer was the only one who had these landraces. At present these two rare landraces have not been collected again.
Two factors that could reduce the risk of genetic erosion in some of the rare landraces are dark seed color and their mixed management by Mayan farmers. Both aspects favor the entrance of wild alleles through formation of wild-weedy-domesticated complexes and the generation of weedy forms (Martínez-Castillo et al. 2004). Two special cases in the use of seed mixtures are the Bacalar and Balche landraces. These have become a kind of “genetic dump” as they contain seeds similar to many different landraces, such as Box-petch, Putsica-sutsuy, Chak-petch, Chak-saac, Pool-santo and Chak-uolis, among others. Indeed, in 2007 weedy forms were observed among the seeds harvested of Bacalar in CEQROO (Fig. 4).
The abundant landraces group had the lowest values of genetic diversity among the three groups for all estimators, except for H that showed the same value showed for the rare landrace group (Table 3). These low values could be reflecting a germplasm selection influenced by external market demands. Martínez-Castillo et al. (2004) reported that one of the main selection criteria applied to the three most abundant landraces (Mulición, Sac and Putsica-sutsuy) is seed production for sale. As a result of this, Mayan farmers currently tend to plant white seeded landraces (Mulición, Sac, Mejen, Nuk). This leads to a selection against weedy forms produced from crosses between landraces and the wild populations surrounding milpas, consequently limiting introgression of wild alleles and increasing the risk of genetic erosion. In relation to dominant Lima bean landraces, Debouck (1979) collected at least ten different landraces in the Mayan community of Nohalal, Campeche, but currently only three have been observed and these are dominated by Mulición and Sac (direct observation). Informal interviews with Mayan producers in Nohalal suggest that this loss of landraces is associated with the introduction of mechanized agriculture and monoculture of improved varieties of corn. Recent field observations indicate that even the planting of abundant Lima bean landraces such as Mulición and Sac is decreasing in response to low prices. A similar case is happening in SYUC, where the Mejen landrace has been replacing the other landraces with color of seed different from white (Martínez-Castillo et al. 2004). Recently, Mejen has decreased in cultivated area as a result of a low in demand markets. Even though in this study Mejen is considered as a landrace, there are evidences that it could be a improved variety introduced approximately 25 years ago: (1) it was not found by Debouck in 1979, (2) it is a variety planted as a monocrop (an aspect non common in the traditional Mayan agriculture) and it is no associated with maize as all the other landraces, and (3) it is a variety with a very short productive cycle that depends on a lot of water, a limited resource in the Yucatan Peninsula. This decrease in the number and density of planted populations may mean that a new genetic bottleneck is soon to come for the abundant landraces.
One little-studied factor in the genetic erosion of crops is change in the food preferences of the rural populations. For Lima bean in the Yucatan Peninsula this currently takes three forms: (a) young adults and children do not eat it; (b) only the elderly plant many of the rare landraces for their own use; and (c) cowpea (Vigna unguiculata (L.) Walpers, locally known as x-pelon), introduced to the region from Africa in the 20th century, has been replacing P. lunatus. In fact, Lima bean is progressively being replaced in some regions of Latin America by other food legumes (Maquet et al. 1997). As it is a long process to re-introduce a crop plant, in a study conducted in Cuba, Esquivel and Hammer (1988) proposed to maintain Lima bean landraces as part of the traditional horticultural system. In several Mayan towns of the Yucatan Peninsula, some landraces are planted into the home gardens. Inclusive, the Madza-kitam landrace is named X-Konan jonal (keeper of the house) too, because this landrace can be planted into the home gardens or the milpa. However, it is not a common agricultural practice. On the other hand, loss of landraces is also apparently linked to the different generations in human populations. Reports document that the Mayan farmers planting a large variety of rare landraces are elderly and their death almost surely means the loss of these landraces (direct observation).
