Abstract
Backgroup
Superfine grinding (SG) technology has attracted considerable attention in food and medicine researcher fields.
Methods
Polysaccharides in superfine powder of Gynostemma pentaphyllum Makino (GPP) were extracted using three methods, including hot water extraction (HWE), ultrasound-assisted hot extraction (UAE), and microwave-assisted hot extraction (MAE), and the purified polysaccharides were specially denoted as GPWP, GPUP, and GPMP, respectively. The possible structures of polysaccharides were investigated by FT-IR, HPLC and SEM. In addition, the antioxidative and immunomodulatory activities were evaluated by in vitro radical-scavenging activity assay and immune cell functional evaluation.
Results
We observed that the yield of GPUP (20.31%) was relatively higher than that of GPWP (15.34%) and GPMP (16.96%). Among all products, GPWP exhibited the highest antioxidative activities against DPPH, hydroxyl, and superoxide anion radicals. GPWP could also preferably chelate Fe2+ and protect against the oxidative damage by increasing the cellular levels of antioxidant enzymes (SOD, CAT and GSH-PX) and decreasing the content of oxidation product (MDA). Three polysaccharides presented some extent of immunoregulatory activity by promoting the phagocytosis of mononuclear macrophages and elevating the levels of NO, TNF-ɑ, and IL-6, and among which GPWP showed the best.
Conclusion
These results indicate that the HWE method is an excellent technique for extracting GPP with high bioactivities that would be suitable for various industrial applications.

Graphical Abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gynostemma pentaphyllum Makino (G. pentaphyllum), also known as Jiaogulan, belongs to the family Cucurbitaceae [1]. In China, Jiaogulan is considered as a medical and edible plant that has many health benefits. Polysaccharides, a kind of macromolecular compounds with relatively less toxic and highly bioactive, are an important bioactive component of Jiaogulan (GPP) [2]. Many studies indicated that GPP have exhibited several biological functions, such as antioxidant [3], antitumor [4], antifatigue [5] and immunomodulatory [6] activities.
Before the extraction of polysaccharides, the raw materials were ground and crushed into powder for efficient separation. Recently, superfine grinding (SG), a new technology, was widely used for obtaining superfine powders with sizes ranging from 1 nm to 100 μm [7]. Compared with traditional grind technology, SG greatly decrease particle size and increase reactive surface to the largest content, resulting to higher extraction yield [8]. Accordingly, SG technology has a high application potential in food and medicine fields. However, there are few reports of the SG technology applied for preparing superfine powders from Jiaogulan.
As we know, extraction methods, the fist and crucial step of polysaccharides research, can influence the yield, physicochemical properties, and bioactivities of polysaccharides. [9]. Currently, hot water extraction (HWE) is a conventional method that has some disadvantages, such as long extraction time and low extraction yield. Ultrasound-assisted hot extraction (UAE) and microwave-assisted hot extraction (MAE) are considered effective strategies that can overcome the above limitations. However, several reports have suggested that UAE and MAE can cause the degradation of polysaccharides and in turn can influence their bioactivity [10]. Thus, a comprehensive evaluation of GPP extractions by HWE, UAE and MAE, is very necessary to screen the optimal research design.
In this study, the superfine powder of G. pentaphyllum was obtained by SG technology and the size distribution of powder was investigated firstly. Subsequently, three polysaccharides (GPWP, GPUP and GPMP) were prepared by different extraction methods (HWE, UAE and MAE), respectively. The yields, structures, and antioxidant, immunomodulatory activities of the polysaccharides were analyzed and compared. The objective of this paper was to provide a theoretical support for further utilization of superfine powder G. pentaphyllum and a guideline for selecting a suitable extraction method for extracting high-activity polysaccharides with high bioactivities.
Materials and methods
Chemicals and reagents
1,1-Diphenyl-2-picryl-hydrazyl (DPPH), phenazine methosulfate (PMS), dihydro-nicotinamide adenine dinucleotide (NADH), and nitrobluetetrazolium (NBT) were purchased from Sigma (St. Louis, MO, USA). Fetal bovine serum (FBS) and penicillin-streptomycin were purchased from Gibco/BRL (Burlington, Canada). Ethanol, sodium salicylate, and other chemicals were purchased from Chengdu Kelong Chemical Factory (Chengdu, China). All standards were purchased from the National Institutes for Food and Drug control (Beijing, China).
Material preparation and pretreatment
Gynostemma pentaphyllum superfine powder was provided by Pingli Shencaoyuan Tea Co., Ltd. (Ankang, Shaanxi, China). The pretreatment of superfine powder was described in supplementary information. The size distribution of G. pentaphyllum powder was measured by dynamic light scattering (DLS) using a laser particle analyzer (Bruker, Germany).
Extraction of polysaccharides by different methods
Hot water extraction (HWE)
50 g of G. pentaphyllum superfine powder was extracted with water at a liquid:solid ratio of 20:1 (mL:g) at 80 °C for 120 min.
Ultrasound-assisted hot extraction (UAE)
50 g of G. pentaphyllum superfine powder was extracted with water at a liquid:solid ratio of 20:1 (mL:g) with an assistance of ultrasonic powder at 200 W for 40 min.
Microwave-assisted hot extraction (MAE)
50 g of G. pentaphyllum superfine powder was extracted with water at a liquid:solid ratio of 20:1 (mL:g) with an assistance from a microwave powder of 800 W for 15 min.
