Abstract
Dental caries remains a major health issue and the Gram-positive bacterium Streptococcus mutans is considered as the major pathogen causing caries. More recently, S. mutans has been recognised as a cause of endocarditis, ulcerative colitis and fatty acid liver disease along with the likelihood of increased cerebral hemorrhage following a stroke if S. mutans is present systemically. We initiated this study to examine the vaccine candidacy of the serotype specific polysaccharides elaborated by S. mutans. We have confirmed the carbohydrate structures for the serotype specific rhamnan containing polysaccharides from serotypes c, f and k. We have prepared glycoconjugate vaccines using the rhamnan containing polymers from serotypes f and k and immunised mice and rabbits. We consistently obtained a robust immune response to the glycoconjugates with cross-reactivity consistent with the structural similarities of the polymers from the different serotypes. We developed an opsonophagocytic assay which illustrated the ability of the post-immune sera to facilitate opsonophagocytic killing of the homologous and heterologous serotypes at titers consistent with the structural homologies. We conclude that glycoconjugates of the rhamnan polymers of S. mutans are a potential vaccine candidate to target dental caries and other sequelae following the escape of S. mutans from the oral cavity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptococcus mutans is a dental pathogen that causes dental caries and periodontal disease [1]. It is also implicated in several diseases when it escapes the oral cavity and spreads via blood including endocarditis [2], increased risk of severe cerebral haemorrhage [3], ulcerative colitis [4] and non-alcoholic fatty liver disease [5]. There are currently no licensed vaccines available to combat this pathogen, and current treatments include regular oral hygiene methodologies including brushing, flossing and mouthwashes. Antibiotics are also utilised to combat endocarditis [6]. Although some mouthwashes have been found to completely kill S. mutans [7], this and other approaches are clearly insufficient or inaccessible as dental caries is still occurring at a significant rate worldwide. The increased recognition of a link between this dental pathogen and the other serious sequelae beyond the oral cavity also points to the importance of the need for new treatments as we become increasingly aware of the fine balance within the microbiome of harmless commensal and opportunistic pathogens. Several virulence factors have been suggested for S. mutans including collagen binding protein [2, 3, 8], antigen I / II (adhesin P1) [9], and glucosyltransferases and their product glucans [10, 11].
There are currently no licensed vaccines or biologics available to combat S. mutans. Planet Biotechnology [12] developed a synthetic antibody against S. mutans, branded CaroRx, which reached phase II trials. Oragenics developed a genetically modified strain of Streptococcus mutans as a probiotic, which is incapable of producing lactic acid, and has recently been taken up by ProBiora Health [13]. Other approaches include a DNA based vaccine against glucosyltransferases and surface proteins [14], and a recombinant fragment of the P1 adhesin [9], and peptide vaccine targeting the glucosyltransferases and glucose binding proteins [15]. Studies in humans have shown that oral immunization with carbohydrate antigens from S. mutans incorporated into liposomes results in mucosal IgA responses, however, the magnitude of the immune responses was shown to be low and their persistence limited [16].
Our proposal is to develop glycoconjugate vaccines based on the cell surface rhamnan polysaccharides of S. mutans. These vaccines could provide systemic immunity to kill the pathogen when it enters the bloodstream, and it is also anticipated that the antibodies produced following conjugate immunisation will access the dental cavity; killing the pathogen in its preferred niche [17]. The rhamnan surface polymers are responsible for the serology of S. mutans, which is classified into four serotypes c, e, f and k based upon the structure of these rhamnose based glyco-polymers [18]. Prior structural analyses had suggested repeat unit structures for the c [19], e [20] f [21] and k [18, 22] serotypes of S. mutans and part of this study was to confirm and extend this structural knowledge. Armed with this improved structural knowledge we set out to investigate if the rhamnan glyco-polymers had any utility as glycoconjugate vaccine antigens.