Genetic relationships
The UPGMA (Fig. 5) did not show the presence of the Potato and Sieva cv-gr, which was also the case in the PCoA (Fig. 6). This coincides with other studies based on morpho-phenological (Martínez-Castillo et al. 2004), alloenzyme (Lioi et al. 1998; Maquet et al. 1997) and seed storage protein (Gutiérrez-Salgado et al. 1995; Maquet 1995) data that also did not detect cultigroups, even though some included material from throughout the Americas. Only Fofana et al. (1997), using RAPD markers, have reported low but significant differentiation between the Potato and Sieva accessions from America. These authors state that the lack of clear differentiation is because these cultigroups are involved in a sympatric differentiation process which has not been sufficiently pronounced to generate two more divergent groups. About the clear existence of these cultigroups, in a study of Lima bean landraces made in Peru, Debouck et al. (1987) mentioned that either more cultigroups would exist in small seeded Lima beans or the distinction between Sieva and Potato cultigroups would lack validity.
Scatter plot of 21 landraces based on first and second components of principal coordinate analysis (PCoA) using 90 ISSR loci. The letters correspond to the letters between brackets identifying to the landraces on Fig. 5
One aspect that leads to the lack of differentiation between cultigroups in the domesticated Lima bean pool from the Yucatan Peninsula is inter-landrace gene flow. Martínez-Castillo et al. (2004) reported the planting of up to seven landraces in a single milpa and the existence of large numbers of hybrid seeds. Using eight microsatellite loci, Martínez-Castillo (2005) observed very high levels of gene flow among domesticated Lima bean populations within each of the four agricultural regions (CEQROO, Nm = 18.1; SEYUC, Nm = 10.7; NECAMP, Nm = 6.1; SYUC, Nm = 2.9). Although Lima bean is mainly an autogamous species, crossing rates of up to 48% have been reported, depending on genotype, growth conditions, plant spacing, prevailing wind direction, and native insect populations (Baudoin et al. 1998). There are recent reports of crossing rates of up to 73% in domesticated populations from CEQROO, values probably due to the plant spacing (usually zero meters between neighbor plants due to the indiscriminate growth pattern of landraces) and the high diversity and abundance of local pollinator insect species in the region (Chimal Chan 2008). These high crossing rates could be favoring the intraspecific hybridization among landraces of the Yucatan Peninsula. On the other hand, seed exchange between producers, and typical agricultural practice among traditional Mayan farmers living in the same region, could be increased the gene flow among landraces.
The Figs. 5 and 6 also do not show any group associated with geographic origin of collections or other groups that could be explained by the existing ethnobotanical, morphological or phenological data (Martínez-Castillo et al. 2004). A group consisting of the Tabaco and Balche landraces was observed at the minor group level and may be associated with geographic origin since both are from CEQROO (Fig. 5), but the bootstrap value supporting this clade is low (0.18) (Fig. 5). The bootstrap low values present in the Fig. 5 supports no existence of landrace groups clearly differentiated at the molecular level in the domesticated gene pool of the Lima bean of the Yucatan Peninsula, Mexico.
Mejen landrace separated in a different clade to the other landraces (Fig. 5). This supports the hypothesis that Mejen is an improved variety introduced to this region of Mexico 30 year ago, approximately. If this is true, this study give evidences about the negative role of the introduction of improved varieties in the displacement and genetic erosion of landraces in their domestication and diversity centers.
Within the domesticated gene pool analyzed, there are three landrace groups with a very similar seed morphology: (1) Mulición and Sac; (2) Putsica-sutsuy, Tsisibal and Balam-pach; and (3) Bacalar, Balche and Tabaco (Martínez-Castillo et al. 2004). The genetic relationship analysis using ISSR markers, however, did not group them together (Figs. 5 and 6). This highlights the need for broader sampling across the entire Peninsula to collect the largest possible allelic diversity for these landraces. Any collection based solely on seed morphology and/or local names could exclude a large part of the allelic diversity of these landraces.
