The sol-gel process of obtaining magnesium-aluminate spinel fibers from a fiber-forming precursor based on aluminum and magnesium polycarboxylates was studied. It was shown that in principle discrete fibers of stoichiometric spinel can be obtained by means of aerodynamic spraying. The results of surface morphology, fine structure, and phase composition studies of the fibers are presented. It is shown that the obtained fibers have a uniform and polycrystalline structure with practically no nonfibrous inclusions in the fibrous mass. The phase composition corresponds to the stoichiometry of spinel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
The operating conditions of aviation and space hardware predetermine the extremely stringent requirements of functional materials on whose quality the reliability and competitiveness of aircraft depend [1]. At the same time the development of new functional materials, including refractory, with fundamentally improved properties is a key avenue for a technological breakthrough in electric-power engineering, metallurgy, shipbuilding, and other industries not associated with aircraft [2]. It is impossible to satisfy the requirements of materials without developing new chemical products with enhanced properties and energy efficient and competitive technologies for their fabrication and processing.
The development of lightweight refractories with working temperatures above 1700°C that are serviceable in acidic media, resistant to corrosive median (including metallurgical slags), withstand significant temperature differentials and at the same time comparatively inexpensive has been a topical problem for many years.
In the Russian Federation the development of fibers and heat-resistant fibrous refractory materials is conducted as part of the complex scientific trend 14.3 ‘Multifunctional heat shielding and heat insulating materials’ [2]. An assortment of heat shielding materials, based on mullite-corundum and quartz fibers, with working temperature reaching 1700°C has been developed within this scientific trend [2,3,4].
Spinel is an excellent refractory material. The congruent melting point of spinel is 2135°C, which is higher than the melting temperature of the conventional compositions of heat shielding lining materials — mullite and corundum [5, 6]. Spinel is not subject to oxidation and is capable of operating for a long time in contact with oxygen at high temperatures, reaching the melting point. Materials based on magnesium-aluminate spinel are resistant to acids and alkali, and possess low electric conductivity. Chemically, spinel is resistant to mineral acids, carbon, many metals, alkalineearth, and a number of oxides. With respect to many slags, specifically those containing ferrous oxide, spinel is more stable than corundum and possesses comparable hardness, equal to 8 – 9 on the Mohs scale [5], and for this reason spinel-based refractories are used in metallurgy, for example, for lining casting ladles and other elements of the process equipment used in the manufacture of steel [7]. Spinel is an excellent material for heat resistant coatings of metal surfaces. Spinel fibers, just as other fibers based on aluminum oxide, can be used for the manufacturer of heat insulating materials and the cores of sealing cords, operating at extreme temperatures with exposure to chemicals [4].
Compared with refractories based on Y2O3 and ZrO2 spinel-based materials are much cheaper, which is a result of the abundance of Al and Mg in the Earth’s crust and smaller specific mass. For example, the theoretical density of tetragonal zirconium oxide is about 6.0 gcm3, while the density of spinel is only about 3.6 gcm3, so that spinel-based refractories will possess 40% smaller specific mass and comparable porosity.
Magnesia spinel is formed upon the interaction of MgO and Al2O3, contains 71.7% Al2O3 and 28.3% MgO, and is the only chemical compound in the binary system MgO–Al2O3. Spinel possesses a cubic crystal lattice with face-centered packing of the oxygen atoms [5]. This state of the crystal lattice is energetically very favorable. For this reason spinel is abundant as a mineral in nature. The conventional processes used for the manufacture of spinel-based refractories are extremely energy-intensive since they require high temperatures for structural consolidation. For example, the reaction forming spinel from powder precursors and sintering of the structure proceed at temperatures 1650 – 1805°C. In addition, as reported in [7] the production of 1 kg of a refractory by the powder technology requires the expenditure of 6000 to 9700 kJ of energy.
