Abstract
Soil dispersion is a phenomenon in which soil particles become afloat when they are exposed to water, and are carried away by the force of seepage. In spite of that soil dispersion is due to its chemical composition, the results obtained from the chemical methods, especially from the most widely used, Sherard method does not match with the results of well-known Pinhole test. This study tries to evaluate and modify the Sherard diagram for determination of dispersion potential of clayey soils. For this purpose, several natural soil samples were collected from different regions of Iran and some artificial soil samples were made by adding different percentages of four chemical agents, including sodium chloride, sodium carbonate, sodium sulfate, and sodium polyphosphate to a natural soil. The physical, chemical and index properties of all samples were determined and for determination of dispersion potential, the commonly used chemical test (Sherard method) and Pinhole test were employed. The results obtained from the tests showed that the Sherard chemical method which is solely based on the amount and type of the existing cations, is not able to determine soil dispersion correctly since the role of some anions, especially chloride is neglected. It was also found that among the existing anions in the soil, the chloride on the contrary to sodium acts as a flocculating factor. The results showed that by converting the vertical axis of the Sherard chart from sodium% to (sodium chloride)%, its conformity to the results of Pinhole tests increases considerably.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dispersive soils are one of the most common problematic soils in several countries around the world which can cause severe damages to hydraulic structures such as earth dams and irrigation structures. Due to a specific chemical nature of dispersive clays, i.e., the interparticle force of repulsion (Electrical surface forces) exceed those of attraction (Van der Waals attraction), soil particles would disperse when comes in contact with water and are carried away by flowing water resulted from leakage or surface runoff Bhuvaneshwari (2007). Shanmuganathan and Oades (1983) presented dispersions of clay are stable when most of the counter ions exist in the diffuse double layer and the thickness of this layer is of the same order of magnitude as the diameter of the individual colloidal particle. Repulsive forces created by overlapping double layers which are increased by increasing the surface charge density prevent the close approach and coalescence of individual particles. Dispersive soils have a wide geographical distribution across Iran and are found in most provinces including Khuzestan, Ardebil, Fars, and Isfahan. In earth dams and embankments in contact with water, piping will start by concentration of flow towards the cracks on the downstream side of the structure which progresses to the upstream side in the form of a pipe until it reaches the water source, at which time a rapid catastrophic failure may result (Knodel 1991). In regard to the irrigation canals founded on dispersive soils, the severe erosion of the soil begins from the bed of the lining or the berm, which eventually cause complete destruction due to elimination of lining bed. In concrete lined canals, destruction begins with formation of cracks, rupture and displacement of lining in the final stages (Rahimi and Abbasi 2008).
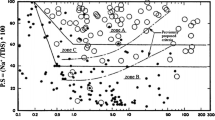
The identification and diagnosis of dispersion phenomenon is the fundamental measure for preventing early destruction of hydraulic structures. Although a number of methods have been suggested for identification of dispersive soils, careless tests and/or inappropriate interpretation of their results may lead to incorrect assessment of the problem. Pinhole, Double Hydrometer and Sherard chemical method are the most commonly used laboratory methods for determining the dispersion potential of soils; each having their own advantages and disadvantages (Knodel 1991). In Pinhole test which the dispersion potential of the soil is evaluated directly, water is allowed to flow through a small hole created in the soil sample. The water flow through the Pinhole simulates flow through a crack or leakage channel in the impervious core of a dam or other earthen structures. For dispersive soils, the flow emerging from the soil sample is cloudy and the hole rapidly enlarges. For non-dispersive soils, the outflow is clear and the hole does not enlarge. The discharge and transparency of the out flow and the diameter of the hole at the end of the test are three main criteria for evaluation of soil dispersion potential. On the basis of this test, the clayey soils are classified in six different categories named as: D1, D2, ND4, ND3, ND2, and ND1 with respect to the intensity of dispersion, where; D1 is most dispersive and ND1 is the most resistance to dispersion. In Double Hydrometer test, gradation curve of the soil is drawn in two cases; using standard hydrometer test and hydrometer test without employing dispersing agent and mechanical mixer. The percentage of particles smaller than 0.005 mm in both methods is then determined and dispersion ratio is calculated as the ratio of the percentage of particles smaller than 5 micron in the method without using dispersing agent and mixer to the percentage of particles smaller than 5 micron in the standard method. If the dispersion ratio is more than 50 %, the soil has dispersion potential, and if the result is between 30 and 50 %, it shows intermediate dispersion potential and <30 % shows non-dispersive nature of the soil (Knodel 1991). According to Sherard chemical method, the dispersion potential of the soil is evaluated with respect to the type and quantity of cations present in pore water of the soil sample. For this purpose, saturation extract is prepared from the soil sample, then its four main cations namely; calcium, magnesium, sodium, and potassium are determined in terms of milliequivalents per liter and the amount of total dissolved salts (TDS) and the percentage of sodium (PS) are calculated. The dispersion potential of the soil sample is evaluated from the Sherard diagram as shown in Fig. 1. The diagram is divided into three zones: Dispersive (zone A), Non-dispersive (zone B), and the Intermediate (zone C) based on PS and TDS of the soil extract (Knodel 1991).
