Abstract
The possible origins and differentiation of a ZZ/ZW sex chromosome system in Semaprochilodus taeniurus, the only species of the family Prochilodontidae known to possess heteromorphic sex chromosomes, were examined by conventional (C-banding) and molecular (cross-species hybridization of W-specific WCP, Fluorescence in situ hybridization (FISH) with telomere (TTAGGG)n, and Rex1 probes) cytogenetic protocols. Several segments obtained by W-specific probe were cloned, and the sequences localized on the W chromosome were identified by DNA sequencing and search of nucleotide collections of the NCBI and GIRI using BLAST and CENSOR, respectively. Blocks of constitutive heterochromatin in chromosomes of S. taeniurus were observed in the centromere of all autosomal chromosomes and in the terminal, interstitial, and pericentromeric regions of the W chromosome, which did not demonstrate interstitial telomeric sites with FISH of the telomere probe. The Rex1 probe displayed a compartmentalized distribution pattern in some chromosomes and showed signs of invasion of the pericentromeric region in the W chromosome. Chromosomal painting with the W-specific WCP of S. taeniurus onto its own chromosomes showed complete staining of the W chromosome, centromeric sites, and the ends of the Z chromosome, as well as other autosomes. However, cross-species painting using this WCP on chromosomes of S. insignis, Prochilodus lineatus, and P. nigricans did not reveal a proto-W element, but instead demonstrated scattered positive signals of repetitive DNAs. Identification of the W-specific repetitive sequences showed high similarity to microsatellites and transposable elements. Classes of repetitive DNA identified in the W chromosome suggested that the genetic degeneration of this chromosome in S. taeniurus occurred through accumulation of these repetitive DNAs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The family Prochilodontidae comprises many commercially important migratory fish, with most of the species demonstrating wide geographic distribution, including all hydrological basins in South America (Mago-Leccia 1972). The family comprises 21 species of three genera: Ichthyoelephas, Prochilodus, and Semaprochilodus (Castro and Vari 2004).
Prochilodontidae demonstrate conserved chromosome numbers (2n = 54) and chromosome morphologies of the meta- and submetacentric type. Many authors consider this family to be karyotypically stable, but this apparent conservation is not seen at the cytogenomic level in two species of Semaprochilodus (Terencio et al. 2012).
The species of the Prochilodontidae do not possess heteromorphic sex chromosomes, with exception to Semaprochilodus taeniurus that is the only with a system of ZZ/ZW feminine heterogamy (Feldberg et al. 1987; Pauls and Bertollo 1990; Oliveira et al. 2003; Terencio et al. 2012). The process of sex-chromosome evolution has attracted considerable interest over the years, and an important question has been what the evolutionary forces are that act to make a pair of autosomes cease recombining in one sex, eventually leading to the formation of two discrete chromosome types (Ellegren 2011). It is thought that heterochromatin plays a fundamental role in the differentiation of the proto-sex chromosome, as it contains large numbers of repetitive sequences that have been demonstrated to be significant promoters of sex chromosome differentiation in a number of organisms, including fish such as Hoplias malabaricus (Cioffi and Bertollo 2010), Characidium spp. (Machado et al. 2011), Oryzias javinucos (Takehana et al. 2012), and members of the Parodontidae (Schemberger et al. 2011).
Several cytogenomic tools have been useful as aids to understanding the origin and evolution of sex systems in various groups of organisms, especially fish (Diniz et al. 2008; Cioffi et al. 2011; Machado et al. 2011). Among these techniques, chromosome microdissection has allowed identification of sex-specific sequences by cloning and/or cross hybridization using whole chromosome painting (WCP).
The present work was designed to generate a W-specific probe of S. taeniurus to investigate possible origins of its differentiation using both classical and molecular cytogenetic tools. These included WCP with the W-specific probe of S. taeniurus to examine three species of the Prochilodontidae: S. insignis, Prochilodus lineatus, and P. nigricans, Fluorescence in situ hybridization (FISH) of telomeric and Rex1 probes in S. taeniurus and the sequencing of clones with W-specific fragments to investigate the genomic composition of this chromosome.
