Abstract
Optimal utilization of animal manures as a plant nutrient source should also prevent adverse impacts on water quality. The objective of this study was to evaluate long-term poultry litter and N fertilizer application on nutrient cycling following establishment of an alley cropping system with eastern black walnut (Juglans nigra L.), pecan [Carya illinoensis (Wangenh.) K. Koch], and northern red oak (Quercus rubra L.) trees and orchardgrass (Dactylis glomerata L.). One half of a 4.25-ha site in northwestern Arkansas USA received broadcast applications of 3.9–6.7 Mg ha−1 fresh poultry litter and the other half 50–76 kg ha−1 N as NH4NO3 fertilizer each spring from 2001 to 2008. Macronutrient (N, P, K, Ca, Mg, and S) and micronutrient (Na, Fe, Mn, Zn, and Cu) concentrations in soil, forage, and tree leaf tissue were monitored along with NO3-N in soil water and groundwater. Poultry litter application resulted in significantly increased concentrations of each macronutrient except S with increases from 6.3 (N) to 121 % (P). Nitrogen fertilizer application resulted in decreased concentrations from 2.1 (N) to 60.9 % (S) for all macronutrients except Ca. Patterns of nutrient content in forage and tree leaf tissue did not generally follow patterns of soil nutrient concentrations suggesting nutrient sufficiency in most years and that climate and plant growth had a greater effect on nutrient uptake. Soil P with litter application increased 41.3 mg kg−1 over 7 years (from 34.1 to 75.4), which may necessitate a lower litter application rate to avoid excessive P runoff.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agroforestry, with its potential for multi-faceted food, fiber, and biomass production, continues to evolve as a popular land use option especially for forest and arable lands in transition. In particular, alley cropping systems have great potential to integrate a wide variety of trees and crops to optimize space, light capture, water use, and nutrient utilization (Kang et al. 1990; Jose et al. 2000). Alley cropping of forages is well-suited to large areas of the southeast and mid-south U.S., which have significant forest cover and limited areas of low-productivity arable land that is often better suited for forage production than for row crops. Considerable research has already been completed addressing cultural practices associated with the economics, establishment, and management of forage alley cropping systems in temperature North America (Gray and Garrett 1999; Garrett and Harper 1999; Garrett et al. 2004).
Northwestern Arkansas is an area of intensive poultry production where the poultry litter (bedding material and manure) is used to fertilize permanent pastures grazed by beef cattle (Bos taurus). In Ozark Highland pastures excessive nutrient accumulation in soils have led to concern for regional surface water (Sauer et al. 1999, 2000; Slaton et al. 2004; Menjoulet et al. 2009; McMullen et al. 2014) and groundwater quality (Chapman 1996; Peterson et al. 2002). Alley cropping systems offer a two-tiered rooting system for nutrient utilization as differences in rooting depth and growth intervals between trees and forages create greater potential for nutrient uptake and therefore reduced likelihood for offsite transport to water resources. Of the numerous possible tree/forage combinations, eastern black walnut (Juglans nigra L.) with either tall fescue (Festuca arundinaceae Schreb.) or orchardgrass (Dactylis glomerata L.) has emerged as a promising combination for forage alley cropping systems in the Ozark Highlands (Dey et al. 1987; Kurtz and Garrett 1990; Garrett and Harper 1999).
Various aspects of black walnut and orchardgrass production have been studied in detail. Ponder (1985) and Dey et al. (1987) reported on soil water effects on black walnut growth confirming the importance of proper site selection (deep and moist but not water-logged soil). Brauer et al. (2006) evaluated nut production from open-canopy black walnut trees in Kansas, Missouri, and Tennessee and found nearly half of the high producing trees bore in an alternate, biennial pattern. Ponder et al. (1998) and Ponder and Jones (2001) reported that N, P, K fertilization (especially P) of black walnut resulted in a moderate increase in nut production and interruption of the alternate-year nut crop. Gray and Garrett (1999) found that black walnut trees receiving low rates of N fertilization during the spring or late summer had greater numbers of pistillate flowers, nut retention, and 2.9–4.8 times more walnuts than control trees. Thus, black walnut nut production has consistently responded favorably to nutrient supplementation.
