The results of studies of the chemical and mineralogical characteristics of alumina-containing wastes of the gas processing industry and the possibilities of their use for the synthesis of aluminum-magnesium spinel are presented. The optimal condition for the synthesis of MgAl2O4 spinel by the sol-gel method using alumina-containing waste is found to be 1000°C temperature and 120 min soaking.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Magnesium spinel MgAl2O4 is an important functional material. Due to its high chemical inertness, unique thermomechanical and optical properties, and high melting point (2135°C) is of great importance as a dopant and semitransparent optical and special highly refractory material for modern technology [1 – 4]. Spinel is mainly an essential component of refractory materials for lining steel-smelting ladles in metallurgy, transition and combustion zones of cement rotating and regenerators of glass-melting furnaces [5].
In industry, more than 70 – 80% of MgAl2O4 spinel is produced by the solid-phase reaction method. The synthesis of spinel requires homogeneous, highly reactive, and non-agglomerated powders of the starting components with firing temperature > 1600°C, which is necessary in order to achieve complete spinelization by means of solid-phase reactions. Consequently, high-temperature firing promotes grain growth and solid agglomeration of aluminum-magnesium spinel powders [6].
Many methods for synthesizing aluminum-magnesium spinel nanopowder have been developed and used in the last decades, including hydrothermal methods [7], combustion synthesis [8], lyophilization [9], Pechini method [10], and precipitation [6] as well as the sol-gel method [3, 11]. It should be noted that, year after year, a decrease in the synthesis temperature of MgAl2O4 spinel becomes a very real problem.
Since the sol-gel method makes it possible to form the necessary phase compositions and structure of the material at lower temperatures, the results of the synthesis of aluminum-magnesium spinel powder by the sol-gel method using alumina-containing waste from the gas processing industry are presented here. Along with temperature reduction the sol-gel method promotes lower consumption of primary native raw materials and less environmental pollution.
The chemical reagents magnesium nitrate hexahydrate Mg(NO3)2 ∙ 6H2O (chemically pure grade) and an alumina-containing spent catalyst from the gas processing industry were used as a source of cations. Nitric acid HNO3 (chemically pure grade) was used as a solvent, citric acid monohydrate as a chelating and polymerizing agent, and distilled water as a hydrolyzing agent.
X-ray phase analysis (LABX XRD-6100 SHIMADZU diffractometer, CuKα radiation, β-filter-Ni, wavelength 1.5418 Å, tube current and tube 30 mA, 30 kW) was used to identify the phase composition of the components used and the samples obtained. The constant rotation speed of the detector was equal to 4 deg/min with 0.02° steps (ω/2θ –mesh) and the scanning angle varied from 4 to 80°. For all samples, the scans were conducted under constant conditions. The international handbooks of x-ray powder diffraction patterns were used in the calculations and to identify phases [12].
In practice, the Claus method is used to purify natural gas from hydrogen sulfide. In particular, at the Shurtan Gas Chemical Complex (SGCC) in Uzbekistan catalytic oxidation of the latter by atmospheric oxygen is conducted on the surface of high-alumina ‘bauxitic’ catalyst with ‘gaseous’ sulfur obtained as a byproduct. High-porosity synthetic granular aluminum hydroxide is used as a catalyst, which, when operating at 400°C and above, partially dehydrates to the oxide γ-Al2O3 (Table 1). The weight content of Al2O3 in this product is equal to 82 – 90%, and after calcination at 900°C usually reaches at least 95% [13 – 15].
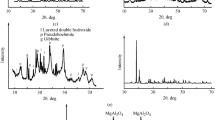
X-ray phase analysis showed that the mineralogical composition of the original spent catalyst includes gibbsite minerals with interplanar distances d = 0.618, 0.317, 0.241, 0.185, 0.145, 0.143, and 0.131 nm and gamma alumina with d = 0.455, 0.288, 0.236, 0.226, 0.197, 0.152, and 0.139 nm (Fig. 1a ).