Materials and methods
Materials
Specimens of Semaprochilodus insignis, S. taeniurus, Prochilodus nigricans and P. lineatus collected with the authorization of the ICMBio SISBIO (10609-1/2007) were cytogenetically examined (Table 1). The fish were anesthetized in ice-cold water and killed. Voucher specimens were deposited in the INPA Animal Genetics Laboratory fish collection (10034, 10037, 10047, 10696).
Obtaining chromosomes and detection of heterochromatin
Chromosome preparations were obtained from anterior kidney cells using an in vivo colchicine treatment (Bertollo et al. 1978). The heterochromatin was analyzed by C-banding (Sumner 1972).
Microdissection
The W chromosomes of S. taeniurus were microdissected using an inverted microscope IX51 (Olympus) equipped with a mechanical micromanipulator (Narishige) from metaphase to allow identification of sex chromosomes. Microdissection was carried out with glass capillaries approximately 0.7 μm in diameter, prepared using a micropipette puller (Narishige). Five W chromosomes of S. taeniurus were microdissected, transferred to a microtube, and amplified through Genome Plex Single Cell—Whole Genome Amplification WGA4 (Sigma-Aldrich) following the manufacturer’s instructions.
Cloning and sequencing of W products
The amplification W products were cloned using an pMOS Blue Blunt Ended Cloning Kit (GE Healthcare), purified using the GFX PCR Purification Kit (GE Healthcare), and sequenced using the Big Dye Kit (Applied Biosystems) in ABI 3130. Sequence alignment was performed using Clustal W (Thompson et al. 1994), which is included in the BioEdit 7.0 software program (Hall 1999). Each clone was used as a query in BLASTN searches against the NCBI nucleotide collection (http://www.ncbi.nlm.nih.gov) and the searches against the Repbase database (Jurka et al. 2005) at the Genetic Information Research Institute (Giri) (http://www.girinst.org/repbase/) using CENSOR software (Kohany et al. 2006).
DNA Extraction and amplification of Rex1 and (TTAGGG)n
Total genomic DNA was extracted from the muscles of S. taeniurus following the phenol–chloroform protocol (Sambrook and Russell 2001). Amplification by PCR of the Rex1 retrotransposon was conducted using the primers RTX1-F1 (5′-TTCTCCAGTGCCTTCAACACC) and RTX1-R1 (5′-TCC CTC AGC AGA AAG AGT CTGCTC) (Volff et al. 2000). The PCR reactions were carried out in a final volume of 25 μl consisting of 2 μl of genomic DNA (100 ng), 2.5 μl of 10 × buffer with magnesium chloride (1.5 mM), 0.25 μl of Taq DNA Polymerase (5 U/μl), 1.5 μl of dNTP (1 mM), 1.5 μl of each primer (5 mM), and milli-Q water to complete the volume. A probe from the general telomeric DNA sequence of the vertebrate (TTAGGG)n was generated by PCR in the absence of a template, using (TTAGGG)5 and (CCCTAA)5 (Ijdo et al. 1991) as primers. Cycling conditions were as follows: (a) Rex1: 2 min at 95 °C (denaturation), 35 cycles of 1 min at 95 °C, 40 s at 55 °C (annealing) and 2 min at 72 °C (extension), 5 min at 72 °C (final extension), and (b) (TTAGGG)n: 1 min at 95 °C, 30 s at 50 °C, 1 min at 72 °C, 35 cycles of 1 min at 94 °C, 30 s at 60 °C, 2 min at 72 °C, and 5 min at 72 °C.