Orchardgrass has been evaluated as forage for grazing in the Ozark Highlands since the 1940’s (Sandal et al. 1953; Hileman 1973). Sandal et al. (1953) reported that, over the 5-year study, a fertilized orchardgrass-legume pasture had similar carrying capacity and nutrient yield as a tall fescue pasture and was superior in animal gains. Burner (2003) compared persistence, yield, and quality of orchardgrass, tall fescue, and a 1:1 binary mixture in an alley crop environment under 10 year-old loblolly (Pinus taeda L.) and shortleaf (Pinus echinata Mill.) pine trees near Booneville, AR. Orchardgrass persisted better in loblolly pine alleys (72 % of stand) compared to the unshaded control while tall fescue persisted better under control (30 % of stand) than in loblolly pine alleys. Yields of orchardgrass and binary mixture were not different in the pine alleys (~1300 kg ha−1 harvest−1) and were usually greater than the tall fescue yields (<700 kg ha−1 harvest−1).
A simultaneous, integrated assessment of nutrient cycling, forage production, and tree growth is needed to better understand the potential of hardwood-forage alley cropping systems in the Ozark Highlands. Given the concentration of poultry production in the region and the vulnerable water resources, such an evaluation needs to include poultry litter as a nutrient source and assessment of potential water quality impacts. The objective of this study was to evaluate nutrient cycling in soil, forage, trees, soil water, and groundwater following establishment of a forage alley cropping system with eastern black walnut, pecan [Carya illinoensis (Wangenh.) K. Koch], and northern red oak (Quercus rubra L.) trees and orchardgrass receiving annual applications of poultry litter or inorganic N fertilizer.
Materials and methods
Site establishment and management
This study was completed in a 4.25-ha paddock located at the University of Arkansas Agricultural Research and Extension Center in Fayetteville, AR (36°5′N, 94°11′W, 380 m above msl, Fig. 1). Local climatological data was available from a station of the National Oceanic and Atmospheric Administration network located at the Northwest Arkansas Regional Airport approximately 15 km away. For 1981–2010, the average annual precipitation was 1,217 mm and mean air temperature was 13.7 °C. The soil in most of the paddock is mapped as Captina silt loam (fine-silty, siliceous, active, mesic Typic Fragiudults) with some Pickwick silt loam (fine-silty mixed, semiactive, thermic Typic Paleudults) and small areas of Nixa cherty silt loam (loamy-skeletal, siliceous, active, mesic Glossic Fragiudults) and Johnsburg silt loam (fine-silty, mixed, active, mesic, Aquic Fragiudults) along the margins (Harper et al. 1969).
The paddock had been an idle, ungrazed pasture for several years prior to initiation of this study in 1999. Site preparation began with glyphosate herbicide application to kill the existing vegetation followed by cultivation, disking, and land-leveling operations and two aglime applications (8.4 Mg ha−1). Fifteen tree rows oriented east–west at a 15 m-spacing were delineated and a single-tine subsoiler pulled by a small bulldozer was used to fracture the subsoil to the depth of 0.4 m in each row. Eastern black walnut and pecan trees were planted in the southern 10 rows (5 rows of each species) at 9.1 m-spacing within rows. Pecan was selected for evaluation as it is a valued nut crop tree and is well-suited for moderately well-drained soils in the region. The eastern black walnut trees were of various graft combinations of 1 year-old scions (primarily Kwikkrop and Sparrow) on 2 year-old roostocks (primarily open-pollinated seedlings of Sparrow, Kwikkrop, and Thomas) (Thomas et al. 2008). The pecan trees were also 1 year-old scions (Kanza or Peruque) on 2 year-old roostocks (Colby seedlings). At planting a small fertilizer packet containing 1.6, 0.3, and 0.7 g of N, P, and K, respectively, was placed at the bottom of each hole. Landscape fabric (1.3 by 1.3 m) was placed around each tree to control weeds, and a 1 m-tall cylindrical plastic tree shelter anchored with a wooden stake was installed to protect the trees for the first year.
Prior to planting of the eastern black walnut and pecan, the alleys between the tree rows were seeded to winter wheat (Triticum aestivum L.) that received 56 kg ha−1 N as NH4NO3 fertilizer in October 1999 and again in February 2000 and was harvested for forage later that spring. The site was fertilized again in September 2000 (50.4, 67.2, and 33.6 kg ha−1 N, P, and K, respectively) prior to seeding of orchardgrass (var. Benchmark) in late September 2000. Alleys between rows of trees were then managed for forage production with hay or silage crops harvested mechanically 2–3 times annually from 2002 to 2005. In March 2000, northern red oak seedlings were planted in the remaining five tree rows on the north side of the paddock at a 2.4 m-spacing within rows. The oak was included in the study because there is an established local market for this timber. Landscape fabric was also used with the oak seedlings, but not tree shelters. During the first two growing seasons, trees were watered by placing 20-L buckets with small holes in their bottom next to the trees and filling with water as needed. Mowing and herbicides were used to control weeds within ~1 m of all trees.
