Abstract
Lectins are carbohydrate binding proteins that are gaining attention as important tools for the identification of specific glycan markers expressed during different stages of the cancer. We earlier reported the purification of a mitogenic lectin from human pathogenic fungus Cephalosporium curvulum (CSL) that has complex sugar specificity when analysed by hapten inhibition assay. In the present study, we report the fine sugar specificity of CSL as determined by glycan array analysis. The results revealed that CSL has exquisite specificity towards core fucosylated N-glycans. Fucosylated trimannosyl core is the basic structure required for the binding of CSL. The presence of fucose in the side chain further enhances the avidity of CSL towards such glycans. The affinity of CSL is drastically reduced towards the non-core fucosylated glycans, in spite of their side chain fucosylation. CSL showed no binding to the tested O-glycans and monosaccharides. These observations suggest the unique specificity of CSL towards core fucosylated N-glycans, which was further validated by binding of CSL to human colon cancer epithelial and hepatocarcinoma cell lines namely HT29 and HepG2, respectively, that are known to express core fucosylated N-glycans, using AOL and LCA as positive controls. LCA and AOL are fucose specific lectins that are currently being used clinically for the diagnosis of hepatocellular carcinomas. Most of the gastrointestinal markers express core fucosylated N-glycans. The high affinity and exclusive specificity of CSL towards α1-6 linkage of core fucosylated glycans compared to other fucose specific lectins, makes it a promising molecule that needs to be further explored for its application in the diagnosis of gastrointestinal cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many key biological processes including cell adhesion, molecular trafficking and clearance, receptor activation, signal transduction and endocytosis involve the active participation of glycans. Understanding the diversity of these glycans expressed on the mammalian glycoconjugates provides useful information in deciphering the cellular-molecular recognitions and underlying signaling mechanisms. Lectins are the carbohydrate binding proteins of non immune origin, and are known to recognize such glycans that are specifically either expressed on cell surface or free in solutions. This glycan recognition property of lectins has made them a useful tool in different fields of life sciences. Some lectins recognize tumor associated glycans and therefore have the potential to serve as biomarkers for malignant tumors and also assist in the study of changes in the glycosylation motif in cancer line [1].The alteration/expression of specific glycans has been observed in many pathophysiological conditions including cancer. Many of these specific glycans are considered as disease markers and are targets for diagnosis as well as for therapeutics. The role of glycans has been well established in the cancer progression and glycans are known to be involved in tumour proliferation, invasion, haematogenous metastasis and angiogenesis. Malignant transformation is often accompanied by the expression of oncofetal antigen-epitopes that are expressed on tumour cells and sometimes released into the serum [2–4].
Alterations of both N- and O-linked glycans have been observed in many cancer markers noticeably in mucin glycoconjugates such as; MUC4, MUC1, MUC16 (CA125), CA19-9 and CA15-3 [5]. For example, α fetoprotein (AFP) and haptoglobin are the serum glycoproteins that are known to express altered N-glycans in gastrointestinal cancer especially core fucosylated N-glycans during transformation. Increased fucosylation, specifically core fucosylation on many serum glycoproteins and membrane receptors is observed in gastrointestinal cancer including hepatocellular and pancreatic carcinoma and hence is becoming an important diagnostic marker [6, 7]. Lectins like, Lens culinaris agglutinin-A (LCA), Aleuria aurantia lectin (AAL), Aspergillus oryzae lectin (AOL), Rhizopus stolonifer lectin (RSL), and Pholiota squarrosa lectin (PhoSL) are known to recognize such altered core fucosylation of N-glycans [6, 8, 9]. Apart from these alterations observed during the transformation, there may be many more altered glycans specifically expressed which needs to be deciphered by using glycan binding proteins like lectins with unique affinity for such glycans.
Recently, we reported purification and characterization of a mitogenic lectin (CSL) from Cephalosporium curvulum, a pathogenic fungus causing mycotic keratitis. CSL had shown complex sugar specificity as analysed by hapten inhibition assay. Here we report the exquisite specificity of CSL to core fucosylated N-glycans as analysed by the glycan array data using version 4.2, which includes screening its affinity for a total of 511 glycans. To validate the glycan array data, interaction of CSL with human hepatocellular carcinoma cell lines with and without FUT8 gene silencing and colon cancer epithelial cell lines by flow cytometry and binding with human colon cancer tissues by lectin histochemistry were performed to explore its possible potential in the diagnosis of gastrointestinal cancer.