Fluorescence in situ hybridization (FISH)
The W-specific probe of S. taeniurus, isolated by microdissection, the Rex1, and (TTAGGG)n probes were labeled with digoxigenin-11-dUTP (Dig-Nick Translation mix; Roche) by nick translation reactions, following the manufacturer’s instructions (in probes, the mix comprised 1,500 ng of template, 4 μl of enzyme mix and milli-Q water for complete 20 μl). The antibody anti-digoxigenin rhodamine (Roche) was used to detect the signal. FISH was performed on mitotic chromosome spreads (Pinkel et al. 1986). Homologous and heterologous in situ fluorescent hybridizations were carried out with 77 % stringency (2.5 ng/μl of DNA W-specific, 50 % deionized formamide, 10 % dextran sulfate, and 2XSSC at 37 °C for 18 h). The chromosomes were counterstained with DAPI (2 μg/ml), in Vectashield mounting medium (Vector).
Microscopy/image processing
Hybridized chromosomes were analyzed using an Olympus BX51 epifluorescence microscope, and the images were captured with a digital camera (Olympus DP71), using Image-Pro MC 6.3 software. Mitotic metaphases were processed using Adobe Photoshop CS4, with the chromosomes measured by the Image J public domain program. The chromosomes were classified as metacentric (m) and submetacentric (sm), according to Levan et al. (1964).
Results
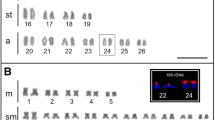
The constitutive heterochromatin of S. taeniurus was localized in the centromeric region of all its chromosomes and in the terminal regions of four chromosomes. Heterochromatin was also identified in the centromeric and terminal regions of the long arm of the Z chromosome, while the W chromosome, which is twice the size of its Z homolog, demonstrated large quantities of constitutive heterochromatin in the two terminal regions as well as in the interstitial and pericentromeric regions (Fig. 1a).
Fluorescence in situ hybridization of the repetitive telomeric sequences (TTAGGG)n of S. taeniurus demonstrated typical sites on the telomeres of all chromosomes, while interstitial telomeric sites (ITS) were not found (Fig. 1b).
The Rex1 element of S. taeniurus displayed a compartmentalized distribution in some chromosomes, with hybridization signals at the centromeric and telomeric regions in both males and females. The W chromosome exhibited clear signals along its full length. No sites of Rex1 were found on the Z chromosome (Fig. 1c).
The amplification of the genomic product corresponding to the W chromosome generated fragments ranging from 100 to 1,000 base pairs (bp) at a concentration of 1,000 ng/μl. Cloning and sequencing of W-specific products revealed several clones that showed high similarity with sequences that are deposited in public sequence database NCBI. However, most of these fragments are short (<200 bp) and were not included, since GenBank does not accept sequences less than 200 bp in length. The analysis revealed repeated sequences, such as microsatellites and transposable elements (TEs), as well as a gene fragment that showed high similarity to the transcription factor of the SOX9 gene (Table 2).
Four species analyzed (S. taeniurus, S. insignis, P. lineatus and P. nigricans) possessed 2n = 54 chromosomes organized as 40 m + 14 sm (Fig. 2). Whole chromosome painting (WCP) with the W-specific probe on metaphase chromosomes demonstrated positive signals in S. taeniurus in the terminal regions of some chromosome pairs and in the centromeric regions of all autosomal chromosomes in both males and females. The Z sex chromosome demonstrated large positive sites in the terminal and centromeric regions, while the W chromosome demonstrated positive signals along its entire length (Fig. 2a).
Cross-species hybridization with the W-specific probe in metaphase chromosomes of S. insignis (males and females) demonstrated positive signals in terminal regions of some chromosomal pairs and along the entire length of the short arm of pair 25. With the exception of pairs 4 and 22, no positive signals were observed in the centromeric regions (Fig. 2b).
Positive signals to the W-specific probe were seen in P. lineatus, principally in the terminal regions of most chromosome pairs. Positive signals were observed along most of the length of the long arms of chromosome pairs 1, 10 and 21 and in the centromeric regions of pairs 11, 16, 18, 26, and 27. Positive signals were also seen on the B chromosomes concentrated in the terminal regions on the first chromosome, but were dispersed on the second (Fig. 2c).