Materials and methods
Growth of bacteria and preparation of purified polysaccharide
S. mutans serotype k strains OM42X, OM88X & OM98X (NRCC # 6749, 6748 & 6747 respectively), serotype f strain OMZ175 (NRCC # 6847) and serotype c strain 10449 (NRCC # 6849) were grown from frozen stock by inoculation of 20 Oxoid (MP0330) chocolate agar plates. Proceeds from overnight plates were used to inoculate 1 L Todd-Hewitt broth, 30 g/L (Difco 249,240) with 2% w/v glucose and 0.3% NaCl in a 4 L baffled flask with 0.8% NaHCO3 and 0.15% K2HPO4. Flask was incubated at 37 °C at 165 RPM in a Forma Scientific floor model shaker until A600 1.0 or greater (2–3 h). Fermenter (30 L newMBR) containing 23 L of media as above (without 0.8% NaHCO3 and 0.15% K2HPO4) and containing 1.0 ml DF204 (Mazer Chemicals) antifoam agent was inoculated with the 1 L shake flask culture. The fermenter culture was grown at 200 RPM, 37 °C, dissolved oxygen control at 5% by aeration only, pH control at 6.9 with 5 N NaOH. Culture was grown until stationary phase A600 > 4.0 (10–20 h strain dependent) and inactivated with addition of 2% phenol. Harvesting was completed with Cepa Z41continuous centrifugation, yielding ~7.5 g/L wet weight.
The polysaccharides were isolated according to the method of Hamada [23]. Briefly, cells ~150 g wet wt were lyophilised (giving ~50 g) and suspended in 1 L of 0.15 M NaCl and autoclaved for 20 min. The mixture was cooled and residual cells pelleted (6.5 K, 30 min.) and the resulting supernatant was dialysed overnight against running water and lyophilised (giving ~4 g). A 1% solution in water was prepared and treated with DNase and RNase at 37 °C for 3.5 h. Proteinase K was then added and the mixture incubated for a further 3.5 h. The mixture was then dialysed overnight against running water and lyophilised (giving ~2.5 g). The resulting material was dissolved at 25 mg/ml in 50 mM NH4CO3 buffer and loaded onto a DEAE Sephadex A-25 column (bed volume 100 mL., 2.6 × 20 cm) at ~50 mg per run. The column was washed and the neutral polysaccharides eluted with 225 mL of the same 50 mM NH4CO3 buffer. The combined eluates were dialysed as above and lyophilised (giving ~0.5 g). The resulting material was dissolved at 30 mg/ml in pyridinium acetate buffer and was loaded onto a Sephadex G-50 column (bed volume 200 mL., 2.6 × 37 cm) at ~150 mg per run. The column was washed with pyridinium acetate buffer and the eluate monitored by a refractometer. The positive fractions from the combined runs were pooled and lyophilised (giving ~25 mg).
Analytical methods
Sugars were determined as alditol acetate derivatives by GLC-MS as described previously [24].
HPLC
Analysis was performed on a Superose 12 10/30 GL column (GE Healthcare Bioscience AB) with PBS as eluent at a rate of 0.4 ml/min, and with UV and RI detectors.
Nuclear magnetic resonance spectroscopy and mass spectrometry
NMR spectroscopy was performed on the purified PS after an additional de-salting step on a G-25 column as described previously [25]. Matrix-assisted laser desorption ionization- time of flight (MALDI-TOF) mass spectra were obtained using a Voyager DE-STR mass spectrometer (Applied BioSystems, Foster City, CA, U.S.A.). The instrument was operated in positive, linear ion mode under delayed extraction conditions (200 ns) using an accelerating voltage of 25,000 V. Each spectrum is the average of approximately 100 laser shots. The matrix used was 3,5-dimethoxy-4hydroxy cinnamic acid (sinapinic acid), prepared at a concentration of 10 μg / μl in 30% acetonitrile and 0.1% formic acid (v/v). These solutions were spotted directly on the MALDI target in a 1:3 ratio with matrix.
Preparation of conjugates from purified PS
Conjugates were prepared separately with both with the k and f purified PS. The size of the purified PS was established by HPLC. The PS was then oxidised with periodate at 10 and 50 mM periodate concentrations to establish the oxidation conditions to facilitate optimum loading of the resulting glycoconjugate. Briefly, the purified PS (5 mg) was dissolved in 0.5 ml of sodium acetate buffer (50 mM, pH 7.5) and 0.5 mL of sodium metaperiodate (either 10 or 50 mM in 50 mM sodium acetate buffer). The oxidation was left in the dark at room temperature and the reaction quenched by addition of 0.5 mL of ethylene glycol and left in the dark for an additional hour at room temperature. The reaction mixture was purified on a G-25 column and lyophilised. The resulting oxidised PS (~ 4.5 mg) was conjugated to BSA (~1.1 mg) by mixing the carbohydrate and protein in ~1 mL of water and left for 1 h at room temperature before lyophilising overnight. The resulting material was immediately dissolved in ~1 mL of 0.2 M sodium phosphate buffer at pH 8 containing ~5 mg/mL of sodium cyanoborohydride and left at 37 °C for 16 h. The reaction product was converted to water through a 30 kDa molecular weight cut off spin column (4 x) and examined by MALDI-MS.