Sherard diagram for evaluation of dispersion potential of soils (Knodel 1991)
As presented in the literature Double Hydrometer test and Sherard diagram are not reliable for evaluation of soil dispersion, where, Pinhole test is the reliable method as determine dispersion potential of soils directly(Craft and Acciardi 1984; Elges 1985; Rahimi and Delfy 1992; Farzaneh et al. 2001; Lashkaripour et al. 2006; Djdiek 2007). But considering the fact that the nature of clay dispersion phenomenon is chemical and that tendency to erosion depends on chemical properties of dissolved salts in pore water, accurate and proper interpretation of the chemical tests results would lead to reliable assessment of soil dispersivity (Ingles and Aitchison 1969; Sherard et al. 1976). Furthermore, the chemical methods have a lower cost as in many projects the chemical analyses of soil are performed for other purposes as well. Farzaneh et al. (2001) believe that the reason for mismatch between chemical (Sherard method) and physical tests is that in the former method it is recommended the tests to be conducted on soil extraction taken at moisture equivalent to the liquid limit; whereas it is usually performed at saturation moisture which is much higher than the liquid limit. Frenkel and Levy (1992) presented that the kaolinite clay is the most sensitive to dispersion of soils and the minerals of smectite and illite are in the next order from this point of view. They also showed that different anions have special relevance to soil dispersion, in which, their dispersive can be greater than the difference between groups of clay minerals. Ouhadi et al. (2006) shown that the dispersivity potential of low electrolyte concentration increases due to repulsive forces overcoming attractive forces. With a further increase in sodium sulfate salt concentration, the dispersivity potential decreases. Lashkaripour et al. (2006) have shown that the results of Pinhole and chemical tests do not match; while the results of Double Hydrometer and Pinhole have a good overlap. Based on the findings of different researchers, it is concluded that there is still a great unreliability in chemical methods, especially the most commonly used Sherard method, for evaluation of soil dispersion. In the present study, the ability and reliability of Sherard method and the effects of some anions and cations of the pore water on soil dispersion is investigated. An attempt has also been made to modify the Sherard method for a more accurate evaluation of soil dispersion.
2 Materials and Method
2.1 Collection and Preparation of Soil Samples
In an attempt to investigate the validity of chemical methods in identification of dispersion phenomenon, 26 natural soil samples with a wide range of physical and chemical properties were collected from different regions of Iran. In the first stage index tests including grain size distribution, Atterberg limits, compaction and classification of samples were performed in accordance with ASTM standards. Table 1 shows the physical and index properties of natural soil samples. In the next stage, the chemical analysis of the soil pore water was also carried out and the quantities of different cations, anions, electrical conductivity, and acidity were determined using the standard methods (Anon 2000). The chemical characteristics of the tested samples are presented in Table 2. In order to prepare samples with desired chemical properties to study the effect of the type and quantity of some salts on the dispersion potential of soils, some artificial soil specimens were also made. The artificial specimens were made by adding four different chemical agents including; sodium chloride, sodium carbonate, sodium sulfate and sodium polyphosphate which all have sodium cation but different anions, to a clayey soil. Mineral type of this soil was determined using X-ray diffraction (XRD) test and found to be Quartz, Calcite and Feldspar. Table 3 shows type and percentages of the chemicals used in making artificial soil specimens. In order to prepare the artificial specimens (A1 to A17), the required quantity of the chemical agents were determined according to the dry weight of the soil. The material was then dissolved in warm water and the dry soil was added to the solution, to form homogeneous slurry. The prepared mixture was kept in the open air to be dried. The dried soil was then crushed and passed through sieve no. 10 (2 mm opening). Two extra artificial specimens (A18 and A19) were also made by adding 5 and 10 % of sodium chloride (NaCl) to one of the natural dispersive soil (sample no. 11). Then, chemical tests were performed on the soil pore water to find the exact concentration of different ions as presented in Table 3.