Prochilodus nigricans showed positive signals to the W-specific probe in the terminal regions of most chromosome pairs. Definite signals were observed on all submetacentric pairs. Centromeric signals were only seen on pairs 15, 20, and 25 (Fig. 2d).
Discussion
In last decades, heterochromatin has become recognized as a fundamental part of the genome, with many functions related to chromosomal segregation, nuclear organization, and the regulation of gene expression (Grewal and Jia 2007). Research has demonstrated that heterochromatin contains high densities of repetitive elements such as satellites, microsatellites, and transposable elements that are principally concentrated in the centromeric and telomeric regions of the eukaryotic chromosomes (Skipper 2007). The heterochromatic patterns seen in the centromeric region of the chromosomes of S. taeniurus corroborated earlier results in terms of both the autosomal and sex chromosomes (Terencio et al. 2012). The W chromosome demonstrated heterochromatic patterns distinct from its Z homologue, with large quantities of heterochromatin along the chromosome length, a characteristic common to organisms having differentiated sex chromosomes (Fig 1a).
Several hypotheses have been proposed to explain the origin of sex chromosomes. Their differentiation from an autosomal pair and/or the partial or total degradation of sex-specific chromosomes (Ohno 1967; Muller 1964), are the most accepted hypotheses for many groups of organisms.
The differentiation of autosomal pairs may occur through two principal mechanisms. The first would involve chromosomal rearrangement, with resulting heteromorphism (Charlesworth et al. 2005). Parodontid fish have experienced an inversion event that rearranged the terminal repetitive DNAs to the proximal region of the proto-sex chromosomes and led to the amplification of these sites near the short arms, resulting in W chromosome differentiation (Schemberger et al. 2011). The absence of interstitial telomeric sites in S. taeniurus suggests that rearrangements by chromosome fusion and/or inversion are not involved in the origin of the W chromosome (which is twice the size of the Z chromosome). However, chromosomes originating from rearrangements can lack interstitial signals due to the loss of the interstitial telomeres during the molecular processes eroding active (Carvalho et al. 2012). The fact that all species of the Prochilodontidae have the same diploid number and karyotypic formula also suggests the absence of chromosomal rearrangements in the origin of W chromosome.
A second mechanism proposed for the differentiation of autosome pairs into sex chromosomes involves a gradual reduction in their recombination (Brooks 1988). Some studies have indicated that the suppression of recombination strongly biases the distribution of retrotransposons in the genome, indicating that the major force driving the evolution of sex chromosomes are repetitive sequences, remodeling euchromatic chromosome structures into heterochromatic (Steinemann and Steinemann 2005; Bohne et al. 2012). It is possible that the suppression of recombination of the proto-sex chromosomes of S. taeniurus promoted the accumulation of heterochromatin in the W chromosome that formed as a consequence of genomic defense against invasive parasitic elements (which frequently occur in non-recombinant genomic regions) (Dasilva et al. 2002; Steinemann and Steinemann 2005; Caputo et al. 2011) (Fig. 3). Thus, the W chromosome in S. taeniurus has significantly increased in size due to the accumulation of repetitive DNAs, such as Rex1 retroelement, with the consequent differentiation of the ZZ/ZW system of sex chromosomes through the degradation of its genetic activities. This hypothesis has merit since the most W-specific fragments of S taeniurus showed high similarity to repetitive sequences classified as microsatellites, transposons and retrotransposons (Table 2). Data accumulated from recent studies indicated that these repetitive sequences have had significant influence on the evolution of genomes, particularly by controlling gene activity (Valente et al. 2011). Transposons and retrotransposons have been found on the sex chromosome of many fish species: Xiphophorus maculatus (Volff et al. 2000; Bohne et al. 2012), Leporinus elongatus (Marreta et al. 2012), Oryzias hubbsi (Takehana et al. 2012), and S. taeniurus in the present study. One of the W-specific fragments showed high similarity with the partial sequence of the transcription factor of the SOX9 gene in Takifugu rubripes. The SOX9 is a gene related to sex determination in many organisms.