From 2001 to 2007, the east half of the paddock received broadcast applications of 3.9–6.7 Mg ha−1 fresh poultry litter obtained from a university broiler house and the west half of the paddock received 50–76 kg ha−1 N as NH4NO3 fertilizer annually each spring. An exception was 2005 when fertilizer and litter applications were made in both spring and fall (total 123 kg ha−1 N in fertilizer and 8.9 Mg ha−1 litter). Litter application rate was dependent on availability. Fertilizer application rate was adjusted when necessary to better match N input from litter. In the spring of 2007, 50 kg ha−1 P and 101 kg ha−1 K were added to the fertilizer-treated side of the paddock as soil P and especially K levels were below optimum for forage growth. The poultry litter application matches the local recommended practice and the fertilizer rate was intended to provide a comparable amount of plant-available N. Litter samples were analyzed for pH (1:2 litter:water), moisture content (105 °C until constant weight), and total C and N (dry combustion). Total P, K, and Ca content were determined by nitric acid digestion with extracts analyzed by inductively coupled plasma emission spectroscopy (CIROSFootnote 1, SPECTRO Analytical Instruments, Inc., Marble Falls, TX). Litter and fertilizer were broadcast-applied to cover the entire area (alleys and tree rows) within their respective treatment areas. The imposition of fertility treatments on such a large scale was necessary to ensure that soil water and groundwater monitoring were not compromised by lateral flow of subsurface water between treatment areas. Beginning in 2004, additional fertilizer was provided to the trees as an annual application of a slow-release fertilizer (Osmocote, The Scotts Miracle-Grow Co., Marysville, OH) containing 5.6, 2.4, and 4.6 g of N, P, and K, respectively, was surface-applied to the ground near each tree in June.
Soil, forage, and tree measurements
Soil cores were taken in March of 2001, 2002, and 2004–2008 prior to annual spring litter and fertilizer applications. A 3.3-cm-diameter probe was used to collect nine 15 cm-deep cores in a zig-zag pattern from each tree row (including ½ of alley on each side) within each of fertilizer- and litter-treated areas. Fertilizer and litter applications were considered the treatments and tree rows within each treatment the replicates (i.e. 15 samples composed of nine cores each were analyzed per litter- and fertilizer-treated areas per year). Areas between treatments in the center of the paddock (~10 m wide), within 2 m of trees, and along paddock boundaries were not sampled.
The nine cores for each row/treatment were composited, air-dried, passed through a 2-mm sieve, and analyzed for macronutrients (P, K, Ca, Mg, and S) and micronutrients (Na, Fe, Mn, Zn, and Cu) using Mehlich 3 extraction (Mehlich 1984) with extracts analyzed by inductively coupled plasma emission spectroscopy. A ~15 g sample of the air dry <2 mm-diameter soil was placed on a roller mill for 12 h to create a fine powder for total C and N analysis using the dry combustion method (Fison NA 1500 Elemental Analyzer, ThermoQuest Corp., Austin, TX). Samples of air dry, <2 mm-diameter soil also were used to determine pH in water (1:2 soil:water).
Forage growth and quality were assessed by measurements made in the middle two alleys of each set of five rows of trees from 2002 to 2005 (Fig. 1). Although the young trees at this stage of the study were not large enough to affect forage growth via shading or root competition, this sampling strategy was chosen as it could be continued as tree shading and root competition effects become significant. Forage yield was determined using the calibrated disk meter method of Bransby et al. (1977). This was accomplished by walking each replicate (two alleys per tree species, i.e. six replicates each for litter- and fertilizer-treated areas) in a zig-zag pattern and then estimating forage mass at 12 locations distributed evenly across each field replicate. Representative forage samples were collected at six of these locations by clipping forage to a 2.5-cm stubble height with hand shears (i.e. six forage samples were analyzed per litter- and fertilizer-treated areas per harvest). There were three forage harvests each year except 2002 when low rainfall prevented significant fall regrowth. In the other years, the first harvest was removed and ensiled during early May, followed by a second harvest in June or July, and a third harvest in early October. Forage was packaged in conventional small square bales for most secondary (regrowth) harvests each year. Disk meter measurements were not completed for the July 2003 harvest, nor are forage quality data available for that harvest. Forage samples were dried to constant weight at 50 °C, and then ground in a Wiley mill (Arthur H. Thomas, Philadelphia, PA) fitted with a 1-mm screen before analysis for macronutrient and micronutrient content by digestion in nitric acid and quantification of nutrient concentration in extracts by inductively coupled plasma emission spectroscopy.