Following optimisation of the oxidizing conditions, conjugation reactions for immunisations were performed following 50 mM oxidation as described above but with 7.5 and 14 mg of the oxidised polysaccharides with 2.5 and 3.5 mg of HSA protein for serotypes f and k, respectively. A small aliquot of the reaction product was converted to water through a 30 kDa spin column as described above for characterisation by MALDI, whilst the remaining reaction products were concentrated using Dulbecco’s PBS (Gibco) containing 10 mM sodium citrate (Sigma) through a 30 kDa molecular weight cut off spin column (4×) and characterised by SDS-PAGE and protein assay. The final concentrate was stored at 4 °C.
SDS-PAGE
The conjugates were separated on 10% Tris-HCl pre-cast gels under reducing conditions with the buffer system of Laemmli [26]. SDS-PAGE was stained with Bio-Safe Coomassie.
Immunisation
New Zealand white female rabbits were immunised subcutaneously with the glycoconjugates. Each rabbit received 50 μg of conjugated carbohydrate as 2 × 0.25 mL per immunisation with incomplete Freunds adjuvant for the prime and boosts. Rabbits were boosted on day 28 and 56 and sera recovered following a trial bleed from the middle ear artery on day 42 and via terminal heart puncture on day 70.
Balb/C female mice (6–8 weeks old) were immunised intra-peritoneally with the glycoconjugates: Each mouse received 10 μg of conjugated carbohydrate per immunisation with Sigma adjuvant for the prime immunisation and boosts. The mice were boosted on days 21 and 42; sera were recovered following trial bleed on day 35 and terminal heart puncture on day 56. Additionally, mice received control immunisations, which consisted of HSA alone (13 μg) or HSA (13 μg) admixed with the purified serotype k PS (10 μg) and adjuvant, all with the same boosting and sera recovery schedule.
Antigen ELISA
Purified and well-characterized conjugates of the purified serotype f and k PS to BSA were used as the coating antigens in a solid-phase indirect ELISA to determine the binding profiles displayed in the mice and rabbit sera. NMR, HPLC, MALDI and / or SDS-PAGE confirmed the structural integrity of each antigen utilised. 96-well Nunc Maxisorp EIA plates were coated with 1.0 μg of purified conjugate (based on protein content) in 0.05 M carbonate buffer containing 0.02 M MgCl2, pH 9.8 at 4 °C overnight. Wells were then blocked with 1% BSA-PBS for 1 h at room temperature, washed with PBS-0.05% Tween 20 (PBS-T) and sera added for 1 h at room temperature. Following washing with PBS-T, alkaline phosphatase labelled goat anti-mouse IgM and/or IgG or goat anti-rabbit Ig were added for 1 h at room temperature. The plates were then washed and developed with Phosphatase Substrate System (Kirkegaard and Perry Laboratories, Gaithersburg, MD). After 60 min the absorbance (A405-410nm) was determined.
Whole cell ELISA
Whole cell ELISA was performed to determine whether sera recognized whole cells from various strains of S. mutans. Briefly, wells of Nunc Maxisorp EIA plates were coated with 100 μL of formalin-killed bacteria (optical density at 620 nm [OD620] of 0.08) in H2O overnight in a 37 °C drying oven and then brought to room temperature before use. Plates were blocked with 1% bovine serum albumin (BSA)-PBS for 1 h at room temperature, wells were washed with PBS–0.05% Tween 20 (PBS-T), and incubated with sera for 3 h at room temperature. Following washing with PBS-T, alkaline phosphatase-labeled goat anti-mouse IgM and / or IgG (or goat anti-rabbit Ig) (Cedarlane Laboratories) diluted 1:2500 or 1:6000 (mice) or 1:3000 (rabbits) in 1% BSA-PBS was added for 1 h at room temperature. The plates were then washed and developed with Phosphatase Substrate System (Kirkegaard and Perry Laboratories). After 60 min OD was measured at A405nm using a microtiter plate reader.