2.2 Determination of Dispersion Potential of Samples
In order to determine the dispersion potential of the samples, three commonly used laboratory test methods including Double Hydrometer, the test of dissolved salts (Sherard method) and Pinhole were performed on all natural and artificial soil samples. Figure 2 shows different parts of the Pinhole test device in a schematic form. Figure 3 shows the photo of Pinhole test device used in this study. In the present study, sample preparation and testing procedure were all in accordance with ASTM standard (Anon 2000).
3 Results and Discussion
3.1 Comparison of the Results of Different Testing Methods
Since the Pinhole method presents a more realistic physical model simulating the dispersion phenomenon, it is accepted as the most accurate and reliable method for determining soil dispersion potential (Knodel 1991). Therefore, in the present study, the results of Pinhole test are taken as the control measure and the results of other methods are compared with them. Table 4 shows the results of different dispersion tests carried out on natural soil samples. As it can be seen from the table, in most of the samples (17 samples out of 26) the results of the three aforementioned testing methods do not match one another, to the extent that in some cases, samples were categorized as dispersive by Sherard chemical method, while they were categorized as ND1 (completely non-dispersive) by Pinhole method. However, all samples were categorized as non-dispersive by Double Hydrometer method. According to the results shown in Table 4, based on Double Hydrometer tests all samples are categorized as non-dispersive, while the results of other tests especially Pinhole show the opposite. As a result, it could be concluded that for the samples tested in this study the Double Hydrometer test is not a reliable method for evaluation of dispersion potential. Moreover, there are many contradictions (for over 70 % of the samples) between the results of Pinhole and Sherard chemical methods. The samples with a large percentage of sodium were classified as dispersive by Sherard chemical method, whereas some of the same samples were categorized as non-dispersive in Pinhole test. After detailed assessment of the chemical characteristics of the samples which were classified dispersive in the Sherard method, but non-dispersive in the Pinhole test, it was found that although the sodium percentage is high in these samples, but the type and quantity of their anions are different. It was therefore concluded that the dispersion of the soil does not only depend on the type and quantity of cations (especially sodium percentage which plays the main role in the Sherard chemical method) but also depends on the type and quantity of the anions present in the soil. With respect to the aforementioned points, it can be realized that the results of Double Hydrometer and Sherard chemical methods, apart from being inefficient, can also be confusing in some cases.
3.2 Effect of Quantity and Type of Anions on Dispersion
As it was mentioned earlier, the results of the experiments conducted in the present study showed that anions have an important role on dispersion potential of the soil. In order to investigate the effects of different anions on dispersion of the soils, artificial samples were made with the characteristics presented in Table 3. The dispersion potential of the artificial samples was also determined using the same aforementioned methods and the results are shown in Table 5. According to Table 5 and considering the fact that salts used for preparing artificial samples have the same major cation (sodium), all artificial samples are categorized as dispersive on the basis of Sherard chemical method. However, based on the results of Pinhole tests, some of the samples were classified as non-dispersive depending on the type and quantity of their anions. In order to determine the extent of effect of anions on dispersion, it will be discussed separately.
Samples A1, A2, and A3 which were made by adding 8, 4, and 1 % by weight of NaCl, in spite of having high sodium content, are all classified as completely non-dispersive according to the Pinhole test, while based on Sherard diagram, they should be dispersive. That is to say that chloride ion neutralizes dispersive effect of sodium, and prevents soil from dispersion. In order to confirm this finding, 5 and 10 % of NaCl salt was added to a naturally dispersive soil (Sample no. 11), and tested afterwards (Samples A18 and A19). Results showed that by increasing the amount of chloride anion in the pore water, the soil tends to flocculate, so that the artificial sample A19 having 5 % of salt is categorized as ND3 (closer to non-dispersive) and sample A18, having 10 % of salt was classified as completely non-dispersive. Therefore, it can be concluded that although NaCl salt provides a large amount of sodium cation to the soil, it leads to reduction of soil dispersion.