To increase the content of single-phase Al2O= the alumina-containing waste was heat treated at 900°C with 120 min soaking. As a result, aluminum oxide was obtained in the γ-form, which crystallizes in a cubic structure. The x-ray diffraction patterns of the samples show that the lines of diffraction maxima corresponding to the mineral Al2O3 were found after the alumina-containing waste underwent heat-treatment: d = 0.456, 0.280, 0.238, 0.227, 0.197, 0.151, and 0.139 nm (Fig. 1b ); the diffraction peaks corresponding to the gibbsite minerals vanish due to dehydration.
The results of thermogravimetric and differential thermal analysis indicate that weight loss is observed mainly at a temperature of 150 – 615°C. The differential thermal analysis of samples of alumina-containing waste showed (Fig. 2) that the heating curve of the sample contains two endothermic effects — at 169 and 578°C — associated with the removal of H2O molecules. The two exothermic effects appearing at 315 and 489°C are due to the oxidation and burnout of organic substances, as well as the recrystallization of gibbsite to boehmite γ-AlO(OH).
The second weight loss associated with the endothermic peak at 578°C is explained by the crystallization of boehmite into γ-Al2O3 (as seen in the x-ray diffraction pattern in Fig. 1). Weight loss occurs by the reaction 2AlOOH → Al2O3 + H2O by removing the H2O molecule, which is accompanied by the appearance of thermal effects due to weight reduction. According to the thermogravimetric curve the total weight loss in the temperature range 150 – 615°C is 14.2%. No thermic event is observed on raising the temperature to 1100°C.
The resulting γ-form of aluminum oxide was comminuted in an agate mortar to the microlevel (0.7 – 1.0 μm), dissolved in distilled water, and acidified with nitric acid HNO3. Magnesium nitrate hexahydrate Mg(NO3)2 ∙ 6H2O was dissolved in distilled water. The resulting solutions were stirred at room temperature, after which citric acid was added and then stirred with a magnetic stirrer at 70 – 80°C until a gel-like mass was obtained. The resulting gel was dried at 125°C in an SNOL 3.5/350 drying oven for 8 h.
The dried gel was kept at 1000°C for 30, 60, and 120 min in order to determine the formation of the structure of crystalline magnesium aluminate and the effect of the soaking time during heat treatment on the process of synthesis of magnesium aluminum spinel, obtained by the sol-gel method, and full completion of the formation of the spinel crystal structure (Fig. 3).
The x-ray diffraction patterns show that the most intense effects of diffraction lines appear near grazing angle 2θ (36.9, 44.5, and 65.3°) in all samples at three soaking intervals (see Fig. 3).
These diffraction lines with interference indices hkl (311), (400), and (404) correspond to the magnesium aluminate phase. However, for the soaking times 30 min (Fig. 3a ) and 60 min (Fig. 3b ) a high intensity I is observed for the diffraction lines d = 0.210, 0.248 (2θ = 42.9; 62.4°), corresponding to magnesium oxide that do not participate in the spinel phase transformation. However, on increasing the soaking time to 120 min these lines diminish, resulting in an intensification of the diffraction lines d = 0.466, 0.285, 0.233, 0.201, 0.164, 0.155, and 0.142 nm, corresponding to spinel (Table 2). This confirms that the formation of the spinel crystal structure is fully complete.
It should also be noted that diffraction lines corresponding to the γ-form of aluminum oxide are not observed in the x-ray diffraction patterns of samples fired to 1000°C, which indicates that it reacts actively as compared with magnesium oxide.
In summary, the possibility of low-temperature synthesis of a fine powder of aluminum-magnesium spinel by a sol-gel method using alumina-containing waste, magnesium nitrate, and citric acid was investigated. It was found that at 400°C the citrate precursors decompose and the formation of aluminum-magnesium spinel commences. In addition, the completion of the reactions leading to the formation of cubic crystalline phases of aluminum-magnesium spinel is due to heat treatment of the prepared mixture at 1000°C with 120 min soaking.