Preparation of polysaccharides
The solutions obtained from HWE, UAE and MAE were filtered using a Büchner funnel (100 mm) and subsequently were centrifuged (5000 rpm/min, 10 min) to further remove the raw materials. Next, the rusting solutions were concentrated to about one-quarter (250 mL) of the original volume by a rotary evaporator and precipitated by 4 volumes of 95% ethanol at 4 °C for 24 h. Subsequently, the precipitates were dried at 60 °C to obtain crude polysaccharides. The crude polysaccharides were re-dissolved in distilled water to obtain polysaccharide solutions, for which proteins were removed using Sevag reagent (chloroform:butyl alcohol, 4:1) [11]. After that, the deproteinated solutions were dialyzed against distilled water (MW cut off = 8–14 kDa) for 48 h to further remove small molecules. The non-dialyzable fractions, which were were lyophilized, and named GPWP, GPUP and GPMP, respectively. The yield was calculated by the following equation:
Where A is the weight of dried polysaccharides and B is the weight of G. pentaphyllum powder.
Preliminary characterization of polysaccharides
Chemical composition
The chemical compositions of GPWP, GPUP and GPMP, including the content of total polysaccharides, proteins and uronic acids, were determined according to previous methods using D-glucose, bovine serum albumin and D-glucuronic acid as standards, respectively [12,13,14].
Monosaccharide analysis and determination of molecular weight
The monosaccharide components of GPWP, GPUP, and GPMP were analyzed by HPLC using 1-phenyl-3-methyl-5-pyrazolone (PMP) pre-column derivatization method described previously with minor modifications [15]. Briefly, 2 mL of samples (4 mg/mL) was hydrolyzed by 2 mL of TFA at 100 °C for 6 h in an ampoules bottle. Subsequently, the TFA was removed by a rotary evaporator using methanol at 50 °C. The above residues were then dissolved in 0.4 mL of distilled water and derivatized with 0.5 mL of PMP-methanol (0.5 M) and 0.6 mL of NaOH (0.3 M) at 70 °C for 70 min. Finally, the reaction solution was neutralized with 0.5 mL of 0.3 M HCl. After that, it was filtered through a 0.45-μm membrane and then subjected to HPLC analysis (Agilent Technologies, USA). The molecular weight (Mw) of GPWP, GPUP, and GPMP were determined following a previous report by HPLC equipped with an evaporative light scattering detector (ELSD) and a ReproSil 200 SEC column (300 × 8 mm, 5 μm). A series of dextran standards with different concentrations were prepared and used to construct the standard curve [16].
Fourier transform infrared (FT-IR) spectroscopy
The function groups of GPWP, GPUP, and GPMP were analyzed by FT-IR (PerkinElmer, USA) at a frequency range of 4000 to 400 cm−1.
Scanning electron microscopy (SEM)
The morphology of GPWP, GPUP, and GPMP were observed at 70× and 140× magnifications by an SEM (S-3400 N, Japan) [17] operated at an accelerating voltage of 10 kV. Prior to the observation, the samples were sputtered with gold powder.
Congo red test
The conformational structures of GPWP, GPUP, and GPMP were determined by Congo red test [17]. Briefly, 1 mL of samples (1 mg/mL) were mixed with 2 mL of Congo red solution (80 μM). After that, 1 M NaOH was added to the mixture to final concentrations of 0 to 0.5 M. Finally, the maximum absorption wavelength (λmax) of the reaction solutions were determined by a spectrophotometric microplate reader (SpectraMax M5, USA) operated at a wavelength range of 200 to 700 nm.
In vitro antioxidative activity of polysaccharides
Radical-scavenging activity
The DPPH-, hydroxyl-, and superoxide anion-scavenging activities, and the Fe2+-chelating abilities of GPWP, GPUP, and GPMP were determined by in vitro chemistry model. The test protocols were described in supplementary information.
Cell culture
The embryonic fibroblast NIH-3T3 cell line, which was obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China), was cultured in DMEM medium (Sigma-Aldrich) supplemented with 10% FBS (Hyclone, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 mM L-glutamine. The cells were incubated at 37 °C in an incubator saturated with 5% CO2.
Before use, the samples were dissolved in the culture medium, and then filtered through a 0.22-μm membrane filter.
Cytotoxicity evaluation
The cell toxicity of GPWP, GPUP, and GPMP were evaluated using cell counting kit-8 (CCK-8). Briefly, cells were cultured in 96-well plates at 5 × 103 cell/well. After that, they were treated with different concentrations of samples (125–2000 μg/mL) for 24 h. The cell viability was determined using CCK-8 kit.
H2O2 was used to induce oxidative stress in cells [18]. Briefly, cells were cultured in 96-well plates at 5 × 103 cell/well for 24 h. Subsequently, they were treated with different concentrations of H2O2 (100–900 μM) for 24 h. The cell viability was then determined by CCK-8 kit. In evaluation of the protective effects of polysaccharides against H2O2-induced cell oxidative stress, the cells (5 × 103 cell/well) were pre-treated with GPWP, GPUP, and GPMP in 96-well plates for 24 h. After the medium was removed, the cells were exposed to 800 μM H2O2 for 24 h, and their viability was then determined by CCK-8 assay.
Flow cytometry
NIH-3T3 cells were divided into three groups: the control group (medium), the model group (800 μM H2O2), and the treated group (polysaccharides + H2O2). The cells (3 × 104 cell/well) in each group were cultured in 24-well plates for 48 h. After that, they were harvested, washed three times with PBS, and then subjected to further analysis.
Intracellular ROS production was examined using 2, 7-dichlorofluorescein diacetate (DCFH-DA) according to the reported method [19]. The cells in each group were incubated with DCFH-DA (10 μM) at 37 °C for 30 min in darkness. After washing with PBS to remove the probe, the cells were analyzed by a flow cytometer (NovoCyte, ACEA Biosciences, San Diego, CA, USA).