Materials and methods
Bovine serum albumin (BSA), streptavidin-horseradish peroxidase, fetuin and L-fucose were obtained from Sigma Chemical Co. (St. Louis, USA).Fetal calf serum (FCS) and DMEM and Lipofectamine 2000 were from Gibco Invitrogen, siRNA against FUT8 from Dharmacon, GE Life Sciences, USA, 3-3′ diaminobenzidine chromogen/H2O2 substrate in buffered solution (pH 7.5) (DAB kit) was obtained from Bangalore Genei, India. LCA was obtained from Vector labs, USA and AOL was obtained from TCI Japan.
Purification, biotinylation and FITC conjugation of CSL
CSL was purified from Cephalosporium curvulum culture in a single step by affinity chromatography on asialofetuin-Sepharose 4B column as described earlier [10]. Biotinylation of CSL and AOL was carried out according to the procedure described by Duk et al. [11]. The biotinylated lectin was stored at −20 °C till further use. Conjugation of CSL, AOL and LCA with FITC was conducted as described by Goldman et al. [12]. Asialofetuin(ASF) used for preparation of affinity column and binding studies was prepared by desialylation of fetuin according to the method described by R.G. Spiro and V. D. Bhoyroo [13].
Glycan array screening of CSL
Glycan array analysis of CSL was carried out at Consortium for Functional Glycomics (CFG) using version 4.2. This version had 511 different glycans covalently attached to N-hydroxysuccinamide (NHS)-activated glass slides (www.functionalglycomics.org). These glycans are natural/synthetic glycan sequences representing major glycan structures of glycoproteins and glycolipids [14]. Streptavidin-Alexa Fluor 488 was used to detect the biotinlyated CSL and the fluorescence was detected using a confocal micro array scanner (Perkin Elmer Microscan XL 4000) and the integrated spot intensities were determined using IMAGENE image analysis software (BioDiscovery, EI Segundo CA, USA). Relative fluorescence units (RFU) for CSL binding to each of the glycans were plotted using Microsoft EXCEL software.
Cell culture
Human colon epithelial cancer HT29 cells were obtained from the European Cell Culture Collection via the Public Health Laboratory Service (Porton Down, Wiltshire, UK). HT29 cells were cultured in DMEM supplemented with 10 % FCS, 100 units/ml penicillin, 100 μg/ml streptomycin (complete DMEM) at 37 °C in 5 % CO2. Hepatocarcinoma HepG2 cells were a kind gift from Dr.Milind Vaidya and were cultured in DMEM supplemented with 10 % FCS, 100 units/ml penicillin, 100 μg/ml streptomycin (complete DMEM) at 37 °C in 5 % CO2.
Flow cytometry
Hepatocellular carcinoma HepG2 and human colon epithelial cancer HT29 cells were incubated with 3 % BSA to block non-specific sites, for 1 h at 4 °C. Cells (1 × 105) were incubated with FITC labelled CSL, AOL and LCA (2 μg/100 μl) for 1 h on ice and were washed thoroughly with 1X PBS. Carbohydrate-mediated binding of lectins was tested by preincubating the FITC conjugated lectins (CSL, AOL and LCA) with competing glycoconjugate, asialofetuin (100 μg/ml) and monosaccharide L-fucose (200 mM) for 1 h at 37 °C before cell staining. Data was acquired for 10,000 events using Beckman Coulter FC500 flow cytometer and was analysed using CXP Analysis software. Unstained cells processed similarly were used as negative control.
RNA mediated FUT8 gene silencing
Synthesis of fucosyl transferase 8, responsible for the addition of L-fucose on the membrane oligosaccharide, was inhibited using siRNA towards FUT8 gene.
Briefly, HepG2 cells were seeded in a 3.5 cm dish (0.15X106/ml) and were grown at 37 °C for 48 h. These cells were transfected with 200 nM siRNA for FUT8 using Lipofectamine 2000 and allowed to grow for 72 h. Transfected cells with reduced fucosylation were used to test their binding to FITC-CSL and to confirm specificity of CSL.