References
J. F. Rufner, R. H. R. Castro, T. Holland, and K. van Benthem, “Mechanical properties of individual MgAl2O4 agglomerates and their effects on densification,” Acta Mater., No. 69, 187 – 195 (2014).
J. A. Wollmershauser, B. N. Feigelson, S. B. Qadri, and G. R. Villalobos, “Transparent nanocrystalline spinel by room temperature high-pressure compaction,” Scr. Mater., No. 69, 334 – 337 (2013).
L. Zarazúa-Villalobos, J. L. Téllez-Jurado, N. Vargas-Becerril, and G. Fantozzi, “Synthesis of magnesium aluminate spinel nanopowder by sol-gel and low-temperature processing,” J. Sol-Gel Sci. Technol., No. 85, 110 – 120 (2018).
R. Sarkar, “Refractory applications of magnesium aluminate spinel,” Int. Ceram. Refract. Manual, No. 1, 11 – 14 (2010).
R. Sarkar and S. Sahoo, “Effect of raw materials on formation and densification of magnesium aluminate spinel,” Ceram. Int., 40, 16719 – 16725 (2014).
S. Adison, J. Sirithan, J. Supatra, and S. Karn, “Synthesis and sintering of magnesium aluminate spinel nanopowders prepared by precipitation method using ammonium hydrogen carbonate as a precipitant,” Key Eng. Mater., 690, 224 – 229 (2016).
X. Zhang, “Hydrothermal synthesis and catalytic performance of high-surface-area mesoporous nanocrystallite MgAl2O4 as catalyst support,” Mater. Chem. Phys., 116, 415 – 420 (2009).
R. Iano°, I. Lazâu, and C. Pâcurariu, “Solution combustion synthesis of MgAl2O4 using fuel mixtures,” Mater. Res. Bull., 43, 3408 – 3415 (2008).
C. T. Wang, L. S. Lin, and S. J. Yang, “Preparation of MgAl2O4 spinel powders via freeze-drying of alkoxide precursors,” J. Am. Ceram. Soc., 75, 2240 – 2243 (1992).
S. Sanjabi and A. Obeydavi, “Synthesis and characterization of nanocrystalline MgAl2O4 spinel via modified sol-gel method,” J. Alloys Compd., 645, 535 – 540 (2015).
W. Liu, J. Yang, H. Xu, et al., “Effects of chelation reactions between metal alkoxide and acetylacetone on the preparation of MgAl2O4 powders by sol-gel process,” Adv. Powder Technol., 24, 436 – 440 (2013).
ASTM Standards. Pt 17. Refractories, Glass, Ceramic Materials, Carbon and Graphite Products, ASTM, Philadelphia (2005), pp. 7 – 9, 51 – 61.
Z. R. Kadyrova, S. K. Tuganova, and A. A. Éminov, “High-temperature interaction between calcium and strontium titanodisilicates,” Glass Ceram., 68(11 – 12), 413 – 415 (2011) [Steklo Keram., No. 12, 31 – 33 (2011)].
Sh. M. Niyazova, Z. R. Kadyrova, Kh. L. Usmanov, and F. G. Khomidov, “Ñhemical and mineralogical studies of magmatic rocks of Uzbekistan for obtaining heat-insulating materials,” Glass Ceram., 75(11 – 12), 491 – 495 (2019) [Steklo Keram., No. 12, 40 – 44 (2018)].
Al. A. Eminov, Z. R. Kadyrova, and M. Iskandarova, “Gas processing waste is a promising raw material for formulating the composition of ceramic grinding bodies,” Steklo Keram., No. 1, 43 – 48 (2021).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 6, pp. 48 – 52, June, 2021.
Rights and permissions
About this article
Cite this article
Khomidov, F.G., Kadyrova, Z.R., Usmanov, K.L. et al. Peculiarities of Sol-Gel Synthesis of Aluminum-Magnesium Spinel. Glass Ceram 78, 251–254 (2021). https://doi.org/10.1007/s10717-021-00389-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-021-00389-7