Apoptotic cells were analyzed by Annexin V-FITC/PI kit (Keygen Technology Co, Ltd., China) according to the manufacturer’s instruction. Briefly, the cells (100 μL) in each group were stained with annexin V (1 μL) and PI (1 μL) at 37 °C for 20 min in darkness. After washing with PBS to remove the dyes, the cells were analyzed using a flow cytometer.
Biochemical assay
After being treated with GPWP, GPUP and GPMP, as described in Flow cytometry part, the cells were sonicated in an ice-water bath and then centrifuged at 3000 rpm/min for 10 min. The supernatant was collected and stored at 4 °C before use. The protein content was determined by the BCA method. The antioxidant activities of enzymes SOD, CAT, and GSH-Px, and the oxidation product (MDA), were measured by the corresponding kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the manufacturer’s manual.
Immunostimulatory activity of polysaccharides
Cell culture
The murine macrophage RAW264.7 cells, which were obtained from the Chinese Academy of Sciences Cell Bank (Shanghai, China), were cultured in DMEM medium (Sigma-Aldrich) containing 10% FBS (Hyclone, USA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 1 mM L-glutamine. The cells were incubated in an incubator at 37 °C under 5% CO2 atmosphere.
Prior to use, the GPWP, GPUP and GPMP samples were dissolved in the medium and then filtered through a 0.22-μm membrane filter. LPS (1 μg/mL) was used as a positive control.
Cytotoxicity assay
The cell toxicity of the samples was evaluated by CCK-8 kit. Briefly, the cells were cultured in 96-well plates at 1 × 104 cell/well and were then treated with the samples at different concentrations (125–1000 μg/mL) for 24 h. After that, the cell viability was determined by CCK-8 kit.
Phagocytosis assay
The phagocytic activity of RAW264.7 was evaluated by neutral red uptake assay. The experimental cells, were divided into three groups: the control group (medium), the positive control group (LPS), and the polysaccharides treated group. After co-culture for 24 h, the medium was replaced with 100 μL of neutral red (0.1% w/v) and incubated for 1 h. Subsequently, the cells were washed with PBS and then lysed using 100 μL of ethanol solution (50%) containing acetic acid (0.1% v/v) for 12 h. The absorbance of the lysed cells was measured at 540 nm thereafter.
NO and cytokine assays
Cells were cultured in 24-well plates at 2 × 104 cell/well and were divided into three groups according to Phagocytosis assay part. After incubation for 24 h, the culture supernatants were collected and stored at 4 °C before use. The NO content was measured by NO assay kit, and the content of TNF-ɑ and IL-6 were determined by ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) based on the manufacturer’s instruction.
Data analysis
All data were the averages from at least three repeats experiments presented as means ± SDs. All data analyses were performed using SPSS 23.0 software. *P < 0.05 and **P < 0.01 were considered significantly different and highly significant difference, respectively.
Results and discussion
Characterization of polysaccharides
Chemical composition
As shown in Fig. S1, the average diameter of G. pentaphyllum superfine powder was 1184.62 ± 153.92 nm. The extraction yields and chemical compositions of GPWP, GPUP, and GPMP are summarized in Table 1. The yield of GPUP, GPWP and GPMP was 15.34%, 16.96% and 20.31%, respectively. Previous review summarized the polysaccharides yield from G. pentaphyllum with ranging from 2.49% to 11.44%, which significantly was lower than our experimental yield [20]. Superfine powder showed good surface properties, such as dispersibility and solubility, increasing the yield of bioactive compounds [8]. Additionally, the yield of GPUP (20.31%) was significantly higher than that of GPWP (15.34%) and GPMP (16.96%), which is in agreement with the findings in most previous studies [21]. Ultrasound, which is a type of mechanical wave that can produce intense pressures, shear forces, and temperature gradient due to bubble cavitation, can rupture plant cell walls and accelerate the release of intracellular polysaccharide into water, thus in turn increase the extraction efficiency [22]. In addition, the contents of carbohydrate, protein, and uronic acid in all three polysaccharides were significantly different. GPMP consisted of lower uronic acid content (3.06%) compared to GPWP (4.35%), which might be due to the degradation of polysaccharide during microwave treatment [23]. HPLC analysis showed that the three samples exhibited a single meristic peak, indicating that the obtained polysaccharides were homogeneous (Table. 1 and Fig. S2A-C). Comparison of the Mw, which were calculated using the formula: log Mw = −0.281 t + 8.279 (R2 = 0.9839), where Mw represents the molecular weight of the dextran standard and t is the retention time, obviously showed that GPWP had the highest Mw among all three samples.
Constituent monosaccharides
Monosaccharides are the basic units that contribute to the unique structures and properties of polysaccharides. As shown in Table. 1 and Fig. S3, the peaks of eight PMP-derived standard monosaccharides were well separated within 60 min. Man, Rha, GalA, Glu, Gal and Ara in GPWP were 2.36%, 6.55%, 10.92%, 50.95% 14.02%, and 15.20%, respectively, whereas those in GPUP were 1.48%, 3.21%, 5.26%, 73.46%, 7.66%, and 8.93%, respectively, and those in GPMP were 2.84%, 6.59%, 10.30%, 50.82%, 14.22%, and 15.24%, respectively. Obviously, the Gal and GalA content of GPUP was lower than that of GPWP and GPMP, which probably was associated ultrasound to induce the degradation of polysaccharide chains, breaking of intermolecular hydrogen bonds and atomic rearrangement [24]. These results are consistent with the with the report by Guo et al. [25], suggesting that the utilized extraction processes had no influence on the types of monosaccharides, but had some influence on their percentages.