Conclusion
Genetic diversity in the genetic pool of domesticated Lima bean P. lunatus in the Yucatan Peninsula, Mexico, remains high, although much lower than its wild counterpart. The rare landraces are in a higher risk of genetic erosion because, with few individuals living per landrace and with moderate genetic diversity, it represents the greatest loss of unique alleles if these landraces go to local extinction. On the other hand, the abundant landraces have the lowest genetic diversity levels and are thus at great risk of genetic erosion due to selection criteria imposed by an external market, too. Although the high gene flow levels observed in the Lima bean domesticated gene pool could be limiting the genetic erosion of its landraces, we have to think that the landraces are not just a group of not-related alleles. Instead, each is a package of alleles selected during centuries by traditional Mayan farmers to cope to different environmental restrictions.
If the data about relative abundance reported by Martínez-Castillo et al. (2004) reflect the current condition of the domesticated Lima bean pool in the Yucatan Peninsula, then this species is at very high risk of genetic erosion since this region is one of its main centers of genetic diversity in Mesoamerica. If current trends continue in the region, many Lima bean landraces may cease to be grown into the milpa in two to three generations. To prevent this, in situ conservation programs are needed that address: (a) an emergency collecting effort to save all landraces ex situ, as a back up for the in situ conservation activities, (b) in situ conservation of landraces and the alleles they consist of; (c) generation of wild-weedy-domesticated complexes that allow introgression of wild alleles into landraces; and (d) reintroduction of rare landraces and programs to promote their planting and acceptance among young Mayan producers and their families. To do this, areas need to be selected that favor in situ conservation while considering the natural, economic, social and cultural factors that contribute to this conservation. In the case of Yucatan Peninsula, we considerate that the route SEYUC-CEQROO can be the area for this in situ conservation, and not only for P. lunatus, but also for many domesticated plants present in the Mayan milpa of this region of Mexico, too.
References
Arnold ML (1992) Natural hybridization as an evolutionary process. Annu Rev Ecol Syst 23:237–261
Ballesteros GA (1999) Contribuciones al conocimiento del frijol Lima (Phaseolus lunatus L.) en América Tropical. Ph.D. thesis, Colegio de Posgraduados, Montecillos, Estado de México, México
Baudet JC (1977) The taxonomic status of the cultivated types of Lima bean (Phaseolus lunatus L.). Trop Grain Legume 7:29–30
Baudoin JP, Degreef J, Hardy O, Janart F, Zoro Bi I (1998) Development of an in situ conservation strategy for wild Lima bean (Phaseolus lunatus L.) populations in the central valley of Costa Rica. In: Owens SJ, Rudall PJ (eds) Reproduction biology. Royal Botanic Garden Press, Kew, pp 417–426
Bellón MR, Taylor JE (1993) Farmer soil taxonomy and technology adoption. Econ Dev Cult Change 41:764–786
Brush SB (1991) A farmer-based approach to conservation crop germplasm. Econ Bot 45:153–165
Caicedo AL, Gaitán E, Duque MC, Toro Chica O, Debouck DG, Tohme J (1999) AFLP fingerprinting of Phaseolus lunatus L. and related wild species from South America. Crop Sci 39:1497–1507
Castiñeiras L, Guzmán FA, Duque MC, Shagarodsky T, Cristóbal R, De Vicente MC (2007) AFLPs and morphological diversity of Phaseolus lunatus L. in Cuban home gardens: approaches to recovering the lost ex situ collection. doi:10.1007/s10531-006-9025-x