Immunofluorescence
In order to determine if antibodies in immune serum could access epitopes on the bacterial cell surface, immunofluorescence on live S. mutans cells was performed. S. mutans was grown for 18 h on chocolate blood agar at 37 °C. The cells were re-suspended in PBS to an OD600 of 1.0 and then 10 μL was air dried onto glass coverslips. The bacteria were heat fixed to the coverslip by passing through a Bunsen flame 5–6 times, and then were blocked with 5% Bacto skim milk (Difco, Sparks, USA)-PBS for 30 min at room temperature. The cells were incubated for 45 min at room temperature in 50 μL of either the pre- or post-immune (D70) anti-serotype k rhamnan serum (RRHV3,) at a dilution of 1:500 in PBS. The coverslips were washed with PBS-0.1% Tween 20 (PBS-T) then incubated for 45 min at room temperature with 50 μL goat anti-rabbit IgG Alexafluor 488 antibody (Invitrogen, Eugene, Oregon, USA) at a 1:1000 dilution. The coverslips were washed with PBS, mounted with Vectashield-DAPI (Vector Laboratories, Burlington, Canada) then examined with a Zeiss microscope (Axiovert 200 M).
Opsonophagocytic assay
S. mutans bacteria were resuscitated from -80 °C frozen stocks in BHI with 20% glycerol onto chocolate agar plates and incubated at 37 °C with 5% CO2 overnight. Colonies were transferred into 5 mL Todd-Hewitt broth (TH) medium in a 50 mL Falcon tube at 37 °C and gently shaken at 200 rpm for about 3.5 h and then 1 mL was transferred into 4 mL TH broth in another 50 mL tube and incubated at 37 °C at 200 rpm for an additional 2 h. The culture was monitored by OD580 against blank TH medium until an OD580 = 0.1 was reached. The resulting culture was diluted to give ~500 cfu/10 μL of log phase S. mutans cells.
HL60 cells (ATCC, VA) were kept in culture at ~2–4 × 105 cells / mL in RPMI with 10% FCS at 37 °C in a 5% CO2 atmosphere. 5 days before use the cells were differentiated into phagocytic cells by adding 100 mM DMF in the culture media without antibiotics. 8–10 × 107 phagocytic cells were required for each assay and were harvested immediately prior to use by centrifugation at 1000 rpm (300 g). The pelleted cells were washed in 50 ml 1X HBSS (−Ca/−Mg) at 1000 rpm (300 g) for 7 min, and washed again in 50 ml HBSS (+Ca/+Mg), counted with a hemocytometer and centrifuged at1000 rpm (300 g) for 7 min and adjusted to 1 × 107 phagocytic cells / mL of opsonisation buffer (OB; 1X HBSS with Ca+/Mg+, containing 1% gelatin and 5% FCS).
Freshly thawed 4 week old baby rabbit serum (Pel-freez Biologicals, Rogers, AR, USA) was maintained at 0 °C until use.
Eight 2-fold serial dilutions were made for each test serum in OB in a sterile 96 well U bottom culture plate (Falcon, 353,077, Corning, NY, USA) and 20 μL / well of OB buffer was added. 20 μL / well of diluted test serum was transferred to duplicate wells of the test plates and 10 μL / well of S. mutans cells containing ~500 cfu of viable bacteria were added and the plate was shaken at 700 rpm for 30 min at room temperature. 50 μL / well at 1:4 v/v of heat inactivated complement / HL-60 cells were added to the test and control wells and the plate was shaken at 700 rpm for 45 min at 37 °C in a 5% CO2 atmosphere. The plates were placed on ice for 20 mins and then 10 μL was dropped onto tilted agar plates and incubated at 37 °C with 5% CO2 for ~36 h before counting.
Results
Isolation of polysaccharides and structural analyses
Polysaccharide (PS) from serotypes c, f and k were isolated as described in the experimental section. The PS was examined by HPLC, which revealed that it eluted at a late retention time consistent with a polymer size of approximately 10,000 Da (data not shown). Sugar analyses of the serotype k strain’s PS revealed rhamnose and galactose in an approximate 3:1 ratio as the only two sugars observed, whereas the serotype f and c strains PS were shown to only elaborate rhamnose and glucose, with an equimolar ratio of the two sugars in serotype f, but only a minor amount of glucose in serotype c. In order to elucidate the exact locations and linkage patterns of the sugars in the PS repeating units, NMR studies were performed (Supplementary Fig. 1). Assignments of the chemical shifts based on standard 2D NMR experiments are detailed in Tables 1, 2 and 3 and identified the major and minor polymers for each serotype (Fig. 1).