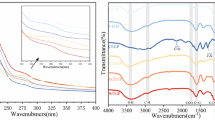
FT-IR spectra
The FT-IR spectra of the three polysaccharides were not significantly different. They revealed the typical absorption peaks of carbohydrates (Fig. 1a). Bands at 3300 cm−1 and 2930 cm−1 are due to O-H stretching vibrations and C-H stretching and bending vibrations, respectively [26]. Moreover, a band at 1737 cm−1 is caused by C=O stretching vibration of esterified groups, and a band at 1606 cm−1 was assigned to C=O asymmetric stretching of COO−, which indicates the presence of uronic acids [23]. These results are consistent with the monosaccharide composition and m-hydroxyphenyl assay results. In addition, a band at 1148 cm−1 is due to the asymmetric C-O-C stretching vibration, which shows the existence of -OCH3 group. A band at 1420 cm−1 indicates the bending vibration of C-H or O-H [27, 28]. Overall, the extraction process had no effect on the characteristic organic groups of the polysaccharides.
FT-IR spectrums (a) and Conformational structure (b) of polysaccharides obtained from three extraction methods: GPWP, polysaccharide extracted by hot water extraction; GPUP, polysaccharide extracted by ultrasound-assisted hot extraction; and GPMP, polysaccharide extracted by microwave-assisted hot extraction
Conformational structure
The conformational structure is important for polysaccharides to maintain their bioactivities [29]. Polysaccharides with conformational structures that can form special complexes with Congo red, then lead to a red-shift of λmax of Congo red. However, the λmax of the complexes can decrease with the increase of NaOH concentration, because NaOH can disrupt the conformational structures of polysaccharides. As shown in Fig. 1b, among all three polysaccharides, only GPWP showed a red-shift of λmax, indicating that its conformational structure was altered. This also suggest the polysaccharides in GPWP rather than GPUP and GPMP, kept the native conformation after extraction process.
SEM
The surface topography of three polysaccharides were observed by SEM. As shown in Fig. 2, the surface of GPWP was smooth and flat, whereas that of GPUP and GPMP were rough with many cavities, which may be caused by ultrasonic cavitation and microwave stimulation. A previous report has suggested that the change of surface of polysaccharides is likely caused by the changes of physicochemical properties and the extraction methods [30].
SEM images of polysaccharides obtained from three extraction methods: (A:70×, a:140×) GPWP, polysaccharide extracted by hot water extraction; (B: 70×, b: 140×) GPUP, polysaccharide extracted by ultrasound-assisted hot extraction; and (C:70×, c:140×) GPMP, polysaccharide extracted by microwave-assisted hot extraction
Antioxidative activities
Radical-scavenging activity
DPPH•-scavenging activity is examined to assess the ability to supply hydrogen of the samples. As shown in Fig. 3a, three polysaccharides (GPWP, GPUP, and GPMP) at concentrations from 62.5 to 2000 μg/mL exhibited good DPPH•-scavenging activity in a concentration-dependent manner with the half-inhibition concentration (IC50) values of 297.48, 405.92, and 275.79 μg/mL, respectively. This also indicates that GPWP and GPMP could better supply hydrogen than GPUP.
Antioxidant activities of polysaccharides obtained from three extraction methods: (a) DPPH radical; (b) Hydroxyl radical; (c) Fe2+ chelating and (d) Superoxide anion radical. DPPH, 1,1-diphenyl-2-picrylhydrazyl; GPWP, polysaccharide extracted by hot water extraction; GPUP, polysaccharide extracted by ultrasound-assisted hot extraction; and GPMP, polysaccharide extracted by microwave-assisted hot extraction
As one of the most harmful ROS, •OH can attack all biomolecules, such as lipids, proteins, and DNA, in turn causing cell death and tissue damage [31]. Thus, it is important to assess the hydroxide radical-scavenging ability of the samples. As shown in Fig. 3b, GPWP displayed a relatively higher and dose-dependent •OH-scavenging activity than GPUP and GPMP, with the concentrations ranging from 62.5 to 2000 μg/mL, the •OH-scavenging activity was concentration-dependent. The IC50 value of GPWP was 1086.14 μg/mL. However, the IC50 values of GPUP and GPMP could not be calculated. Additionally, Fe2+ can generate •OH through the Fenton reaction (Fe2+ + H2O2 = Fe3+ + OH− + •OH). Thus, the metal chelating ability is widely recognized as one of the antioxidant activities. As shown in Fig. 3c, all samples exhibited excellent Fe2+ chelating activity in a concentration-dependent manner. The IC50 values were 51.08, 47.27, and 37.37 μg/mL, respectively. Qu et al. have suggested that the chelating ability of polysaccharides is associated with the formation of cross-bridges between the carboxyl group in uronic acid and the divalent ions [32]. Polysaccharides, which contain many active hydroxyl groups, can directly scavenge •OH, or can suppress the generation of •OH by chelating Fe2+.
Superoxide anion radical, which is a free radical, can form stronger ROS, including •OH, singlet oxygen, and H2O2, and can ultimately cause many diseases [33]. As shown in Fig. 3d, both GPWP and GPMP exhibited a better •O2−-scavenging activity than GPUP. The IC50 values of GPWP and GPMP were 898.79 and 1738.65 μg/mL, respectively.
Cytotoxicity and protective effect
The cytotoxicity of GPWP, GPUP, and GPMP at different concentrations (125–2000 μg/mL) on NIH-3T3 cell are shown in Fig. 4a. At the maximum concentration (2000 μg/mL), all three samples had negative effect on cell viability. Thus, only the samples at concentrations of 125–1000 μg/mL were selected for further experiments.