Lectin histochemistry
Human colon tissue samples (normal, primary and metastatic cancer tissues) were procured from S. L. Raheja Hospital, Mumbai, India, with the approval of the ethical committee (IRB No.08/2009). Tissues were obtained during surgery or colonoscopic polypectomy, fixed in buffered formalin and embedded in paraffin for routine pathological examination. Additional 5 μm sections were prepared for lectin histochemistry after the pathological diagnosis was confirmed.
Lectin histochemistry of biotinylated CSL and AOL (10 μg/ml) was carried out as described by Boland et al. [15]. CSL and AOL binding was then evaluated using Streptavidin-horseradish peroxidase –DAB system through optical analysis.
Results
Biotinylated CSL was used for glycan array analysis and the carbohydrate specificity of CSL was established by measuring the fluorescence intensity after the addition of Streptavidin-A488. Relative fluorescence units (RFU) measured for total 511 glycans are presented in Fig. 1 and the acquired data of RFU for individual ligands can be found on consortium website (www.functionalglycomics.org/primscreen_3703).
CSL shows strong binding affinity to the core fucosylated N-glycans
The results of glycan array analysis reveals that CSL is specific towards N-glycans and all the top seventeen glycans have core fucosylation as common structure (Table 1). Trimannosyl pentasaccharide core with fucosylation at the reducing end (Glycan# 477) is the basic structure required for the binding of CSL. Binding affinity of CSL is enhanced for extended fucosylated core by Galβ1-4GlcNAcβ1-2 at both the mannose residues (Glycan# 350). This is an important structure, which is found on many of the secretory glycoproteins (α-fetoprotein, α1-antitrypsin, haptoglobin, E-cadherin etc.). The presence of fucose residues in the side chain of the extended core fucosylated structure (Glycan# 465 and 457) enhances the avidity of CSL enormously. Changes in the glycosidic linkage of fucose, galactose or N-acetylglucosamine at the non-reducing end have negligible effect on the affinity of CSL towards these glycans (Glycan# 350, 455, 456, 457 etc.). Substitution of fucose with sialic acid at the non-reducing end slightly decreases the binding of CSL (Glycan# 475 and 476) suggesting preferred affinity of CSL towards fucose in the side chain.
Significance of core fucosylation for CSL binding
Binding of CSL to non-core fucosylated glycans but with side chain fucosylation is presented in the Table 2. It is interesting to note that none of the glycans tested without core fucosylation are recognized by CSL. Glycans with fucose residues in the side chain of the non-core fucosylated glycans and with all the possible orientations did not show any affinity to CSL. Affinity of CSL was reduced enormously between core fucosylated glycans that have shown highest affinity for CSL and the respective non-core fucosylated glycans. Noticeably, the glycan# ‘465 and 359′, ‘477 and 50′, ‘454 and 367′, ‘457 and 370′, ‘455 and 371′, ‘466 and 372′, ‘417 and 358′, ‘423 and 357′, and ‘424 and 368′ are similar in their structure except for core fucosylation (Table 2). These observations demonstrate the importance of the core fucosylation for CSL binding.
CSL shows no affinity for fucosylated O-glycans
All the tested O-glycans with or without fucosylation at reducing or non-reducing end did not show any binding to CSL. These results confirm the exclusive specificity of CSL towards core fucosylated N-glycans but not to O-glycans.
CSL shows no affinity for monosaccharides
None of the tested monosaccharides showed affinity towards CSL. The components of the trimannosyl core; mannose, N-acetylglucosamine and fucose have shown negligible binding to CSL. These results showed that the affinity of CSL is directed towards more extended glycans.