Structures of the polysaccharide repeating units for the serotype c, f and k polymers. For serotype c major polymer was the disaccharide, for serotype f major polymers were the tetra and tri-saccharides in equimolar amounts and for serotype k major polymer was the trisaccharide. Letter designations are same as in the NMR tables
Preparation of the glycoconjugate
The polysaccharides were activated by treatment with periodate as described in the experimental section. The conditions were optimised in trial reactions to obtain conditions that resulted in optimal loading of carbohydrate on the BSA carrier protein as revealed by MALDI-MS analyses (Fig. 2). Conjugation following 10 mM oxidation facilitated MALDI identification of a conjugate with 1–5 polysaccharide units attached with each unit being approximately 9.5 kDa in size (Fig. 2b). Conjugation with 50 mM oxidised polysaccharide resulted in higher loading of the BSA carrier protein, with 3–10 polysaccharide units attached (Fig. 2c). As the 50 mM periodate oxidation led to higher loading, this was the concentration utilised for the glycoconjugates of the f and k PS with HSA as the carrier protein, MALDI analyses revealing a mass increase from 66 kDa to ~110 kDa for both glycoconjugates (Figs. 2d & e). The conjugation products were also characterised by HPLC (data not shown), SDS-PAGE (Fig. 3) and quantified by protein assay (data not shown). SDS-PAGE (Fig. 3) revealed a change in migration consistent with the MALDI data.
MALDI-MS analyses of (a) BSA, (b) BSA-serotype k rhamnan conjugate following 10 mM periodate oxidation, (c) BSA-serotype k rhamnan conjugate following 50 mM periodate oxidation, (d) HSA-serotype k rhamnan conjugate following 50 mM periodate oxidation, (e) HSA-serotype f rhamnan conjugate following 50 mM periodate oxidation
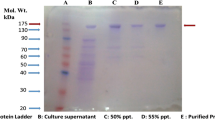
SDS-PAGE analyses of carrier proteins and conjugates for (a) serotype k rhamnan conjugation: Lane1, MW ladder; Lane 2, HSA; Lane 3, HSA-serotype k rhamnan conjugate following 50 mM oxidation; Lane 4, BSA-serotype k rhamnan conjugate following 10 mM oxidation; Lane 5, BSA-serotype k rhamnan conjugate following 50 mM oxidation; and (b) serotype f rhamnan conjugation: Lane 1, HSA; Lane 2, HSA-serotype f rhamnan conjugate following 50 mM oxidation; Lane 3, BSA; Lane 4, BSA-serotype f rhamnan conjugate following 50 mM oxidation; Lane 5, ladder
Immunogenicity of glycoconjugates
Mice and rabbits were immunised with the glycoconjugates as described in the experimental section. All ten mice that received the serotype k and all five mice that received the serotype f glycoconjugate vaccines had seroconverted to an IgG response that recognised the homologous PS at good titers (data not shown). Control sera did not recognise the homologous antigens (data not shown). Recognition of whole cells was also tested with the mice sera and this revealed that serotype f conjugate derived sera could recognise serotypes c and k strains at similar titers to the homologous cells. Although the mice sera titers from the serotype k conjugate derived sera were slightly lower than those derived from the f conjugate sera, it was clear that serotype k conjugate derived mice sera similarly recognised the homologous and heterologous serotype strains equally well (Table 4). Control sera did not recognise the whole cells (Table 4).
Rabbit sera recognised the homologous antigens at good titers (data not shown). Recognition of whole cells was also tested with the rabbit sera and this revealed that serotype f conjugate derived sera could recognise the serotype k strain at similar titers to the homologous cells, but the titers to the serotype c strain were slightly lower (Table 4). Serotype k conjugate derived rabbit sera recognised the homologous serotype strains somewhat better than the heterologous serotype strains (Table 4).
Immunofluorescence at the bacterial cell surface
In order to determine if antibodies in immune serum could access epitopes on the bacterial cell surface, immunofluorescence on live S. mutans cells was performed. The accessibility to the cell surface and cross reactivity to live cells of S. mutans serotype k strain OM 88X was demonstrated by the binding activity of the post immune serum in immunofluorescence experiments comparing pre- and post- (D70) immune serum from rabbit RRHV3 (Fig. 4). No fluorescence was observed with the strain when pre-immune serum was used whereas significant fluorescence was observed with the post-immune sera. This illustrates that the derived sera is specifically recognising an accessible epitope on the surface of live S. mutans cells.