Leaf tissue samples of all tree species were collected in July of each year from 2004 to 2010. Nine composite samples were assembled from 6 to 10 trees within each of the row areas where growth measurements were made. Each sample was considered a replicate within the litter- and fertilizer-treated areas and (i.e. nine samples were analyzed for each area). Samples were made by either collecting recently matured, whole, individual leaves from multiple branches (oak) or the middle pair of leaflets from the middle leaf on multiple individual branches (walnut and pecan) of the current season’s growth between 1 and 2 m height. Each sample was composed of at least 50 leaves or 100 leaflets. Leaf samples were dried to constant weight at 50 °C, and then ground in a Wiley mill. Procedures for nutrient analysis of the tree leaf tissue were the same as described previously for forages. Tree growth was monitored by measuring stem circumference 1.37 m aboveground to calculate the diameter at breast height (DBH).
Soil water and groundwater monitoring
Porous ceramic cup soil water samplers (SPS 200, Earth Systems Solutions LLC, Lompoc, CA) were installed at 0.55 and 0.8 m depths at two locations within each of the middle three tree rows in each species of both litter and fertilizer application areas (Fig. 1). The 18 shallow (0.55 m) and 18 deep (0.8 m) samplers were considered replicates within the litter- and fertilizer-treated areas. The samplers were located near the midpoints between adjacent trees. Holes were augered below the installation depth, silica flour added to create a 0.15 m-thick layer, and samplers inserted into the silica flour. The borehole was backfilled with auger cuttings to within 0.15 m of the surface and then bentonite was added to prevent water flow next to the sampler body. The sampling strategy was to collect samples shortly following significant rainfall events or at least monthly throughout the growing season. Soil water samples were collected six to eight times per year for a total of 25 times from 2002 to 2005. The samplers were emptied and a vacuum (−60 kPa) applied 1 day prior to sample collection. Soil water samples were analyzed for NO3-N by Cd reduction, flow injection analysis (QuikChem 8000, Lachat Instruments, Milwaukee, WI). The soil was often too dry to extract samples during summer months. A total of 324 samples (161 shallow, 163 deep) were collected from the litter-treated area and 327 (155 shallow, 172 deep) from the fertilizer-treated area.
A single 0.05-m-diameter groundwater monitoring well was installed adjacent to each pair of soil water samplers. The wells were installed in a manner similar to the soil water samplers with a gravel/sand pack at the bottom and bentonite seal around the base at ground level. Well depth and well screen length varied due to the presence of chert fragments and depth to bedrock. Average well depth was 2.64 and 1.82 m for the litter- and fertilizer-treated areas, respectively. The 18 wells within each of the litter- and fertilizer-treated areas were considered replicates. The groundwater sampling strategy was similar to the soil water sampling strategy and wells and samplers were often sampled on the same day. Groundwater samples were collected four to 6 times per year for a total of 21 times from 2002 to 2005. Each well was purged on the day prior to sampling. Groundwater samples were analyzed for NO3-N by the same method as for soil water. As for the soil water samplers, many wells did not contain water on summer sampling dates. A total of 158 samples were collected from the litter-treated area and 122 from the fertilizer-treated area.
Due to the project objectives and lack of replication of litter- and fertilizer-treated areas, statistical differences between litter- and fertilizer-treated areas were not tested. Single-Factor ANOVA and Fisher’s Protected LSD were used to test for differences in soil parameters between initial (2001) and final (2008) mean values of poultry litter-treated and fertilizer-treated areas (Steel and Torie 1980). The significance level was P = 0.05 for all analyses.