CSL shows strong binding to HepG2 and HT29 cells
The receptor-mediated lectin binding to the cells namely human hepatocellular carcinoma HepG2 cells and colon epithelial cancer HT29 cells was confirmed by studying the binding of FITC conjugated lectins CSL, AOL and LCA in presence and absence of different competing glycoconjugates or haptens. Flow cytometry histograms of the lectins binding to cells after blocking with asialofetuin and L-fucose are presented in Fig. 2a and Fig. 3, respectively. CSL showed strong binding to HepG2 and HT29 cells. The binding was significantly inhibited (53 % with HepG2 cells and 90 % with HT29 cells) in the presence of asialofetuin, whereas L-fucose failed to completely block the binding of CSL to HepG2 cells confirming the affinity of CSL exclusively towards complex glycans and not to fucose. The fucose specific lectins AOL and LCA used as positive controls also showed strong affinity to both cell lines. AOL and LCA binding to HepG2 cells were inhibited by 68 % and 48 %, respectively, in presence of asialofetuin and by 84 % and 44 % in the presence of fucose. Similarly, the binding of AOL and LCA to HT29 cells was inhibited by 38 % and 64 %, respectively, in presence of asialofetuin and by 84 % and 72 % in presence of fucose. These results show that for AOL, L-fucose is an effective inhibitor compared to asialofetuin, whereas for LCA, asialofetuin and fucose are equally effective in blocking. Hence, unlike CSL, binding of these fucose specific lectins was inhibited in the presence of both asialofetuin as well as L-fucose, which demonstrates their broader specificity.
a CSL shows strong binding to HepG2 cells: HepG2 cells were incubated with FITC labelled CSL (A1), AOL (B1) and LCA (C1) in presence or absence of competing glycoconjugates and fucose. Binding was analysed by flow cytometry. The overlays are representative data with X-axis and Y-axis representing fluorescent intensity and cell count, respectively. Figures A2, B2 and C2 represent inhibition of binding (MFI) values in presence or absence of competing glycoconjugates. b CSL shows reduced binding to FUT8 gene silenced HepG2 cells: HepG2 cells transfected with FUT8 siRNA were incubated with FITC-CSL and AOL and analysed by flow cytometry. The histogram reveals three distinct populations of FUT8 silenced HepG2 cells for CSL (Peak A, B and C) and has negligible effect for FITC-AOL
CSL shows strong binding to HT29 cells: HT29 cells were incubated with FITC labeled CSL (A1), AOL (B1) and LCA (C1) in presence or absence of competing glycoconjugates and fucose and binding was analysed by flow cytometry. The overlays are representative data with X-axis and Y-axis representing fluorescent intensity and cell count, respectively. Figures A2, B2 and C2 represent inhibition of binding (MFI) values in presence or absence of competing glycoconjugates
CSL shows reduced binding to HepG2 cells silenced with FUT8 siRNA
In order to confirm the affinity of CSL towards core fucosylated glycans, its interaction was studied with HepG2 cells silenced using FUT8 siRNA. Histograms reveal binding of CSL to three distinct populations of transfected HepG2 cells that likely represents different levels of gene silencing as shown in Fig. 2b. The majority of the transfected cells show decrease in binding by 86 %,(peak B) compared to untransfected cells, whereas a second population represents almost complete inhibition by 97.5 %, (peak A).The histogram also shows a small population of cells (peak C) with moderate transfection and hence show less decline in MFI 48.5 %, compared to control. The MFI of CSL for siRNA transfected populations is reduced drastically in all the transfected cells, indicating the importance of core fucosylation for CSL binding and thus confirming specificity of CSL. AOL was used as a positive control. Interestingly, there was not much decrease in MFI values for transfected cells, which may be due to the broader specificity of AOL towards linkages other than α1-6.
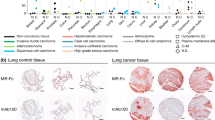
CSL shows strong binding to colon cancer tissues
CSL showed strong binding to cancerous and metastatic tissues and no or very weak binding to normal tissues of human colon. Tissue samples were probed for CSL and AOL binding; representative images are shown in the Fig. 4. AOL, a fucose specific lectin, used as positive control showed similar affinity and binding towards cancer tissues. The preferential binding of CSL to cancer and metastatic tissues similar to AOL not only supports its binding specificity to the core-fucosylated glycans over-expressed in gastrointestinal carcinoma tissues, but also reveals its diagnostic potential.
CSL and AOL show strong binding to colon cancer tissues: CSL (a) and AOL(b) histochemistry shows no binding of lectins to normal human colon tissues but strong binding to primary and metastatic colon tissues. All the images were obtained with 100× magnification. “Arrows” point to lectin binding to apical surface of the secretory gland epithelia
Discussion
The present manuscript describes the fine sugar specificity of CSL by glycan microarray analysis, a more accurate method, which revealed the specificity of CSL towards core fucosylated N-glycans, exclusively for α1-6 linkage unlike other fucose-specific lectins. The glycan array data analysed was further validated by flow cytometry and histochemical studies with human hepatocellular and colon cancer tissues.