(a) Cytotoxicity of polysaccharides obtained from three extraction methods; (b) Oxidative stress model by H2O2, and (c) Protection of polysaccharides obtained from three extraction methods in NIH-3T3 cell. GPWP, polysaccharide extracted by hot water extraction; GPUP, polysaccharide extracted by ultrasound-assisted hot extraction; and GPMP, polysaccharide extracted by microwave-assisted hot extraction. *P < 0.05 and **P < 0.01 were considered significantly different and highly significant difference, respectively
As a primary source of ROS, H2O2 can lead to cellular oxidative stress and induce cell apoptosis. As shown in Fig. 4b, at concentrations ranging from 200 to 900 μM, H2O2 could significantly inhibit the cell viability in a concentration-dependent trend. With H2O2 at a concentration of 800 μM, the cell viability was about 50%. Thus, this concentration was chosen to induce cellular oxidative injury.
The protective effect of GPWP, GPUP, and GPMP against oxidative injury shown in Fig. 4c indicated that the pre-treatment of cells with the three polysaccharides at concentrations from 125 to 1000 μg/mL could significantly increase the cell viability in a concentration-dependent tendency. The viabilities of the cells were 94%, 87%, and 89% when they were treated with GPWP, GPUP, and GPMP (1000 μg/mL), respectively. The results suggest that GPWP had a higher protective effect against oxidative injury than GPUP and GPMP.
Inhibition of ROS and apoptosis
The intracellular ROS was measured by DCFH-DA after different treatments. As shown in Fig. 5, the model group exhibited significantly higher ROS levels (35.12%) than the control group (1.95%). However, when cells were pre-incubated with GPWP, GPUP, and GPMP, the overproduction of ROS was significantly decreased to 6.22%, 8.37%, and 6.36%, respectively. Similarly, the mean DCF fluorescence intensity of the three sample groups were significantly decreased compared with that of the model group.
Effects of polysaccharides obtained from three extraction methods on cell ROS production: (a) Different group; (b) Histogram of intracellular ROS. GPWP, polysaccharide extracted by hot water extraction; GPUP, polysaccharide extracted by ultrasound-assisted hot extraction; and GPMP, polysaccharide extracted by microwave-assisted hot extraction. *P < 0.05 and **P < 0.01 were considered significantly different and highly significant difference, respectively
Flow cytometry was employed to detect cell apoptosis, as shown in Fig. 6. The model group had a higher apoptosis rate (50.03%) than the control group (1.5%). However, when cells were pre-incubated with GPWP, GPUP, and GPMP, the apoptosis rates were significantly decreased to 6%, 11.43%, and 9.13%, respectively. Both the ROS and the apoptosis data were coincided well with the aforementioned protective results, indicating that GPWP has higher antioxidant activity than GPUP and GPMP.
Effects of polysaccharides obtained from three extraction methods on cell apoptosis: (a) Different group; (b) Histogram of apoptotic rate. GPWP, polysaccharide extracted by hot water extraction; GPUP, polysaccharide extracted by ultrasound-assisted hot extraction; and GPMP, polysaccharide extracted by microwave-assisted hot extraction. *P < 0.05 and **P < 0.01 were considered significantly different and highly significant difference, respectively
Antioxidant enzyme activities and MDA level
Normal cells maintain their ROS balance through the enzymatic system, such as the actions of antioxidant enzymes including SOD, CAT, and GSH-PX, as well as through the non-enzymatic system, e.g. through actions of GSH and vitamin E [34]. As shown in Figs. 7a-c, the model group exhibited significantly lower SOD, CAT, and GSH-PX activities than the control group. However, the SOD activity of cells treated with GPWP, GPUP, and GPMP was remarkably increased by 54.5%, 24.0%, and 38.1%, respectively, compared with that of the model group. Similarly, compared with that of the model group, the CAT activity was increased by 46.5%, 31.4%, and 37.0%, respectively, and the GSH-PX activity was increased by 57.4%, 32.5%, and 36.7%, respectively. In addition, MDA as the end product of lipid peroxidation, has been usually used as the indicator of fatty oxidation [35]. As shown in Fig. 7d, the MDA content of the cells pretreated with GPWP, GPUP, and GPMP was significantly decreased by 67.1%, 50.2%, and 57.4%, respectively, compared with that of the model group. These data suggest that the GPWP could preferably enhance the antioxidant enzyme activities and decrease the MDA content, thus could better ameliorate the injury caused by oxidative stress, as compared with GPUP and GPMP.
Effects of polysaccharides obtained from three extraction methods on antioxidant enzymes activities and oxidation product: (a) SOD activity; (b) CAT activity; (c) GSH-Px activity and (d) MDA content. SOD, Superoxide dismutase; CAT, Catalase; Glutathione peroxidase and MDA, Malondialdehyde. GPWP, polysaccharide extracted by hot water extraction; GPUP, polysaccharide extracted by ultrasound-assisted hot extraction; and GPMP, polysaccharide extracted by microwave-assisted hot extraction. *P < 0.05 and **P < 0.01 were considered significantly different and highly significant difference, respectively
Immunomodulatory activity
Cytotoxicity
The cytotoxicity of GPWP, GPUP, and GPMP at different concentrations (250–1000 μg/mL) on RAW264.7 cell is shown in Fig. S4. The viability of cells in the presence of GPWP, GPUP, and GPMP each at the maximal 1000 μg/mL were 106%, 107%, and 104%, respectively, which are not different from that in the control. Additionally, the cell viability in the presence of LPS was significant enhanced to 150%. The results indicate that all samples were not toxic to RAW264.7 cells. Therefore, the sample concentration of 1000 μg/mL was chosen for subsequent analysis.