Immunofluorescence of (a) pre- (D0) and (b) post-immune (D70) rabbit RRHV3 sera vs. live cells of S. mutans strain OM 88X. Anti-rhamnan binding was visualised with anti-rabbit IgG Alexafluor 488. The figures merge 488 fluorescent transmitted light and DAPI stain visualising nucleic acids channels together. Representative cells are shown
Functionality of conjugate derived sera
The sera from mice and rabbits immunised with the conjugates of the different PS serotypes were then examined for their ability to facilitate opsonophagocytic killing (Table 5). For the serotype k conjugate derived mice sera, killing of the homologous k strains was observed at high titers when compared to the control sera from protein and admixed controls. For the serotype f conjugate derived mice sera, killing of the homologous serotype strain was evidenced by an increase in opsonophagocytic titer from pre- to post-immune sera. A similar increase in titers was also observed for the serotype k strain killing with serotype f derived sera, however only weak killing titers were observed for killing the serotype c strain with the serotype f conjugate derived sera (Fig. 5a). For the rabbit sera a similar pattern emerged with excellent evidence of killing of the conjugate-homologous serotypes being observed. Furthermore good evidence that serotype f conjugate derived sera could adequately kill serotype k strains was observed, but with significantly weaker killing of serotype c strains. Serotype k conjugate derived sera killed the heterologous f serotype strain at weaker titers than that observed for the homologous strain killing, but was unable to kill the heterologous c serotype strains at titers greater than 1:320 (Fig. 5b). In order to illustrate the S. mutans specificity of the derived sera (f and k) we performed an opsonophagocytic assay against a Pseudomonas aeruginosa strain and observed no killing whatsoever (data not shown).
Opsonophagocytic assay titration curves of (a) mice pre- and post-immune sera raised to HSA-serotype f PS conjugate (MSMV 6–10) and (b) rabbit pre- and post-immune sera raised to HSA serotype f conjugate (RSMV 4–6) and post-immune sera raised to HSA serotype k conjugate (RRHV 3) against S. mutans type f strain OMZ175, type k strains OM 42X and OM 88X and type c strain 10499
Discussion
This study has confirmed and extended the known structural information for the S. mutans serotype specific polymers. In the case of serotype c the trisaccharide repeat unit was confirmed as identified previously [19], but the identification of a disaccharide variant without the glucose side-chain sugar had not been reported. In this study the disaccharide was actually the major polymer elaborated by the serotype c strain 10499. For serotype f the trisaccharide repeat unit was reported previously [21], but the identification of the tetrasaccharide unit with an additional glucose moiety attached to the side-chain glucose, nor a disaccharide variant without the glucose side-chain sugar had not been reported. The tri- and tetrasaccharide repeat units were the major polymers elaborated by serotype f strain OMZ175 in this study. To our knowledge the structure of the serotype k disaccharide repeat unit had only been reported by inference from immunochemical and genetic data [18, 22]. It was postulated that the repeat unit was a simple rhamnan disaccharide, the backbone of all serotype repeat units if you will. Our results indicated that the major polymer consisted of a trisaccharide repeat unit with a galactose side chain at the 2-position of the 3-linked rhamnose of the polymer backbone, differentiating from serotype f which elaborate a glucose residue at the 2-position of the 3-linked rhamnose. A minor polymer just consisting of the disaccharide repeat unit was also observed. This was the case for three different serotype k strains examined, OM42X, OM88X and OM98X. All serotypes do seem to elaborate the rhamnose disaccharide repeat unit as a major or minor constituent, which might suggest some potential for this antigen as the vaccine candidate. Unfortunately, we were unable to acquire a serotype e strain for this study. Conjugates were prepared from both serotype f and serotype k polymers. No attempts were made to separate the different repeat unit constituents prior to conjugation. At the time of initial conjugate preparation we did not have access to more immunogenic carrier proteins, thus we used HSA for the initial serotype k conjugations and maintained use of that carrier so as not to skew any subsequent conjugates with the serotype f polysaccharide. All serotype polymers elaborated by S. mutans appear naturally to be