In the case of sodium carbonate, Na2Co3, it was found that adding 0.5 % of sodium carbonate (sample A8) did not affect soil dispersion. However, adding 1 % of the same salt (sample A7), made the sample moderately dispersive (ND4). Samples A6, A5, and A4 which were made by adding 4, 8, and 15 % of sodium carbonate are evaluated as dispersive (D2), very dispersive (D1) and dispersion-prone (ND4), respectively. Thus, it can be concluded that sodium carbonate causes dispersion, though its effect differs according to the salt percentage. With respect to the aforementioned results and the findings of chemical analysis, it was realized that increase in carbonate and bicarbonate anions up to a certain level leads to an increase in soil dispersion, and beyond that, it will decrease this potential. The effect of sodium sulfate, Na2So4, on soil dispersion was also studied in the same manner. As it can be seen from Table 5, sample A9 with 20 % sodium sulfate is categorized as moderately dispersive(ND4), and samples A12, A10, and A11 having 12, 8, and 4 % of salt, respectively, are all categorized as slightly dispersive (ND3), while sample A13 with 1 % of salt is classified as completely non-dispersive (ND1). Therefore, it can be concluded that the sulfate tends to act as a dispersing agent. In addition, increasing of sodium sulfate content in large quantities (over 20 %), leads to soil dispersion, while quantities below 4 % do not have any significant effect.
Although, the effect of sodium Polyphosphate (NaPo3) on dispersivity of soils was well known since it is commonly used as dispersing agent in standard hydrometer test, however, it has been studied in the present work as a control measure. The results of the present study showed that the intensity of dispersion increases by increasing this material, as sample A14 with 12 % of Polyphosphate salt was categorized as very dispersive (D1), while samples A15, A16, and A17 with 8, 4, and 1 % of the salt, were categorized as D2 (dispersive), D2 (dispersive), and ND3 (slightly dispersive), respectively. In general, based on the results of the present study, only chloride anion leads to formation of a flocculated structure in the soil and elimination of dispersion potential, while other anions more or less lead to soil dispersion. In other words, presence of chloride anion in the soil acts like an obstacle against the dispersing nature of sodium cation.
One of the most important supports to describe the effect of different anions or cations on clay dispersion is the diffuse double layer theory. The thickness of this layer is affected by the balance between repulsive and attractive forces which the particles experience as they approach each other. Flocculation occurs when the thickness of the double layer is reduced to such an extent that short range attractive forces become dominant and unlike dispersion occurs when the thickness of the double layer is increased (Sparks 2000; Shanmuganathan and Oades 1983). The concentration of electrolyte and sodium absorption ratio, SAR, are two main factors which effect the balance between repulsive and attractive forces and thereby thickness of diffused double layer. Flocculation is induced at high electrolyte concentration, when repulsive force decays and attractive force becomes dominant. While the thickness of the double layer increase and dispersion occurs as SAR increases (Sparks 2000; Panayiotopoulosk et al. 2004).So that when a salt with sodium cation was added to the soil, sodium was exchange with the existing calcium in the soil and then based of the type of its anion different salts having different solubility were produced. Based on the solubility of the produced salt the concentration and sodium absorption ratio, SAR, of the electrolyte and subsequently thickness of diffused double layer and dispersion potential of the soil will be different. In the case of sodium chloride, the produced salt after cation exchange would be calcium chloride which is a high soluble salt and led to increase the concentration and decrease the sodium absorption ratio, SAR, of the electrolyte and so that decrease the dispersion potential of the soil. Unlike in the case of the other salts (sodium carbonate and sodium sulfate, the produced salts will be calcium carbonate and calcium sulfate which are less soluble material and may settle and go out of the electrolyte and subsequently decrease the concentration and increase the sodium absorption ratio, SAR, of the electrolyte where both of them led to increase the dispersion potential of the soils.
3.3 Modification of Sherard Chemical Method
As it was mentioned earlier, among all anions present in the soil, only chloride anion acts as a flocculating agent which makes the soil non-dispersive. Therefore, a part from the total sodium ion existing in the soil employs for neutralization of chloride anion and the surplus amount of chloride anion acts as dispersion factor of soil. This fact was considered in order to modify the Sherard diagram. To do this a new index named as “percentage difference of sodium and chloride” (PSC), is defined as:
In the modified method PSC are replaced by “sodium percentage” (PS), on the vertical axis of the Sherard diagram. Other specifications and indices are the same as used in Sherard method. In order to evaluate the accuracy of the modified method, the dispersion potential of some of the natural soil samples, which mainly showed contradictory results in Sherard and Pinhole methods, were determined according to the proposed method. The results of the new experiments in comparison with the results of Sherard and Pinhole methods are presented in Table 6. As the table shows, the results obtained by the modified method are in a very good agreement with the results of Pinhole test.
4 Conclusion
Based on the overall results of the experiments carried out in this study, the following conclusions are made:
-
1.