Phagocytic activity and release of NO, TNF-ɑ, and IL-6
As the first line of defense during innate immunity, macrophage cells not only have a direct phagocytic ability, but also indirect resort to the production of pro-inflammatory cytokines, such as TNF-ɑ, IL-6, and NO, which can kill pathogenic microorganisms [36]. As shown in Fig. 8a, upon the treatment with the three samples, the phagocytosis was significantly improved. However, the activation of the sample group remained weaker than that of the LPS group. This result shows that GPWP can better enhance the phagocytosis of macrophages cells than GPUP and GPMP.
Effects of polysaccharides obtained from three extraction methods on phagocytic ability and cytokine production. (a) phagocytosis activity; (b) NO production (c) TNF-ɑ production and (d) IL-6 production. No, Nitric Oxide; TNF-ɑ, Tumor Necrosis Factor-ɑ and IL-6, Interleukin −6. GPWP, polysaccharide extracted by hot water extraction; GPUP, polysaccharide extracted by ultrasound-assisted hot extraction; and GPMP, polysaccharide extracted by microwave-assisted hot extraction. *P < 0.05 and **P < 0.01 were considered significantly different and highly significant difference, respectively
The effects of GPWP, GPUP, and GPMP on the macrophage activation were evaluated through the secretion of NO, IL-6, and TNF-α. As shown in Fig. 8B-D, all samples could significantly stimulate the production of the three cytokines in RAW264.7 cells. Specifically, the secretion of NO caused by GPWP, GPUP, and GPMP was significantly increased from 4.24 (control group) to 21.26, 16.33, and 18.33 μM, respectively, that of TNF-α was increased from 43.70 (control group) to 63.35, 53.32, and 57.32 pg/mL, respectively, and that of IL-6 was increased from 6.50 (control group) to 16.56, 11.64, and 13.02 pg/mL, respectively. Similar to the neutral red uptake results, these findings suggest that GPWP can more strongly promote the secretion of NO, TNF-ɑ, and IL-6 than GPUP and GPMP.
In summary, HWE extracts (GPWP) showed better activity and immunomodulator activities than UAE and MAE extracts (GPUP and GPMP). It is well known that the bioactivities of polysaccharides were strongly influenced by various factors, such as chemical components, Mw, monosaccharide composition and ratios, as well as configurations [37].
In general, the radical scavenging activities of polysaccharides were related with their electron- or hydrogen-donating ability. Uronic acid groups in polysaccharides can interact with the hydrogen atom of anomeric carbons and therefore affect the activities of polysaccharide. Previous reports have revealed that the polysaccharides with higher uronic acid contents usually showed stronger activity [38]. Meanwhile, some researches indicated the contents of mannose and galactose were highly relevant to antitumor and immunomodulator activities [39]. Furthermore, it was supposed that polysaccharides with higher Mw showed more significant activities compared with lower ones. For example, Xu et al. used DEAE-52 cellulose obtaining two different polysaccharide fragments EAP-1 N and EAP-2A (eluted with distilled water and 0.5 M NaCl). Their results showed that EAP-2A exhibited a stronger antioxidant capacity both in vitro and in vivo than EAP-1 N, which might be associated with its higher uronic acid content and larger Mw [40]. Additionally, many other studies have also indicated that polysaccharides with helical conformations and smooth surfaces tend to have higher activities [41]. In this paper, we demonstrated that the HWE extracts (GPWP) with highest content (GalA and Gal) and Mw, relatively smooth surface, and helical conformation than other extracts (GPUP and GPMP). Hence, the highest bioactivities of GPWP may be the combination of several factors, rather than just a single factor. The explorations of structure-activity relation of GPWP are currently underway.
Conclusions
In conclusion, we prepared G. pentaphyllum superfine powder with size distribution 1000 nm and demonstrated that the polysaccharides yield (15.34%–20.31%) from superfine powder was significantly higher than common powder (2.49%–11.44%). Next, the effects of three extraction techniques (HWE, UAE, and WAE) on the yields, characteristics, and antioxidant and immunomodulatory activities of polysaccharides (GPWP, GPUP and GPMP) from G. pentaphyllum superfine powder were evaluated. The results showed that all samples had similar functional groups, but their monosaccharide contents, molecular weights, helical conformations, and surface morphologies were significantly different. Whereas GPUP had the highest yield, GPWP exhibited the highest bioactivity, e.g. in scavenging free radicals in vitro, and could ameliorate cellular oxidative injury and enhance host immunity. Collectively, the HWE method is a suitable technique for extracting polysaccharides with high bioactivities, and the GPWP is a potential antioxidant that can be applied as immunoregulatory active ingredients in food, medicine and cosmetics industries.