The sol-gel process makes it possible to form the required phase composition and structure of the material at significantly lower temperatures and, correspondingly, with significantly lower energy consumption. So, according to [7] the formation of the spinel phase occurs when the sol-gel process proceeds in the temperature interval 800 – 900°C, which, evidently, will make the technology less energy intensive. For this reason the present article is devoted to the sol-gel process of obtaining fibers of magnesium-aluminate spinel as the initial component for heat shielding products.
Expensive and combustible materials are not required to obtain nano-sized particles of spinel by the sol-gel method. The basis of the process is to obtain chelate polymer compounds of aluminum and magnesium with water soluble carboxylic acids, predominantly, oxalic acid and citric [8]. Chelate complexes are often combined with nitrate salts and organic polymers for an exothermal oxidation reaction with production of nanodisperse powders and porous materials by means of reactive sintering [9,10,11]. However, the indicated method is not applicable for obtaining fibers because the fibers breakdown in the gassing process.
The use of solid carboxylic acids to obtain fibers is also undesirable. This is based on the fact that the forming solutions manifest fiber-forming processes with high mass content (> 30%), and concentration provokes crystallization of excess acid in the form of a solid phase.
Polymer sol-gel precursors based on ammonium and magnesium polycarboxylates and lower carboxylic acids, such as formic, acetic, propionic, and isobutyric, possess excellent fiber forming properties. So, a method of obtaining continuous polycrystalline fibers of magnesium-aluminate spinel from aluminum tributoxide and magnesium acetate is described in [12]. The authors describe the process of obtaining aluminum polycarboxylate by the reaction of aluminum tributoxide with dilute formic acid. The authors obtained the forming solution by mixing a stoichiometric ratio of aluminum polyformiate with magnesium hydroxyacetate followed by hydrolysis upon heating to sol formation. To obtain a stable colloidal system stabilizers in the form of formic and isobutyric acids as well as ethylene glycol were introduced into the solution. Next, the sol was concentrated up to attainment of fiber-forming properties. The method described in [12] requires the use of aluminum tributoxide, which is a quite expensive product.
A less expensive and simple method of forming polycrystalline nanostructured discrete fibers of magnesium-aluminate spinel from homogeneous fiber-forming solutions preserving sedimentation stability at fiber-forming concentrations is proposed in the present article. Commercially available inexpensive precursors were used in the presented method, which makes the method of obtaining fibers commercially attractive in prospect.
Experimental Methodology
The fiber-forming precursor was synthesized by peptization of gels of a mixture of aluminum and magnesium hydroxides in a mixture of lower fatty carboxylic acids. Commercially available aluminum and magnesium salts were used to obtain gels of metal hydroxides. The gels contained stoichiometric ratio of Al and Mg in order to form spinel. To reach the required viscosity the solution was concentrated with simultaneous hydrolysis by repeatedly adding solvent. Fiber formation was conducted by means of aerodynamic (nozzle) spraying followed by heat-treatment of the gel-fibers up to the transition of the structure into a polycrystalline form.
The rheological properties of precursors were investigated by means of rotary rheometry performed with an MCR 501 rheometer in a rotation and oscillation regime. The surface morphology was investigated and the average diameter of the fibers determined by means of scanning electron microscopy. Transmission electron microscopy was used to study the fine structure of the fibers. Identification of the phase composition of the fibers was performed by x-ray diffraction; the diffraction patterns were recorded using monochromatized CuK radiation in the interval of angles 2 from 20 to 80°. The PDF-2 database was used to interpret the diffraction patterns; the reflection intensities minus the background were compared.
Results and Discussion
It was determined that the synthesized fiber-forming precursor possesses sedimentation stability for a long time, right up to the transition into gel upon curing or removal of the solvent to a dry residue. In the process of removing the solvent the viscosity of the solution increases, and crystallization of the solution is absent. On the basis of organoleptic observations it was hypothesized that the structure of the precursor is polymeric and amorphous. To check this hypothesis a number of studies were performed of the precursor by means of rheometry, optical microscopy, and x-ray diffraction.