Results and discussion
Macronutrients
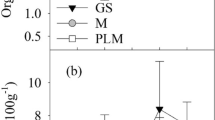
There were relatively few instances of large differences in concentrations of N, P, K, Ca, Mg, and S in soil between successive years in either litter- and fertilizer-treated areas (Fig. 2). Multi-year trends among macronutrients were inconsistent. N concentration increased until 2004 then declined for both treatments while P concentrations increased steadily with litter application and slowly declined in the fertilizer-treated area. Horizontal lines in Fig. 2b, c indicate optimum soil test levels of P and K for forage production (Cooperative Extension Service 1998). To maintain soil P at the optimum level and to increase soil K levels back to optimum levels for forage production, 50 and 101 kg ha−1 of P and K fertilizer, respectively, were broadcast-applied to the fertilizer-treated side of the paddock in 2007. Soil K concentration increased in 2008 but soil P levels did not increase following the P addition. Plant uptake in the year following P addition, low soil P levels, and the relatively small amount of P input may explain why the soil P concentration did not increase. The double application of both litter and fertilizer in 2005 resulted in increased levels of Ca, Mg, P, and K for the litter-treated area but decreases in N and S were also observed. From 2001 to 2008, litter application resulted in significantly increased levels of each macronutrient except S with increases from 6.3 (N) to 121 % (P) (Table 1). Tewolde et al. (2011) and Netthisinghe et al. (2014) also reported similar increasing soil concentrations of P, K, Ca, and Mg with poultry litter application. Fertilizer application over the same interval resulted in decreased concentrations from 2.1 (N) to 60.9 % (S) for all macronutrients except Ca, which increased 37.7 %, perhaps a legacy of lime application.
Mean macronutrient concentrations in soil, forage, and tree leaves for poultry litter- and fertilizer-treated areas. Light and heavy solid horizontal lines in the forage panels indicate cattle nutritional requirements for minimum concentrations and peak lactation, respectively. Horizontal lines for soil P and K concentrations indicate optimum soil test levels for those nutrients. Error bars are one standard error
Trends in macronutrient concentration in forage were inconsistent and often not correlated with soil concentrations of the respective nutrients. Poor correlation between soil and cotton (Gossypium hirsutum L.) nutrient concentrations were also reported for a silt loam soil receiving poultry litter applications (Netthisinghe et al. 2011). There were no large year-to-year changes for forage Ca, Mg, and S and only single years with large differences for N, P, and K. Concentrations of Ca, Mg, and S all tended to increase with time for both treatments. Forage K concentration tended to decrease, likely the result of decreasing soil K. McClain and Blevins (2014) found increased leaf N, K, and P but lower leaf Ca and Mg in stockpiled tall fescue following poultry litter application. To put the forage macronutrient concentrations in perspective, nutritional guidelines for a 544-kg mature beef cow at peak lactation (month 2, 9.1 kg milk day−1) and minimum requirements (month 7) are indicated with dashed and solid lines, respectively (National Research Council 1996). Note that if only a solid line is shown, the peak lactation and minimum requirements are identical. Average annual macronutrient concentrations in the forage of both treatments met the nutritional requirements in all years and for all nutrients with NRC standards.
As for forage, differences between successive years of tree leaf tissue macronutrient concentrations were generally small. Small differences from year-to-year and especially failure to follow trends in soil macronutrient concentrations after 2004 may be due to the slow release fertilizer applications. Overall, tree leaf tissue macronutrient concentration followed similar trends for both litter and fertilizer treatments with N and S increasing until 2007 then decreasing, little change in P and K, and gradual increases in Ca and Mg. Compared to orchardgrass forage, nutrient concentrations in the tree leaves were much greater for Ca, comparable for N, Mg, and S, and much lower for K. The similar year-to-year patterns of macronutrient concentrations in forage and tree leaf tissue for both treatments suggests that plant growth and nutrient uptake were affected more by climate (precipitation and temperature, Table 2) than nutrient source or soil nutrient concentration. The application of slow-release fertilizer to all trees in both treatments also likely reduced differences in leaf tissue nutrient content.
Micronutrients
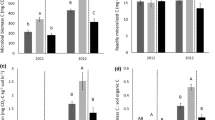
Although micronutrient concentrations were not measured in the applied poultry litter, surveys of poultry litter composition have generally found significant amounts of Fe, Cu, Zn, and Mn present (Stephenson et al. 1990; Kpomblekou et al. 2002; Bolan et al. 2010). Poultry litter applications resulted in significantly greater concentrations of each micronutrient in soil with increases from 26.2 to 82.6 % (Fig. 3; Table 1). Significantly increased concentrations of Cu (30 %) and Zn (76 %) following poultry litter application were also reported by Netthisinghe et al. (2014). Micronutrient concentrations in soil for both treatments had greater year-to-year variability as compared to the macronutrients. The fertilizer treatment also had increased concentrations of each micronutrient (18.5–63 %) from 2001 to 2008 although the increases were less than for the litter treatment for all nutrients except Mn.