References
Yang, X., Zhao, Y., Yang, Y., Ruan, Y.: Isolation and characterization of immunostimulatory polysaccharide from an herb tea, Gynostemma pentaphyllum Makino. J. Agr. Food. Chem. 56(16), 6905–6909 (2008)
Li, B., Zhang, X., Wang, M., Jiao, L.: Characterization and antioxidant activities of acidic polysaccharides from Gynostemma pentaphyllum (Thunb.) Markino. Carbohyd. Polym. 127, 209–214 (2015)
Wang, Z., Wang, Z., Huang, W., Suo, J., Chen, X., Ding, K., Sun, Q., Zhang, H.: Antioxidant and anti-inflammatory activities of an anti-diabetic polysaccharide extracted from Gynostemma pentaphyllum herb. Int. J. Biol. Macromol. 145, 484–491 (2020)
Li, X., Wang, Z., Zhao, Y., Luo, S., Zhang, D., Xiao, S., Peng, Z.: Isolation and antitumor activities of acidic polysaccharide from Gynostemma pentaphyllum Makino. Carbohyd. Polym. 89, 942–947 (2012)
Chi, A., Tang, L., Zhang, J., Zhang, K.: Chemical composition of three polysaccharides from Gynostemma pentaphyllum and their antioxidant activity in skeletal muscle of exercised mice. Int, J. Sprot. Nutr. Exe. 22, 479–485 (2012)
He, X., Wang, Z., Xiao, Y., Zhou, L., Ruan, Z., Chen, X., Hu, M., Zheng, M., Su, X., Deng, X.: Gynostemma pentaphyllum polysaccharide prevents the growth of h22 ascites tumour by enhancing immunity rather than cytotoxicity in mice. Food. Agr. Immunol. 31, 367–378 (2020)
Xiao, W., Zhang, Y., Fan, C., Han, L.: A method for producing superfine black tea powder with enhanced infusion and dispersion property. Food Chem. 214, 242–247 (2017)
Zhang, L., Cheng, Z., Zhao, Q., Wang, M.: Green and efficient PEG-based ultrasound-assisted extraction of polysaccharides from superfine ground lotus plumule to investigate their antioxidant activities. Ind. Crop. Prod. 109, 320–326 (2017)
Zhu, J., Chen, Z., Zhou, H., Yu, C., Han, Z., Shao, S., Hu, X., Wei, X., Wang, Y.: Effects of extraction methods on physicochemical properties and hypoglycemic activities of polysaccharides from coarse green tea. Glycocojugate. J. 37, 241–250 (2020)
Chen, S., Shang, H., Yang, J., Li, R., Wu, H.: Effects of different extraction techniques on physicochemical properties and activities of polysaccharides from comfrey (Symphytum officinale L.) root. Ind. Crop. Prod. 121, 18–25 (2018)
Yang, J., Tu, J., Liu, H., Wen, L., Jiang, Y., Yang, B.: Identification of an immunostimulatory polysaccharide in banana. Food Chem. 277, 46–53 (2019)
Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.T., Smith, F.: Colorimetric method for determination of sugars and related substances. Anal. Chem. 28(3), 350–356 (1956)
Smith, P.E., Krohn, R.I., Hermanson, G., Mallia, A., Gartner, F., Provenzano, M., Fujimoto, E., Goeke, N., Olson, B., Klenk, D.: Measurement of protein using bicinchoninic acid. Anal. Biochem. 150(1), 76–85 (1985)
Blumenkrantz, N., Asboe-Hansen, G.: New method for quantitative determination of uronic acids. Anal. Biochem. 54(2), 484–489 (1973)
Li, Q., Li, Q., Hao, Z., Zheng, X., He, W.: A novel polysaccharide from Rhizoma panacis japonica exerts anti-inflammatory effects via STAT3 signal pathway. RSC Adv. 8(46), 26371–26376 (2018)
Pérez-López, E., Mateos-Aparicio, I., Rupérez, P.: Determination of soluble dietary fibre content of Okara treated with high hydrostatic pressure and enzymes: a comparative evaluation of two methods (AOAC and HPLC-ELSD). J. Food Sci. Technol. 54(5), 1333–1339 (2017)
Feng, S., Luan, D., Ning, K., Shao, P., Sun, P.: Ultrafiltration isolation, hypoglycemic activity analysis and structural characterization of polysaccharides from Brasenia schreberi. Int. J. Biol. Macromol. 135, 141–151 (2019)
Zhang, W., Hu, Y., Zhao, J., Zhang, Y., Guo, D., Gao, C., Gao, C., Duan, J., Li, P.: Immunoregulation and antioxidant activities of a novel acidic polysaccharide from Radix Paeoniae Alba. Glycocojugate. J. 37, 361–371 (2020)
Feng, X., Shi, Y., Xie, L., Zhang, K., Wang, X., Liu, Q., Wang, P.: Synthesis, characterization, and biological evaluation of a porphyrin-based photosensitizer and its isomer for effective photodynamic therapy against breast cancer. J. Med. Chem. 61(16), 7189–7201 (2018)
Ji, X., Shen, Y., Guo, X.: Isolation, Structures, and Bioactivities of the Polysaccharides from Gynostemma pentaphyllum (Thunb.) Makino. A Review. Biomed. Res. Int. 2018, 521–532 (2018)
He, L., Yan, X., Liang, J., Li, S., He, H., Xiong, Q., Lai, X., Hou, S., Huang, S.: Comparison of different extraction methods for polysaccharides from Dendrobium officinale stem. Carbohyd. Polym. 198, 101–108 (2018)
Alboofetileh, M., Rezaei, M., Tabarsa, M., Rittà, M., Donalisio, M., Mariatti, F., You, S., Lembo, D., Cravotto, G.: Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 124, 131–137 (2019)
Yuan, Q., Lin, S., Fu, Y., Nie, X.-R., Liu, W., Su, Y., Han, Q.-H., Zhao, L., Zhang, Q., Lin, D.-R.: Effects of extraction methods on the physicochemical characteristics and biological activities of polysaccharides from okra (Abelmoschus esculentus). Int. J. Biol. Macromol. 127, 178–186 (2019)