Soil dispersion is a physical–chemical phenomenon that is caused due to presence of certain combination of anions and cations of the soil, both in type and quantity. In other words, soil dispersion depends not only on the type and amount of cations, especially sodium, but also on the types and amount of different anions present in the soil.
-
2.
Sherard chemical method is not able to determine soil dispersion potential reliably as it does not consider the role of anions, especially chloride. The results obtained by this method, are mostly in contradiction compared to the results of Pinhole test.
-
3.
It was shown that among different anions present in the soil, the chlorine ion, as opposed to the sodium cation, is a factor that makes the soil non-dispersive. This is due the fact that in combination with chloride, part of sodium settles in the form of sodium chloride, while the surplus sodium acts as a diffuser.
-
4.
The results of the study show that sodium chloride leads to reduction of dispersion potential of the soil, while sodium carbonate leads to its dispersion. This fact should be taken into consideration in modification of dispersive soils in practical cases. Moreover, in the projects in which dispersive soil is needed, this demand could be met by adding sodium carbonate to the existing soils.
-
5.
A modified Chemical Method was presented for evaluation of dispersion potential of soils by introducing a new index named as the “percentage difference of sodium and chloride”, (PSC), to replace “sodium percentage” on the vertical axis of the Sherard diagram.
References
Anon (2000) Annual book of ASTM standards. Vol 04.08, Soil and Rock, ASTM D420, D5779, D4221, D4647
Bhuvaneshwari S, Soundra B, Robinson RG, Gandhi SR (2007) Stabilization and microstructural modification of dispersive clayey soils. First international conference on soil and rock engineering organized by Srilankan Geotechnical Society, Columbo, Srilanka, 5–11 August 2007
Craft D, Acciardi RG (1984) Failure of pore water pressure analyses for dispersion. J Geotech Eng Div ASCE 110(4)
Didiek Djarwadi (2007) Dispersivity test of Duriangkang dam filling material dinamika. TEKNIK SIPIL 7(1):11–19
Elges HFWK (1985) Problem soils in South Africa-State of the Art. Civ Eng S Afr 27(7):347–349
Farzaneh O, Rezvan A, Bicheranloo Reza (2001) The effect of extraction moisture on dispersivity potential of sample using Sherard method. J Tech Coll Tehran Univ 35(4):513–522 (in Farsi)
Frenkel H, Levy GJ (1992) Clay dispersion and hydraulic conductivity of clay-sand mixtyres as affected by the addition of various anions. J Clay Clay Miner 40(5):515–521
Ingles OG, Aitchison GD (1969) Soil−water disequilibrium as a cause of subsidence in natural soils and earth embankments. In: symposium on land subsidence, Tokyo, AIHS Publication no 89, vol 2
Knodel PC (1991) Characteristica and problems of dispersive clay soils. US Department of the Interior, Bureau of Reclamation, Denver, Office, R-91-09
Lashkaripour GR, Khamehchiyan M, Soloki HR, Rahimi E (2006) The characteristics of dispersive soils in Sistan plain, eastern Iran. J Appl Geol 3(1):75–80
Ouhadi Vahid, Goodarzi R, Amir R (2006) Assessment of the stability of a dispersive soil treated by alum. J Eng Geol 85:91–101
Panayiotopoulosk P, Barbayiannis N, Papatolios K (2004) Influence of electrolyte concentration, sodium adsorption ratio, and mechanical disturbance on dispersed clay particle size and critical flocculation concentration in Alfisols. Commun Soil Sci Plant Anal 35(9–10):1415–1434
Rahimi H, Abbasi N (2008) Failure of concrete canal lining on fine sandy soils (A case study for Saveh Project). J Irrig Drainage 57:83–92
Rahimi H, Delfy M (1992) Recognition of dispersive clay in Khuzestan Province. M.Sc thesis, Department of irrigation and drainage, University of Tehran
Shanmuganathan RT, Oades JM (1983) Influence of anions on dispersion and physical properties of the horizon of a Red-brown earth. Geoderma 29(3):257–277
Sherard JL, Dunnigan LP, Decker RS (1976) Identification and natures of dispersive soils. J Geotech Eng Div 102(4):287–301
Sparks Donald (2000) Soil physical chemistry. CRC Press, Florida 33431
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbasi, N., Nazifi, M.H. Assessment and Modification of Sherard Chemical Method for Evaluation of Dispersion Potential of Soils. Geotech Geol Eng 31, 337–346 (2013). https://doi.org/10.1007/s10706-012-9573-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10706-012-9573-7