Glycan array analysis version 4.2 used for the analysis of CSL, has a total of 511 glycans, which are either isolated from the glycoproteins or chemically synthesized. The CSL showed highest affinity towards the core fucosylated N-glycans. The top seventeen glycans recognized by CSL have the core fucosylation and trimannosyl core as the common structure. The trimannosyl core with fucose at the reducing end with α1-6 linkage (Glycan# 477) is the minimum basic structure required for CSL binding. The removal of core fucose (Fucα1-6) from the reducing end of the glycans, with highest affinity completely reduced the binding to CSL in spite of their side chain fucosylation.
Interestingly, all the tested O-glycans with side chain fucosylation are not recognized by CSL and also none of the monosaccharides tested including fucose, mannose and N-Acetylglucosamine showed any binding to CSL. All these results reveal the exclusive specificity of CSL towards core α1-6 fucosylated N-glycans with the trimannosyl core. To substantiate these observations, the affinity of CSL to core fucosylated glycans expressed on gastrointestinal cell lines namely HepG2 and HT29 cells was tested and confirmed. These results were further validated by silencing of fucosyl transferase by FUT8 siRNA that showed the decrease in the binding of the CSL to the transfected cells, whereas for AOL with broader specificity, the decrease in binding was not significant. These results reveal the exclusive specificity of CSL towards core fucosylated N-glycans. The differential binding of CSL towards human colon cancer tissues, comparable with that of the fucose specific lectin-AOL,was also demonstrated.
The increased fucosylation, especially core fucosylation on many serum glycoproteins and membrane receptors is observed in gastrointestinal cancer including hepatocellular and pancreatic carcinoma and hence is becoming an important diagnostic marker [6, 7] A number of gastrointestinal tumor markers have been identified such as; α fetoprotein-L3 fraction (APF-L3), fucosylated haptoglobin, fucosylated α-1-antitrypsin (Fc-AAT), Golgi protein 73 (GP73), Carbohydrate Antigen 19–9 (CA19-9), Carcinoembryonic antigen (CEA) [16–20]. These tumor markers are known for their differential expression of altered glycans, specifically fucosylation pattern in the normal and cancerous conditions. The serum levels of some of these markers are elevated in cancer and hence are becoming important diagnostic targets. Apart from cancer, the expression of some of these markers is also observed in other pathophysiological conditions of liver disease, such as chronic hepatitis (CH) and liver cirrhosis (LC), which gives the false positive results in detection of carcinoma. However, increased expression core fucose and total fucosylation levels of these markers has been observed in carcinoma that provides the means for specific differential identification in cancerous and other pathophysiological conditions. Serum levels of AAL-reactive Hpt are higher in pancreatic cancer patients, whereas those of PhoSL-reactive Hpt are higher in colorectal cancer patients [21]. In view of this, lectins with specific affinity for the fucosylated glycans are gaining application in the diagnosis of gastrointestinal cancer [5, 6]. Fucose-specific lectins from different sources are known to have affinity for the specific type of linkage [Table 3]. Aleuria aurantia lectin (AAL), Ulex uropeus agglutinin (UEA), Lens culinaris agglutinin (LCA), Rhizopus stolonifer lectin (RSL) and Aspergillus oryzae lectin (AOL) are fucose specific lectins, however they differ with respect to their affinity towards specific glycosidic linkage. For example, AAL recognizes α1-3/α1-4 and α1-6 fucose, UEA recognizes α1-2 fucose, LCA recognizes the native form of α1-6 fucose with a mannose arm with preference for α1-6 > α1-3 and AOL recognizes fucose with α1-6> > α1-3 > α1-4 > α1-2 linkages [7]. Apart from recognizing core fucosylated glycans, these lectins are also known to recognize fucose monosaccharide. These lectins other than AOL also recognize non fucosylated N-glycans, but show high affinity for fucosylated glycans. AOL has high specificity towards fucose α1-6 linkage compared to α1-2, α1-3 and α1-4 linkages [22]. Recently, another lectin from the mushroom Pholiota squarrosa (PhoSL) has been reported which has high specificity towards the core fucosylated glycans compared to other fucose linkages [8].