The study of the structure of the precursor by means of optical microscopy showed that upon gelation the precursor transitions into a glassy state; a crystalline phase or inhomogeneity of a different character is not revealed (Fig. 1a ). The method of wide-angle x-ray diffraction confirms that the structure of the precursor is x-ray amorphous (Fig. 1b ). However, unlike the hydroxide from which it is synthesized the precursor possesses the prerequisites for forming supramolecular structures or regions of nucleation of a crystalline phase, which manifest as peaks at small angles (about 10°).
Investigation of the rheological properties of the fiberforming precursor in a viscous state confirms the supposition that its structure is polymeric and, possibly, linear. This conclusion is based on the following results. The character of the flow curve corresponds to a pseudoplastic liquid (Fig. 2a ), manifesting Newtonian flow at low shear rates (< 1 sec – 1 ). At higher shear rates from 1 to 1000 sec – 1 a region of plastic deformation is observed; the precursor manifests a distinct pseudoplastic flow character with the precursor molecules oriented in the direction of the load, and the precursor easily transforms into a fluid state.
The modulus of the fiber-forming precursor losses is greater than the accumulation modulus in a wide range of deformations (Fig. 2b ), i.e. the damping factor tan is always > 1. This fact indicates absence of long-range order in the solution and strong intermolecular bonds capable of storing energy and the forming elastic properties of the solution (Fig. 2b ).
The flow curve shows no evidence of hysteresis. Therefore the precursor has no weak intermolecular bonds that result in the appearance of a three-dimensional cellular gel structure and are capable of irreversible rupture under a load. The linear viscoelasticity range exceeds 200%, which indicates a high degree of elasticity of the polymer (see Fig. 2b ). Thus, the formation of long-range order in the fiber-forming precursor solution can be excluded and it must be assumed that the structure of the molecules is polymeric, predominantly linear, with no significant branching.
Fibers in the form of wool were obtained from the viscous forming solution by means of aerodynamic spraying (Fig. 3). It was determined experimentally that for this process the range of fiber-forming properties corresponds to viscosity from 0.3 to 10.0 Pa·sec. For higher values of the viscosity the fiber-forming properties are improved, but in this case other methods of formation must be used, such as extrusion or centrifugal spraying. Correspondingly, the production of fibers with large diameter, and in the future even continuous fibers, should be expected [12].
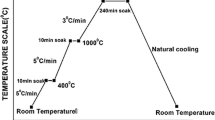
The gel-fibers were heat-treated at 900°C in order to form oxide fiber. The appearance of the fibers is shown in Fig. 3.
Investigation of the fine structure of the fibers showed the formation of a polycrystalline structure (Fig. 4a ), whose nanocrystallites form concentric circles as result of the diffraction of the electron beam (Fig. 4b ). The average size of the crystallites does not exceed 15 nm.
The surface morphology of the fibers is smooth with no visible defects (Fig. 5a ), which is a consequence of the absence of significant stresses of the structure during formation from the highly elastic polymer. The average diameter of the fibers, measured from the electronic photographs, is equal to 1.9μm. The diameter of the bulk of the fibers ranges from 0.6 to 2.6μm, the fiber diameter distribution is very uniformly, and the histogram is unimodal (Fig. 5b ). This form of the diagram also indicates the stability of the formation process. The absence of nonfibrous inclusions in the electronic photographs attests perfection of the process and, therefore, of the fiber-forming precursor. However, the form of the diagram is far from gaussian, so that the process is not ideal and requires additional technological elaboration.
Analysis of the diffraction spectra of the fiber samples obtained in the process of firing at different temperatures for 1 h showed that predominantly a transformation of gel-fibers into a polycrystalline magnesium-aluminate spinel occurs at 900°C (No. 1 sample) (Fig. 6a ). The presence of a halo at small angles attests the presence of amorphous matter, which vanishes after heat-treatment at 1250°C (sample No. 2, Fig. 6b ). Comparing the diffraction spectra of the samples Nos. 1 and 2 shows perfection of the polycrystalline structure with increasing firing temperature in the experimental range. The reflections of the fiber samples No. 1 weaker than the reflections of the fiber sample No. 2, and the lines themselves are wider. These effects indicate smaller coherent scattering regions and a less perfect crystalline structure in the case of sample No. 1 as compared with the sample No. 2. Thus, a complete transition of fibers into a polycrystalline state does not occur at 900°C; higher-temperature firing is required.