The W-specific probe hybridized in heterochromatin regions in autosomal pairs of S. taeniurus suggests that the W chromosome comprises other repetitive DNA families, such as the Rex1 retroelement seen in this study. This hypothesis is reinforced by the fact that signals homologous to those seen on the W chromosome were evident in the subterminal regions of the other species analyzed, and these subtelomeric domains represent dynamic satellite regions with high evolution rates (Torres et al. 2011).
Comparative chromosomal painting indicating the presence of repetitive DNA sequences in the W sex chromosome of S. taeniurus that are shared with S. insignis, P. lineatus, and P. nigricans also revealed the existence of centromeric repetitive sequences in S. taeniurus that is not shared with the other species studied and possibly represents a species-specific repetitive DNA sequence. Species generally demonstrate divergences as a result of concerted evolutionary mechanisms, leading to species-specific DNA sequences (Arnheim 1983; Canapa et al. 2002; Grewal and Jia 2007; Vicari et al. 2010). This would help explain why S. taeniurus demonstrated sites on the majority of its centromeres while the other species did not.
W-specific signals seen in S. insignis, P. lineatus, and P. nigricans were primarily evident in the terminal chromosome regions and did not coincide with the heterochromatic regions revealed by C banding. The heterochromatic blocks in these species are principally located in the centromeric regions of most chromosomes (Venere et al. 1999; Artoni et al. 2006; Vicari et al. 2006; Terencio et al. 2012).
Presence of repetitive elements, chiefly microsatellites, near the telomeres is common in fish and indicates that these repetitive DNA sequences have an important role in genomic evolution (Gross et al. 2010; Jurka et al. 2011; Oliver and Greene 2011). It is probable that the W-specific positive signals present in the terminal chromosome regions of the species S. insignis, P. lineatus, and P. nigricans represent satellites and/or microsatellites specific to those regions. This pattern of telomeric marking has also been reported in three species of Prochilodus whose chromosomes were mapped using the (AATTT)n microsatellite probe (Hatanaka et al. 2002).
The B chromosomes detected in P. lineatus demonstrated positive signals with the W-specific probe, indicating repetitive DNA families. Jesus et al. (2003) isolated and characterized two satellite DNA families (SATH 1 and SATH2) present in the chromosomes of P. lineatus. However, only SATH2 occur on B chromosomes. The presence of repetitive DNAs with the W probe showing terminal localizations in P. lineatus, and visualized on the B chromosomes, indicated that other repetitive DNA classes different from SATH families may also occur on these chromosomes. Some studies have suggested that B chromosomes could influence sex determination in fish (Yoshida et al. 2011; Noleto et al. 2012) although no relationship between the occurrence of B chromosomes and sex determination has been observed in P. lineatus.
Chromosomal painting of the W-specific probe allowed the identification of the Z chromosome in S. taeniurus but did not allow the identification of any proto-W chromosomes in S. insignis, P. lineatus, or P. nigricans. As Semaprochilodus is a phylogenetically derived genus in the Prochilodontidae (Castro and Vari 2004), we suggest that the sex chromosome system seen in S. taeniurus appeared relatively recently in this species, as no proto-W chromosomes could be identified in cross hybridizations among the species analyzed.