Chimal Chan A (2008) Polinización y flujo genético en Phaseolus lunatus L. silvestres y cultivados en la Península de Yucatán. Bachelor thesis, Instituto Tecnológico de Conkal, Yucatán, México
Cuanalo de la Cerda HE, Arias R LM (1997) Cultural and economics factors that affect farmers decision-making in Yucatan, Mexico. In: Jarvis DI, Hodgkin T (eds) Strengthening the scientific basis of in situ conservation of agricultural biodiversity on-farm. Options for data collecting and analysis. IPGRI, Rome, p 14
Culley TM, Wolfe AD (2001) Population genetic structure of the cleistogamous plant species Viola pubescens, as indicated by isozyme and ISSR molecular markers. Heredity 86:545–556
Culley TM, Sbita SJ, Wick A (2007) Population genetic effects of urban habitat fragmentation in the perennial herb Viola pubescens (Violaceae) using ISSR Markers. Ann Bot 100:91–100
Debouck DG (1979) Proyecto de recolección de germoplasma de Phaseolus en México. CIAT-INIA, Centro Internacional de Agricultura Tropical (CIAT), Colombia
Debouck DG, Liñan Lara JH, Campana Sierra A, De la Cruz Rojas JH (1987) Observations on the domestication of Phaseolus lunatus L. FAO/IBPGR Plant Genet Resour Newsl 70:26–32
Doyle J, Doyle J (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Esquivel H, Hammer K (1988) The “conuco” – an important refuge of Cuban plant genetic resources. Kulturpflanze 36:451–463
FAO (1996) The state of the world’s plant genetic resources: diversity and erosion. Third World Resurgence. Farmers’ Rights and the Battle for Agrobiodiversity. Issue No. 72/73 KDN PP6738/1/96. An excerpt from the Report on the State of the World’s Plant Genetic Resources prepared by the FAO Secretariat for the International Technical Conference on Plant Genetic Resources at Leipzig, Germany, 17–23 June1996
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Felsenstein J (2005) PHYLIP (Phylogeny inference package) version 3.6. Distributed by the author. Department of Genome Sciences, University of Washington, Seattle
Fofana B, Vekemans X, du Jardin P, Baudoin J-P (1997) Genetic diversity in Lima bean (Phaseolus lunatus L.) as revealed by RAPD markers. Euphytica 95:157–165
Galván MZ, Bornet B, Balatti PA, Branchard M (2003) Inter-simple sequence repeat (ISSR) markers as a tool for the assessment of both genetic diversity and gene pool origin in common bean (Phaseolus vulgaris L.). Euphytica 132:297–301; 94:597–602
González A, Wong A, Delgado-Salinas A, Papa R, Gepts P (2005) Assessment of inter simple sequence repeat markers to differentiate sympatric wild and domesticated populations of common bean. Crop Sci 45:606–615
Gower JC (1966) Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53:325–338
Gutiérrez-Salgado A, Gepts P, Debouck DG (1995) Evidence for two gene pools of the Lima beans, Phaseolus lunatus L., in the Americas. Genet Resour Crop Evol 42:15–28
Hammer K, Laghetti G (2005) Genetic erosion – examples from Italy. Genet Resour Crop Evol 52:629–634
Hamrick JL, Godt MJW, Murawski DA, Loveless MD (1991) Correlations between species traits and allozyme diversity: implications for conservation biology. In: Falk DA, Holsinger KE (eds) Genetics and conservation of rare plants. Oxford University Press, New York, pp 75–86
Harlan JR (1965) The possible role of weedy races in the evolution of cultivated plants. Euphytica 14:173–176
Harlan JR, de Wit JMJ (1971) Toward a rational classification of cultivated plants. Taxon 20:509–517
Hernández-Xolocotzi E (1959) La agricultura. In: Beltrán E (ed) Los recursos naturales del sureste y su aprovechamiento, vol 3. Instituto Mexicano de Recursos Naturales Renovables, México, D. F.