Conclusions
The possibility of obtaining discrete fibers of magnesium-aluminate spinel from commercially available raw material is the main result of these studies. This opens up prospects for assimilating the production of the type of fibers studied here for use in the manufacture of functional materials.
A fiber-forming precursor with a perfect structure, making possible a stable process of formation and production of high-quality fibers, was synthesized as a result of this research. Using available methods of research, it was hypothesized that the structure of the polymeric precursor is linear, which was confirmed by forming the fibers in practice.
References
E. N. Kablov, “Aerospace materials science,” Vse Materialy. Entsiklopedicheskii Spravochnik, No. 3, 2 – 14 (2008).
E. N. Kablov, “Strategic directions of development of materialsand their processing technologies for the period up to 2030,” Aviatsionnye materialy i tekhnologii, No. S, 7 – 17 (2012).
N. M. Varrik, “Heat-resistant fibers and heat and sound insulating fireproof materials,” Tr. VIAM: Elektron. Nauch.-Tekhn. Zh., No. 6, Art. 07 (2015), URL: http:www.viam-works.ru (accessed: December 03, 2018), DOI: 10.185772307-60462014-0-6-7-7.
V. G. Babashov and N. M. Varrik, “High-temperature flexible fibrous heat-insulating material,” Tr. VIAM: Elektron. Nauch.-tekhn. Zh., No. 1, Art. 03 (2015); URL: http: www.viam-works.ru (accessed: December 03, 2018), DOI: 10.185772307-6046-2015-0-1-3-3.
Ceramics from Magnesium-Aluminate Spinel: Internet Resource; URL: https:markmet.ruogneupornie-materialykeramikaiz-alyumomagnezialnoi-shpineli (Access date: 11062018).
Abdul-Majeed Azard and Teng Wan Dung, “Spinel producedvia self-heat-sustained (SUS) technique,” Mater. Res. Bull., 36, 1417 – 1430 (2001).
Aysun Özkan, Zerrin Günkaya, Gülden Tok, et al., “Life CycleCost Analysis of Magnesia Spinel Bric Production,” Sustainability, 8(7), 662 (2016); URL: https:www.mdpi.com 2071-105087662 (access date: 03.12.20), DOI: https: doi.org10.3390su8070662.
J.-Guang Li, Takayasy Ikagami, Jong Heun Lee, et al., “Awet-chemical process yielding reactive magnesium aluminate spinel (MgAl2O4) powder,” Ceram. Int., 27, 481 – 489 (2001).
Z. Ding, M. Zhang, and J. Han, “Synthesis of magnesium aluminate powders utilizing the solubility relationships in the Mg – Al – oxalic acid-H2O system,” Bulgar. J. Phys., 30, 152 – 157 (2003).
Li-Zhai Peia, Wan-Yun Yinb, Ji-Fen Wanga, et al., “Low temperature synthesis of magnesium oxide and spinel powders by sol-gel process,” Mater. Res., 13(3), 339 – 343 (2010).
Zhang Chunye, Shen Xiangqian, Zhou Jianxin, and Shen Jiangying, “Preparation of spinel ferrite CoFe2O4 fibers by organic gel-thermal decomposition process,” Rare Metal Mater. Eng., 37, 180 – 184 (2008).
Yin Liu and Richard M. Laine, “Spinel fibers from carboxylateprecursor,” J. Europ. Ceram. Soc., 19, 1949 – 1959 (1999).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Steklo i Keramika, No. 8, pp. 28 – 33, August, 2019.
Rights and permissions
About this article
Cite this article
Balinova, Y.A., Lyulyukina, G.Y. & Kolyshev, S.G. Magnesium-Aluminate Spinel Fibers Obtained by the Sol-Gel Method. Glass Ceram 76, 302–306 (2019). https://doi.org/10.1007/s10717-019-00188-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10717-019-00188-1