Gao, W., Zhang, P., Lin, P., Zeng, X., Brennan, M.: Comparison of litchi polysaccharides extracted by four methods: composition, structure and in vitro antioxidant activity. Int. J. Food. Sci. Tech. 55, 1343–1350 (2020)
Guo, H., Yuan, Q., Fu, Y., Liu, W., Su, Y., Liu, H., Wu, C., Zhao, L., Zhang, Q., Lin, D., Chen, H., Qin, W., Wu, D.: Extraction Optimization and Effects of Extraction Methods on the Chemical Structures and Antioxidant Activities of Polysaccharides from Snow Chrysanthemum (Coreopsis tinctoria). Plymers. 11, 215 (2019)
Abuduwaili, A., Rozi, P., Mutailifu, P., Gao, Y., Nuerxiati, R., Aisa, H.A., Yili, A.: Effects of different extraction techniques on physicochemical properties and biological activities of polysaccharides from Fritillaria pallidiflora Schrenk. Process Biochem. 83, 189–197 (2019)
Pereira, P.H.F., Oliveira, T.Í.S., Rosa, M.F., Cavalcante, F.L., Moates, G.K., Wellner, N., Waldron, K.W., Azeredo, H.M.: Pectin extraction from pomegranate peels with citric acid. Int. J. Biol. Macromol. 88, 373–379 (2016)
Zheng, W., Zhao, T., Feng, W., Wang, W., Zou, Y., Zheng, D., Takase, M., Li, Q., Wu, H., Yang, L.: Purification, characterization and immunomodulating activity of a polysaccharide from flowers of Abelmoschus esculentus. Carbohyd. Polym. 106, 335–342 (2014)
Shang, H., Chen, S., Li, R., Zhou, H., Wu, H., Song, H.: Influences of extraction methods on physicochemical characteristics and activities of Astragalus cicer L. polysaccharides. Process Biochem. 73, 220–227 (2018)
Cheng, H., Feng, S., Jia, X., Li, Q., Zhou, Y., Ding, C.: Structural characterization and antioxidant activities of polysaccharides extracted from Epimedium acuminatum. Carbohydr. Polym. 92(1), 63–68 (2013)
Chen, L., Huang, G.: Antioxidant activities of sulfated pumpkin polysaccharides. Int. J. Biol. Macromol. 126, 743–746 (2019)
Qu, Y., Li, C., Zhang, C., Zeng, R., Fu, C.: Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohyd. Polym. 148, 345–353 (2016)
Barroso, M.F., De-Los-Santos-Álvarez, N., Lobo-Castañón, M.J., Miranda-Ordieres, A.J., Delerue-Matos, C., Oliveira, M.B.P.P., Tuñón-Blanco, P.: Electrocatalytic evaluation of DNA damage by superoxide radical for antioxidant capacity assessment. J. Electroanal. Chem. 659(1), 43–49 (2011)
Chen, L., Long, R., Huang, G., Huang, H.: Extraction and antioxidant activities in vivo of pumpkin polysaccharide. Ind. Crop. Prod. 146, 112199 (2020)
Cheeseman, K.: Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Method. Enzymol. 186, 406–413 (1994)
Chen, F., Huang, G.: Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 112, 211–216 (2018)
Xu, Z., Feng, S., Qu, J., Yuan, M., Yang, R., Zhou, L., Chen, T., Ding, C.: The effect of extraction methods on preliminary structural properties and antioxidant activities of polysaccharides from Lactarius vividus. Processes. 7, 482 (2019)
Chen, G., Yuan, B., Wang, H., Qi, G., Cheng, S.: Characterization and antioxidant activity of polysaccharides obtained from ginger pomace using two different extraction processes. Int. J. Biol. Macromol. 139, 801–809 (2019)
Li, X., Qin, G., Cheng, C., Yuan, B., Huang, B., Cheng, S., Cao, C., Chen, G.: Purification, characterization and anti-tumor activities of polysaccharides from Ecklonia kurome obtained by three different extraction methods. Int. J. Biol. Macromol. 150, 1000–1010 (2020)
Xu, Z., Feng, S., Shen, S., Wang, H., Yuan, M., Liu, J., Huang, Y., Ding, C.: The antioxidant activities effect of neutral and acidic polysaccharides from Epimedium acuminatum Franch. On Caenorhabditis elegans. Carbohyd. Polym. 144, 122–130 (2016)
Wang, Y., Wei, X., Wang, F., Xu, J., Tang, X., Li, N.: Structural characterization and antioxidant activity of polysaccharide from ginger. Int. J. Biol. Macromol. 111, 862–869 (2018)
Acknowledgments
This work was supported by National Natural Science Foundation of China (81571834, 31670299), the Fundamental Research Funds for the Central Universities (GK201906008, 2019CSLY028), the National Key Technologies R & D Program for Modernization of Traditional Chinese Medicine (2017YFC1701300, 2017YFC1700706), the Key R&D Program of Shaanxi Province (2019SF-307), Xi’an Science and Technology Project (20NYYF0057), and Research Project on Postgraduate Education and Teaching Reform of Shaanxi Normal University (GERP-20-41).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• We prepared the superfine powder of Gynostemma pentaphyllum Makino.
• G. pentaphyllum polysaccharides (GPP) were extracted and compared by three methods.
• The polysaccharide by hot water extraction (HWE) showed stringer antioxidant and immunomodulatory activities
• HWE method is an excellent technique for extracting high-activity GPP that are suitable for various industrial applications
Rights and permissions
About this article
Cite this article
Wang, B., Niu, J., Mai, B. et al. Effects of extraction methods on antioxidant and immunomodulatory activities of polysaccharides from superfine powder Gynostemma pentaphyllum Makino. Glycoconj J 37, 777–789 (2020). https://doi.org/10.1007/s10719-020-09949-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-020-09949-5