Hernández-Xolocotzi E (1973) Genetic resources of primitive varieties of Mesoamerica: Zea spp., Phaseolus spp., Capsicum spp., and Cucurbita spp. In: Survey of crop genetic resources in their centers of diversity. FAO, Roma, pp 76–115
Jarvis DI, Myer L, Klemick H, Guarino L, Smale M, Brown AHD (2000) A training guide for in situ conservation on-farm. Version 1. International Plant Genetic Resources Institute, Rome
Kimberling DN, Ferreira AR, Shuster SM, Keim P (1996) RAPD marker estimation of genetic structure among isolated northern leopard frog populations in south-western USA. Mol Ecol 5:521–529
Krauss SL (2000) Accurate gene diversity estimates from amplified fragment length polymorphism (AFLP) markers. Mol Ecol 9:1241–1245
Kremer A, Caron H, Cavers S, Colpaert N, Gheysen L, Gribel R (2005) Monitoring genetic diversity in tropical trees with multilocus dominant markers. Heredity 95:274−280
Ku-Naal R (1995) Cambios técnicos en la milpa bajo roza-tumba-quema en Yaxcabá, Yucatán. In: Hernández XE, Bello BE, Levy TS (eds) La milpa en Yucatán: Un sistema de producción agrícola tradicional. Colegio de Postgraduados, México, pp 401–418
Ladizinsky G (1985) Founder effect in crop-plant evolution. Econ Bot 39:191–198
Lioi L, Galasso I (2002) Oligonucleotide DNA fingerprinting revealing polymorphism in Phaseolus lunatus L. Genet Resour Crop Evol 49:53–58
Lioi L, Lotti C, Galasso I (1998) Isozyme diversity, RFLP of the rDNA and phylogenetic affinities among cultivated Lima beans, Phaseolus lunatus (Fabaceae). Plant Syst Evol 213:153–164
Lynch M, Milligan BG (1994) Analysis of population genetic structure with RAPD markers. Mol Ecol 3:91–99
Maquet A (1995) Etude de la diversité génetique de la légumineuse alimentaire Phaseolus lunatus L. par l’analyse de caracteres morphophysiologiques et de marqueurs protéiques. Thèse de Doctorat, Faculté Universitaire des Sciences Agronomiques, Gembloux, Belgique
Maquet A, Zoro Bi I, Delvaux M, Wathelet B, Baudoin JP (1997) Genetic structure of a Lima bean base collection using allozyme markers. Theor Appl Genet 95:980–991
Martínez-Castillo J (2005) Diversidad intraespecífica de Phaseolus lunatus L. e intensificación de la agricultura tradicional en la Península de Yucatán, México. Ph.D. Thesis, Centro de Investigación Científica de Yucatán, A. C., Mérida, México
Martínez-Castillo J, Zizumbo-Villarreal D, Perales-Rivera H, Colunga-GarcíaMarín P (2004) Intraspecific diversity and morpho-phenological variation in Phaseolus lunatus L. from the Yucatan Peninsula, Mexico. Econ Bot 58(3):354–380
Martínez-Castillo J, Zizumbo-Villarreal D, Gepts P, Delgado-Valerio P, Colunga-GarcíaMarín P (2006) Structure and genetic diversity of wild populations of Lima bean (Phaseolus lunatus L.) from the Yucatan Peninsula, Mexico. Crop Sci 46:1071–1080
Martínez-Castillo J, Zizumbo-Villarreal D, Gepts P, Colunga-GarcíaMarín P (2007) Gene flow and genetic structure in the wild-weedy-domesticated complex of Lima bean (Phaseolus lunatus L.) in its Mesoamerican center of domestication and diversity. Crop Sci 47:58–66
Martins M, Tenreiro R, Oliveira MM (2003) Genetic relatedness of Portuguese almond cultivars assessed by RAPD and ISSR markers. Plant Cell Rep 22:71–78
Mazhar F (1997) Nayakrishi Andoland: an initiative of the Bangladesh peasants for a better living. In: Sperling L, Loevinsohn M (eds) Using diversity: enhancing and maintaining genetic resources on-farm. International Development Research Centre, Ottawa
Miller MP (1997) Tools for population genetic analysis (TFPGA) 1.3: a windows program for the analysis of allozyme and molecular population genetic data. Distributed by the author