References
Arnheim N (1983) Concerted evolution of multigene families. In: Nei M, Koehn RK (eds) Genes and Evolution of Genes and Proteins. Sinauer, Sunderland, pp 38–61
Artoni RF, Vicari MR, Endler AL, Cavallaro ZI, Jesus CM, Almeida MC, Moreira-Filho O, Bertollo LAC (2006) Banding pattern of A and B chromosomes of Prochilodus lineatus (Characiformes, Prochilodontidae), with comments on B chromosomes evolution. Genetica 127:277–284. doi:10.1007/s10709-005-4846-1
Bertollo LAC, Takahashi CS, Moreira-Filho O (1978) Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erytrinidae). Braz J Genet 1:103–120
Bohne A, Zhou Q, Amandine D, Schmidt C, Schartl M, Galiana-Arnoux D, Volff JN (2012) Zisupton—A novel superfamily of DNA transposable elements recently active in fish. Mol Biol Evol 29(2):631–645. doi:10.1093/molbev/msr208
Brooks LD (1988) The evolution of recombination rates. In: Michod RE, Levin BR (eds) The Evolution of Sex. Sianauer, Sunderland, pp 87–105
Canapa A, Cerioni PN, Barucca M, Olmo E, Caputo V (2002) A centromeric satellite DNA may be involved in heterochromatin compactness in gobiid fishes. Chromosome Res 10:297–304. doi:10.1023/A:1016519708187
Caputo V, Giovannotti M, Cerioni PN, Splendiani A, Tagliavini J, Olmo E (2011) Chromosomal study of a lamprey (Lampetra zanandreai Vladykov, 1955) (Petromyzonida: Petromyzontiformes): convencional and FISH analysis. Chromosome Res 19:481–491
Carvalho NDM, Gross MC, Schneider CH, Terencio ML, Zuanon J, Feldberg E (2012) Cytogenetics of Synbranchiformes: a comparative analysis of two Synbranchus Bloch, 1795 species from the Amazon. Genetica 140:149–158
Castro RMC, Vari RP (2004) Detritivores of the South American fish family Prochilodontidae (Teleostei: Ostariophysi: Characiformes): A Phylogenetic and Revisionary Study: Smithsonian Contributions and Studies Series. An Imprint of Smithsonian Books, Washington, D. C. N622
Charlesworth D, Charlesworth B, Marais G (2005) Steps in the evolution of heteromorphic sex chromosomes. Heredity 95:118–128. doi:10.1038/sj.hdy.6800697
Cioffi MB, Bertollo LAC (2010) Initial steps in XY chromosome differentiation in Hoplias malabaricus and the origin of an X1X2Y sex chromosome system in this fish group. Heredity 105:554–561. doi:10.1038/hdy.2010.18
Cioffi MB, Sanchez A, Marchal JA, Kosyakova N, Liehr T, Trifonov V (2011) Whole chromosome painting reveals independent origin of sex chromosomes in closely related forms of a fish species. Genetica 8:1065–1072. doi:10.1007/s10709-011-9610-0
Dasilva C, Hadji H, Ozouf-Costaz C, Nicaud S, Jaillon O, Weissenbach J, Roest Crollius H (2002) Remarkable compartmentalization of transposable elements and pseudogenes in the heterocromatin of the Tetraodon nigroviridis genome. Proc Natl Acad Sci USA 21:13636–13641
Diniz D, Laudicina A, Cioffi MB, Bertollo LAC (2008) Microdissection and whole chromosome painting. Improving sex chromosome analysis in Triportheus (Teleostei, Characiformes). Cytogenet Genome Res 122:163–168. doi:10.1159/000163094
Ellegren H (2011) Sex-chromosome evolution: recent progress and the influence of male and female heterogamety. Nat Rev Genet 12:157–166. doi:10.1038/nrg2948
Feldberg E, Bertollo LAC, Almeida-Toledo LF, Foresti F, Moreira-Filho O, Santos AF (1987) Biological aspects of Amazonian fishes. IX. Cytogenetic studies in two species of the genus Semaprochilodus (Pisces, Prochilodontidae). Genome 29:1–4
Grewal SIS, Jia S (2007) Heterochromatin revisited. Nat Rev Genet 8:35–46. doi:10.1038/nrg2008