relatively small, with ~10 kDa units identified in the 10 mM periodate oxidation trial conjugation, thus no sizing of the polymers was required in order to optimise conjugation efficiency. We obtained conjugates with good loading as determined by MALDI-MS analyses following 50 mM periodate oxidation with approximately 45% carbohydrate conjugated in the final conjugates. Antisera derived from immunisations in both mice and rabbits were able to recognise both homologous and heterologous serotypes at similar titers. Immunofluorescence confirmed that the targeted epitopes were visible and recognised on live cells also. Finally, the functionality of the derived sera was examined, by virtue of determining the ability of the antisera to facilitate opsonophagocytic killing of homologous and heterologous strains of S. mutans. For the mice sera derived from the serotype k conjugate we only examined ability to kill the homologous strain, which the sera achieved at titers in the 2 K to 10 K range, significantly better than the control sera. For the serotype f conjugate derived mice sera similar titers were observed against the homologous strain and excellent titers were also observed against the serotype k strains, however titers were not as high when serotype c strain was the target. Pre-immune mice sera were unable to kill the target strains at or only at very poor titers when compared to the post-immune sera. For rabbit sera derived from the serotype k conjugate, excellent killing titers were observed against the homologous serotype k strains and when the best rabbit’s sera was examined against the heterologous serotype c and f strains killing was observed at titers approximately 4–5 two-fold dilutions lower than against the homologous strains, but at titers 4 two-fold dilutions greater than that seen with the pre-immune sera. For rabbit sera derived from the serotype f conjugate good killing titers were observed for the homologous serotype f strain and the heterologous serotype k strains, whereas the serotype c strain although sensitive to the killing effect of the sera was only killed a lower titers. Although the small number of animals utilised in this initial study precludes any statistically significant conclusions it is clear that rhamnan serotype specific conjugates are capable of provoking a good immune response in both mice and rabbits that can recognise the target antigen on the cell surface of S. mutans bacteria. The derived sera are capable of killing both homologous and heterologous serotypes, raising the possibility that a vaccine based on a limited number of serotype specific rhamnan based polymers may be sufficient to combat S. mutans. Further studies are necessary to determine if this humoral immune response can access the oral cavity in sufficient numbers to combat dental caries or is able to “mop up” S. mutans bacteria if they escape the oral cavity before they can cause any of the secondary diseases associated with this pathogen.
References
Taubman, M.A., Nash, D.A.: The scientific and public health imperative for a vaccine against dental caries. Nat. Rev. Immunol. 6, 555–563 (2006)
Nobbs, A.: Getting to the heart of the matter: Role of Streptococcus mutans adhesin Cnm in systemic disease. Virulence. 8, 1–4 (2017)
Nakano, K., Hokamura, K., Taniguchi, N., Wada, K., Kudo, C., Nomura, R., et al.: The collagen binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat. Commun. 2, 485–494 (2011)
Kojima, A., Nomura, R., Naka, S., Okawa, R., Ooshima, T., Nakano, K.: Aggravation of inflammatory bowel disease by oral streptococci. Oral Dis. 20, 359–366 (2014)
Naka, S., Nomura, R., Takashima, Y., Okawa, R., Ooshima, T., Nakano, K.: A specific Streptococcus mutans strain aggravates non-alcoholic fatty liver disease. Oral Dis. 20, 700–706 (2014)
Gould, F.K., Denning, D.W., Elliott, T.S., Foweraker, J., Perry, J.D., Prendergast, B.D., et al.: Guidelines for the diagnosis and antibiotic treatment of endocarditis in adults: a report of the working Party of the British Society for antimicrobial chemotherapy. J. Antimicrob. Chemother. 67, 269–289 (2012)
Elshibly, A., Coulter, W.A., Millar, B.C., Prendergast, B.D., Thornhill, M., Irwin, C., et al.: Effective oral health in infective endocarditis: efficacy of high street mouthwashes against the viridans group streptococci. Oral Microb. 5, 151–153 (2014)
Miller, J.H., Aviles-Reyes, A., Scott-Anne, K., Gregoire, S., Watson, G.E., Sampson, E., et al.: The collagen binding protein Cnm contributes to the oral colonisation and cariogenicity of Streptococcus mutans OMZ175. Infect. Immun. 83, 2001–2010 (2015)