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Nienhuis J, Tivang J, Skroch P, dos Santos JB (1995) Genetic relationships among cultivars and landraces of Lima bean (Phaseolus lunatus L.) as measured by RAPD markers. J Am Soc Hortic Sci 120(2):300–306
Nybom H (2004) Comparison of different nuclear DNA markers for estimating intraspecific genetic diversity in plants. Mol Ecol 13:1143–1155
Page RDM (1996) TREEVIEW: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358
Payro de la Cruz E, Gepts P, Colunga-GarcíaMarín P, Zizumbo-Villarreal D (2005) Spatial distribution of genetic diversity in wild populations of Phaseolus vulgaris L. from Guanajuato and Michoacán, México. Genet Resour Crop Evol 52:589–599
Prevost A, Wilkinson M (1999) A new system of comparing PCR primers applied to ISSR fingerprinting of potato cultivars. Theor Appl Genet 98:107–112
Quiros CF, Ortega R, Van Raamsdonk LWD (1992) Amplification of potato genetic resources in their center of diversity: the role of natural outcrossing and selection by the Andean farmer. Genet Resour Crop Evol 39:107–113
Reyes GD, Aguilar CG (1992) Intensificación de la milpa en Yucatán. In: Zizumbo VD, Rasmussen Ch, Arias RLM, Terán S (eds) La modernización de la milpa en Yucatán: utopía o realidad. CICY-DANIDA, Mérida, pp 347–358
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Slatkin M (1987) Gene flow and the geographic structure of natural populations. Science 236:787–792
Tsegaye B, Berg T (2007) Genetic erosion of Ethiopian tetraploid wheat landraces in Eastern Shewa, Central Ethiopia. Genet Resour Crop Evol 54:715–726
Upadhyay MP, Sthapit BR (1998) Plant genetic resource conservation programs in Nepal: some proposals for scientific basis of in situ conservation of agrobiodiversity. Paper presented on the strengthening the scientific basis of in situ conservation of crop gene pools, from 17–19 July in Rome, Italy, IPGRI
Vavilov NI (1926) Centers of origin of cultivated plants. Bull Appl Bot Genet Plant Breed 16:248
Vekemans X (2002) AFLP-SURV version 1.0. Distributed by the author. Laboratoire de Génétique et Ecologie Végétale, Université Libre de Bruxelles, Belgium
Yeh FC, Boyle TJB (1999) Popgene v. 1.31. Microsoft Windows-based freeware for population analysis. University of Alberta and Centre for International Forestry Research, Edmonton
Zhivotovsky LA (1999) Estimating population structure in diploids with multilocus dominant DNA markers. Mol Ecol 8:907–913
Zietkiewicz E, Rafalski A, Labuda D (1994) Genome finger-printing by simple sequence repeat (SSR)-anchored polymerase chain reaction amplification. Genomics 20:176–183
Zizumbo-Villarreal D, Colunga-GarcíaMarín P, Payró de la Cruz E, Delgado-Valerio P, Gepts P (2005) Population structure and evolutionary dynamics of wild–weedy–domesticated complexes of common bean in a Mesoamerican region. Genet Resour Crop Evol 45:1073–1083
Acknowledgements
This research was done in the Plant Genetic Resources Diversity and Molecular Evolution Laboratory of the Department of Natural Resources-CICY. The authors thank Filogonio May-Pat for fieldwork assistance, Julian Coello and Patricia Flores for labwork assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Martínez-Castillo, J., Colunga-GarcíaMarín, P. & Zizumbo-Villarreal, D. Genetic erosion and in situ conservation of Lima bean (Phaseolus lunatus L.) landraces in its Mesoamerican diversity center. Genet Resour Crop Evol 55, 1065–1077 (2008). https://doi.org/10.1007/s10722-008-9314-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-008-9314-1