Lectin-Ab ELISA kits for AFP-L3 and Fuc-Hpt are the approved kits and are currently used in the diagnosis of hepatocellular carcinoma in combination with other diagnostic tests. AAL is used in recognition of the core fucosylated N-glycans, whereas LCA fraction 3 is used in detecting the core fucosylated AFP [6, 7]. Recently, another ELISA kit developed using AAL and PhoSL for detection of serum levels of fucosylated haptoglobin has shown that the serum levels of AAL-reactive Hpt are higher in pancreatic cancer patients, whereas those of PhoSL-reactive Hpt are higher in colorectal cancer patients [21]. Hence, these lectins are of significance in differentiating the increased fucosylation in different types of cancer.
Compared to these fucose specific lectins, CSL has shown exclusive specificity towards the core fucosylated (α1-6) N-glycans. CSL and PhoSL appear to have similar glycan specificity towards the core fucosylated glycans, which strengthens the potential of CSL to be developed as a diagnostic tool. It is known that trimannosyl pentasaccharide fucosylated core recognized by CSL is expressed in most of the gastrointestinal cancer markers. The exquisite affinity of CSL towards such glycans makes it an important promising molecule that needs to be further explored for its application in the diagnosis of gastrointestinal cancer.
It is surprising to note that there are three glycans with core fucosylation and trimannosyl core (Glycan# 500, 501, 416) which have shown reduced affinity for CSL. Glycan# 500 and 501 (RFU -1 and −2, respectively), are the complex bisecting N-glycans with bulkier side chains, which are not recognized by CSL in spite of core fucosylation. Possibly the hindrance is created due to the bulkier side chains, which may have influenced the binding of CSL. Glycan# 416 (RFU-11) is a core fucosylated glycan with the side chain fucose on the GlcNAc residue through α1-3 linkage. The binding affinity of CSL to this glycan is greatly reduced in spite of almost all the required glycan structure for CSL binding. The ambiguity observed for this glycan is yet to be addressed.
In conclusion, CSL has shown specific binding affinity towards the trimannosyl pentasaccharide fucosylated core, which is expressed on many cancer specific markers. CSL does not recognize any of the monosaccharides or O-glycans tested in the glycan array. The exquisite affinity of CSL towards such glycans makes it an important promising molecule that needs to be further explored in identifying the cancer specific markers with core fucosylation for its possible clinical application.
References
Mody R., Joshi S., Chaney W.: Use of lectins as diagnostic and therapeutic tools for cancer. J. Pharmacol. Toxicol. Methods. 33, 1–10 (1995)
Ohtsubo K., Marth J.D.: Glycosylation in cellular mechanisms of health and disease. Cell. 126, 855–867 (2006)
Fuster M.M., Esko J.D.: The sweet and sour of cancer: glycans as novel therapeutic targets. Nat. Rev. Cancer. 5, 526–542 (2005)
Marth, J. 1999. Glycosylation changes in ontogeny and cell activation. In: Varki, A. et al. (eds) Essentials of Glycobiology: NewYork: Cold Spring Harbor Laboratory Press, p 515–536.
Hollingsworth M.A., Swanson B.J.: Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 4, 45–60 (2004)
Moriwaki K., Miyoshi E.: Fucosylation and gastrointestinal cancer. World J. Hepatol. 2, 151–161 (2010)
Miyoshi E., Moriwaki K., Terao N., Tan C.C., Terao M., Nakagawa T., Matsumoto H., Shinzaki S., Kamada Y.: Fucosylation is a promising target for cancer diagnosis and therapy. Biomolecules. 2, 34–45 (2012)
Miyoshi E., Moriwaki K., Nakagawa T.: Biological function of fucosylation in cancer biology. J. Biochem. 143, 725–729 (2008)
Kobayashi Y., Tateno H., Dohra H., Moriwaki K., Miyoshi E., Hirabayashi J., Kawagishi H.: A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J Biol. Chem. 287, 33973–33982 (2012)