Gross MC, Schneider CH, Valente GT, Porto JIR, Martins C, Feldberg E (2010) Comparative cytogenetic analysis of the genus Symphysodon (Discus Fishes, Cichlidae): chromosomal characteristics of retrotransposons and minor ribosomal DNA. Cytogenet Genome Res 127:43–53. doi:10.1159/000279443
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/96/NT. Nucleic Acids Symp Ser 41:95–98
Hatanaka T, Henrique-Silva F, Galetti PM Jr (2002) A polymorphic, telomeric-like sequence microsatellite in the neotropical fish Prochilodus. Cytogenet Genome Res 98:308–310. doi:10.1159/000071054
Ijdo JW, Wells RA, Baldini A, Reeders ST (1991) Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucleic Acids Res 19:4780
Jesus CM, Galetti PM Jr, Valentini SR, Moreira-Filho O (2003) Molecular characterization and chromosomal location of two families of satellite DNA in Prochilodus lineatus (Pisces, Prochilodontidae), a species with B chromosomes. Genetica 118:25–32. doi:10.1023/A:1022986816648
Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, Walichiewicz J (2005) Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res 110:462–467
Jurka J, Bao W, Kojima KK (2011) Families of transposable elements, population structure and the origin of species. Biol Direct 6:44. doi:10.1186/1745-6150-6-44
Kohany O, Gentles AJ, Hankus L, Jurka J (2006) Annotation, submission and screening of repetitive elements in repbase: repbase submitter and censor. BMC Bioinforma 7:474
Levan A, Fredga K, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52:201–220
Machado TC, Pansonato-Alves JC, Pucci MB, Nogaroto V, Almeida MC, Oliveira C, Foresti F, Bertollo LAC, Moreira-Filho O, Artoni RF, Vicari MR (2011) Chromosomal painting and ZW sex chromosomes differentiation in Characidium (Characiformes, Crenuchidae). BMC Genet 12:65. doi:10.1186/1471-2156-12-65
Mago-Leccia E (1972) Consideraciones sobre la sistematica de la familia prochilodontidae (Osteichthyes, Cypriniformes), con una sinopsia de las especies de Venezuela. Acta Biol Venezuelica 8(Suppl 1):35–96
Marreta ME, Faldoni FLC, Parise-Maltempi PP (2012) Cytogenetic mapping of the W chromosome in the genus Leporinus (Teleostei, Anostomidae) using a highly repetitive DNA sequence. J Fish Biol 80:630–637. doi:10.1111/j.1095-8649.2011.03199.x
Muller HJ (1964) The relation of recombination to mutational advance. Mutat Res 1:2–9
Noleto RB, Vicari MR, Cestari MM, Artoni RF (2012) Variable B chromosomes frequencies between males and females of two species of pufferfishes (Tetraodontiformes). Rev Fish Biol Fisheries 22:343–349. doi:10.1007/s11160-011-9231-9
Ohno S (1967) Sex Chromosomes and Sex-Linked Genes. Springer, Berlin
Oliveira C, Nirchio M, Granado A, Levy S (2003) Karyotypic characterization of Prochilodus mariae, Semaprochilodus kneri and Semaprochilodus laticeps (Teleostei: Prochilodontidae) from caicara del orinoco, Venezuela. Neotrop Ichthyol 1:47–52. doi:10.1590/S1679-62252003000100005
Oliver KR, Greene WK (2011) Mobile DNA and the TE-Thrust Hypothesis: supporting evidence from the primates. Mob DNA 2:8. doi:10.1186/1759-8753-2-8
Pauls E, Bertollo LAC (1990) Distribution of a supernumerary chromosome system and aspects of karyotypic evolution in the genus Prochilodus (Pisces, Prochilodontidae). Genetica 81:117–123. doi:10.1007/BF00226450
Pinkel D, Straume T, Gray JW (1986) Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci USA 83:2934–2938
Sambrook J, Russell DW (2001) Molecular cloning: A laboratory manual, vol I. Cold Spring Harbor Press, Cold Spring Harbor