Batista, M.T., Souza, R.D., Ferreira, E.L., Robinette, R., Crowley, P.J., Rodrigues, J.F., et al.: Immunogenicity and in vitro and in vivo protective effects of antibodies targeting a recombinant form of the Streptococcus mutans P1 surface protein. Infect. Immun. 82, 4978–4988 (2014)
Zhu, F., Zhang, H., Wu, H.: Glycosyltransferase-mediated sweet modification in oral streptococci. J. Dent. Res. 94, 659–665 (2015)
Bowen, W.H., Koo, H.: Biology of Streptococcus mutans-derived glucosyltransferases: role in extracellular matrix formation of cariogenic biofilms. Caries Res. 45, 69–86 (2011)
Weintraub, J.A., Hilton, J.F., White, J.M., Hoover, C.I., Wycoff, K.L., Yu, L., et al.: Clinical trial of a plant-derived antibody on recolonization of mutans streptococci. Caries Res. 39, 241–250 (2005)
Hillman, J.D., McDonell, E., Cramm, T., Hillman, C.H., Zahradnik, R.T.: A spontaneous lactate dehydrogenase deficient mutant of Streptococcus rattus for use as a probiotic in the prevention of dental caries. J. Appl. Microbiol. 107, 1551–1558 (2009)
Luo, W., Wen, S., Yang, L., Zheng, G.: Mucosal anti-caries DNA vaccine: a new approach to induce protective immunity against Streptococcus mutans. Int. J. Clin. Exp. Pathol. 10, 853–857 (2017)
Smith, D.J., King, W.F., Rivero, J., Taubman, M.A.: Immunological and protective effects of di-epitopic sub-unit dental caries vaccines. Infect. Immun. 73, 2797–2804 (2005)
Childers, N.K., Michalek, S.M., Pritchard, D.G., McGhee, J.R.: Mucosal and systemic responses to an oral liposome-Streptococcus mutans carbohydrate vaccine in humans. Reg. Immunol. 3, 289–296 (1990–91)
Challacombe, S.J., Russell, M.W., Hawkes, J.E., Bergmeier, L.A., Lehner, T.: Passage of immunoglobulins from plasma to the oral cavity in rhesus monkeys. Immunology. 35, 923–931 (1978)
Nakano, K., Ooshima, T.: Serotype classification of Streptococcus mutans and its detection outside the oral cavity. Future Microbiol. 4, 891–902 (2009)
Linzer, R., Reddy, M.S., Levine, M.J.: Immunochemical aspects of serotype carbohydrate antigens of Streptococcus mutans. In: Hamada, S.M., Michalek, H., Kiyono, L.M., McGhee, J.R. (eds.) Molecular Microbiology and Immunobiology of Streptococcus mutans, pp. 29–38. Elsevier Science Publishing Inc., Amsterdam (1986)
Pritchard, D.G., Gregory, R.L., Michalek, S.M., McGhee, J.R.: Characterization of the serotype e polysaccharide antigen of Streptococcus mutans. Mol. Immunol. 23, 141–145 (1986)
Linzer, R., Reddy, M.S., Levine, M.J.: Structural studies of the serotype f polysaccharide antigen from Streptococcus mutans OMZ175. Infect. Immun. 55, 3006–3010 (1987)
Fujiwara, T., Nakano, K., Kawaguchi, M., Ooshima, T., Sobue, S., Kawabata, S., et al.: Biochemical and genetic characterization of serologically untypable Streptococcus mutans strains isolated from patients with bacteremia. Eur. J. Oral Sci. 109, 330–334 (2001)
Hamada, S., Michalek, S.M., Torii, M., Morisaki, I., McGhee, J.R.: An enzyme-linked immunosorbent assay (ELISA) for quantification of antibodies to Streptococcus mutans surface antigens. Mol. Immunol. 20, 453–464 (1983)
Sawardeker, D.G., Sloneker, J.H., Jeanes, A.: Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal. Chem. 37, 1602–1604 (1965)
Cox, A.D., Li, J., Brisson, J.-R., Moxon, E.R., Richards, J.C.: Structural analysis of the lipopolysaccharide from Neisseria meningitidis strain BZ157 galE: localisation of two phosphoethanolamine residues in the inner core oligosaccharide. Carbohydr. Res. 337, 1435–1444 (2002)
Laemmli, U.K.: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227, 680–685 (1970)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors. All animal care and accompanying procedures were in accordance with the guidelines and policies of the Canadian Council on Animal Care (CCAC). The study was approved by the Human Health Therapeutics’ Animal Care Committee which is certified by the CCAC.
Electronic supplementary material
Supplementary Fig. 1
a. 1H–NMR spectrum for the glucorhamnan and rhamnan repeating units from Streptococcus mutans serotype c strain 10499. b. 1H–NMR spectrum for the glucorhamnan and rhamnan repeating units from Streptococcus mutans serotype f strain OMZ175. c. 1H–NMR spectrum for the galactorhamnan and rhamnan repeating units from Streptococcus mutans serotype z strain OM42X. (PDF 61 kb)
Rights and permissions
About this article
Cite this article
St. Michael, F., Yang, Q., Cairns, C. et al. Investigating the candidacy of the serotype specific rhamnan polysaccharide based glycoconjugates to prevent disease caused by the dental pathogen Streptococcus mutans . Glycoconj J 35, 53–64 (2018). https://doi.org/10.1007/s10719-017-9798-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-017-9798-z