Nagre N.N., Chachadi V.B., Eligar S.M., Shubhada C., Pujari R., Shastry P., Swamy B.M., Inamdar S.R.: Purification and characterization of a mitogenic lectin from Cephalosporium, a pathogenic fungus causing mycotic keratitis. Biochem. Res. Int. 2010, 854656 (2010)
Duk M., Lisowska E., Wu J.H., Wu A.M.: The biotin/avidin-mediated microtiter plate lectin assay with the use of chemically modified glycoprotein ligand. Anal. Biochem. 221, 266–272 (1994)
Goldman M.: In fluorescent antibody methods. Academic Press, New York. 101-61, (1968)
Spiro R.G., Bhoyroo V.D.: Structure of the O-glycosidically linked carbohydrate units of fetuin. J. Biol. Chem. 249, 5704–5717 (1974)
Blixt O., Head S., Mondala T., Scanlan C., Huflejt M.E., Alvarez R., Bryan M.C., Fazio F., Calarese D., Stevens J., Razi N., Stevens D.J., Skehel J.J., van Die I., Burton D.R., Wilson I.A., Cummings R., Bovin N., Wong C.H., Paulson J.C.: Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc. Natl. Acad. Sci. U. S. A. 101, 17033–17038 (2004)
Boland C.R., Chen Y.F., Rinderle S.J., Resau J.H., Luk G.D., Lynch H.T., Goldstein I.J.: Use of the lectin from Amaranthus caudatus as a histochemical probe of proliferating colonic epithelial cells. Cancer Res. 51(2), 657–665 (1991)
Aoyagi Y., Isemura M., Suzuki Y., Sekine C., Soga K., Ozaki T., Ichida F.: Fucosylated alpha-fetoprotein as marker of early hepatocellular carcinoma. Lancet. 2, 1353–1354 (1985)
Okuyama N., Ide Y., Nakano M., Nakagawa T., Yamanaka K., Moriwaki K., Murata K., Ohigashi H., Yokoyama S., Eguchi H., Ishikawa O., Ito T., Kato M., Kasahara A., Kawano S., Gu J., Taniguchi N., Miyoshi E.: Fucosylated haptoglobin is a novel marker for pancreatic cancer: a detailed analysis of the oligosaccharide structure and a possible mechanism for fucosylation. Int. J. Cancer. 118, 2803–2808 (2006)
Block T.M., Comunale M.A., Lowman M., Steel L.F., Romano P.R., Fimmel C., Tennant B.C., London W.T., Evans A.A., Blumberg B.S., Dwek R.A., Mattu T.S., Mehta A.S.: Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc. Natl. Acad. Sci. U. S. A. 102, 779–784 (2005)
Marrero J.A., Romano P.R., Nikolaeva O., Steel L., Mehta A., Fimmel C.J., Comunale M.A., D’Amelio A., Lok A.S., Block T.M.: GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J. Hepatol. 43, 1007–1012 (2005)
Wang M., Long R.E., Comunale M.A., Junaidi O., Marrero J., Di Bisceglie A.M., Block T.M., Mehta A.S.: Novel fucosylated biomarkers for the early detection of hepatocellular carcinoma. Cancer Epidemiol. Biomark. Prev. 18, 1914–1921 (2009)
Shimomura M., Nakayama K., Azuma K., Terao N., Nishino K., Takamatsu S., Nakano M., Takahashi S., Kobayashi Y., Murata K., Kamada Y., Miyoshi E.: Establishment of a novel lectin-antibody ELISA system to determine core-fucosylated haptoglobin. Clin. Chim. Acta. 446, 30–36 (2015)
Matsumura K., Higashida K., Ishida H., Hata Y., Yamamoto K., Shigeta M., Mizuno-Horikawa Y., Wang X., Miyoshi E., Gu J., Taniguchi N.: Carbohydrate binding specificity of a fucose-specific lectin from Aspergillus oryzae: a novel probe for core fucose. J. Biol. Chem. 282, 15700–15708 (2007)
Acknowledgments
We would like to thank the Consortium for Functional Glycomics (CFG, USA), for the glycan array analysis of CSL. We thank Dr. Anitha Borges and Dr. Praveen Mahajan, S.L Raheja Hospital for providing the human colon tissue samples. The work was supported by the funding from Department of Science and Technology, India (No.SR/S0/BB-0085/2010/ 2012) and partial support from UGC sponsored UPE and CPEPA program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Inamdar, S.R., Eligar, S.M., Ballal, S. et al. Exquisite specificity of mitogenic lectin from Cephalosporium curvulum to core fucosylated N-glycans. Glycoconj J 33, 19–28 (2016). https://doi.org/10.1007/s10719-015-9628-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-015-9628-0