Schemberger MO, Bellafronte E, Nogaroto V, Almeida MC, Schuhli GS, Artoni RF, Moreira-Filho O, Vicari MR (2011) Differentiation of repetitive DNA sites and sex chromosome systems reveal closely related group in Parodontidae (Actinopterygii: Characiformes). Genetica 139:1499–1508. doi:10.1007/s10709-012-9649-6
Skipper M (2007) Mysteries of heterochromatic sequences unravelled. Nat Rev Genet 8:567. doi:10.1038/nrg2161
Steinemann S, Steinemann M (2005) Retroelements: tools for sex chromosome evolution. Cytogenet Genome Res 110:134–143. doi:10.1159/000084945
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304–306
Takehana Y, Naruse K, Asada Y, Matsuda Y, Shin-I T, Kohara Y, Fujiyama A, Hamaguchi S, Sakaizumi M (2012) Molecular cloning and characterization of the repetitive DNA sequences that comprise the constitutive heterochromoatin of the W chromosomes of the medaka fishes. Chromosome Res 20:71–81. doi:10.1007/s10577-011-9259-7
Terencio ML, Schneider CH, Gross MC, Vicari MR, Feldberg E (2012) Stable karyotypes: a general rule for the fish of the family Prochilodontidae? Hydrobiologia 686:147–156. doi:10.1007/s10750-012-1006-3
Thompson JD, Higgins DG, Gibson TJ (1994) Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Torres GA, Gong Z, Iovene M, Hirsch CD, Buell CR, Bryan GL, Novák P, Macas J, Jiang J (2011) Organization and evolution of subtelomeric satellite repeats in the potato genome. Genes Genomes Genet 1:85–92. doi:10.1534/g3.111.000125
Valente GT, Mazzuchelli J, Ferreira IA, Poletto AB, Fantinatti BEA, Martins C (2011) Cytogenetic mapping of the retroelements Rex1, Rex3 and Rex6 among cichlid fish: new insights on the chromosomal distribution of transposable elements. Cytogenet Genome Res 133:34–42. doi:10.1159/000322888
Venere PC, Miyazawa CS, Galetti-Jr PM (1999) New cases of supernumerary chromosomes in characiform fishes. Genet Mol Biol 22:345–349. doi:10.1590/S1415-47572006000400008
Vicari MR, Almeida MC, Bertollo LAC, Moreira-Filho O, Artoni RF (2006) Cytogenetic analysis and chromosomal characteristics of the polymorphic 18S rDNA in the fish Prochilodus lineatus (Characiformes, Prochilodontidae). Genet Mol Biol 4:621–625. doi:10.1590/S1415-47572006000400008
Vicari MR, Nogaroto V, Noleto RB, Cestari MM, Cioffi MB, Almeida MC, Moreira-Filho O, Bertollo LAC, Artoni RF (2010) Satellite DNA and chromosomes in neotropical fishes: methods, applications and perspectives. J Fish Biol 76:1094–1116. doi:10.1111/j.1095-8649.2010.02564.x
Volff JN, Korting C, Schartl M (2000) Multiple lineages of the non-LTR retrotransposons Rex1with varying success in invading fish genomes. Mol Biol Evol 17:1673–1684
Yoshida K, Terai Y, Mizoiri S, Aibara M, Nishihara H, Watanable M, Kuroiwa A, Hirai H, Hirai Y, Matsuda Y, Okada N (2011) B chromosomes have a functional effect on female sex determination in lake victoria cichlid fishes. PLoS Genet 7(8):e1002203. doi:10.1371/journal.pgen.1002203
Acknowledgments
This study was supported by National Council for Scientific and Technological Development (CNPq—141660/2009-0), National Amazon Research Institute/Genetic, Conservations and Evolutionary Biology (INPA/GCBEV), The State of Amazonas Research Foundation (FAPEAM) and Centre for Studies of Adaptation to Environmental Changes in the Amazon (INCT ADAPTA,FAPEAM/CNPq 573976/2008-2), PRONEX/FAPEAM/CNPQ 003/2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Terencio, M.L., Schneider, C.H., Gross, M.C. et al. Repetitive sequences associated with differentiation of W chromosome in Semaprochilodus taeniurus . Genetica 140, 505–512 (2012). https://doi.org/10.1007/s10709-013-9699-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10709-013-9699-4