Mean micronutrient concentrations in soil, forage, and tree leaves for poultry litter- and fertilizer-treated areas. Light and heavy solid horizontal lines in forage panels indicate cattle nutritional requirements for minimum concentrations and peak lactation, respectively. Error bars are one standard error
Micronutrient concentrations in forage, as for the macronutrient concentrations, did not mirror the trends in soil concentrations. Relatively large changes between years were observed on multiple occasions. In contrast to the macronutrient data, there were years with annual average forage micronutrient concentrations below the beef cow nutritional requirements for Na, Zn, and Cu. Some instances of Cu and Zn deficiencies for beef cow nutritional requirements have been observed in poultry litter-treated forages in the Ozark Highlands (McGinley et al. 2004) although this was likely due to low soil fertility and not related to litter application.
Large year-to-year changes in tree leaf micronutrient concentrations were found for Na, Zn, and Cu prior to 2006 but only occasionally after. Again, lack of correlation between tree leaf and soil micronutrient levels is likely due to the application of slow release fertilizer to all trees beginning in 2004 and the effect of climate on plant growth and nutrient uptake. When compared to orchardgrass, micronutrient concentrations in the tree leaves were much greater for Mn, comparable for Fe, Zn, and Cu, and much lower for Na.
Soil pH, C, and N
Soil pH increased from an average value of 6.6 in 2001 to over 7.2 (Fig. 4; Table 1). This increase is the result of aglime application during the site development and the application of poultry litter, which had an average pH of 6.8. Relatively small changes between years occurred in total C and N with the litter-treated area having consistently greater concentrations especially after 2004 when C and N concentrations in the fertilizer-treated area decreased at a greater rate. Sainju et al. (2010), reporting on a study of poultry litter application to cotton in Alabama, found that 10 years of poultry litter application had increased soil N storage and mineralization. Measurement of C content in the poultry litter began in 2003 and averaged 41 % (dry-wt basis) from 2003 to 2007 corresponding to an annual application of 2,196 kg C ha−1 with an average C:N of 13.4. Although the total C in the litter-treated area increased from 14.4 g kg−1 in 2001 to 15.7 g kg−1 in 2008, this increase was not statistically significant. The change in total C in the fertilizer-treated area was also not significant, decreasing from 15.3 to 14.9 g kg−1.
Soil water and groundwater NO3-N
Concentrations of NO3-N in soil water and groundwater showed a consistent pattern of greater concentration in the fertilizer-treated area throughout the study (Fig. 5). Average values for the fertilizer- and litter-treated areas were 8.6 and 5.3 mg L−1, respectively, for the 0.55-m porous cups and 9.4 and 6.2 mg L−1, respectively, for the 0.8-m porous cups. Average values for NO3-N in groundwater were 7.1 and 5.0 mg L−1 for the fertilizer- and litter-treated areas, respectively. There was a trend of increasing concentrations of NO3-N in the soil water collected in the porous cup samplers, especially in the fertilizer-treated area, while NO3-N concentrations in the associated groundwater monitoring wells decreased over time.
The pattern of NO3-N concentration increasing in soil water but decreasing in groundwater has several possible explanations. As the forage and tree roots extended and penetrated deeper into the soil they would be more effective in taking up NO3-N. Van Es et al. (2006) concluded that liquid manure application to orchardgrass on a clay loam or a loamy sand in New York posed a minimal NO3-N leaching concern due to longer periods of uptake and higher rates of N uptake compared to corn (Zea mays L.). Increased denitrification in the soil below 0.8 m or in the shallow aquifer would also effectively remove NO3-N as increased groundwater recharge (with low NO3-N water) would dilute the NO3-N in groundwater. However, below-normal rainfall especially in 2003 and 2005, would make this explanation for decreasing groundwater NO3-N less likely. Another possible explanation is that the fertilizer NH3 is rapidly nitrified and, with the NO3 already present, more rapidly leached from the root zone whereas the NO3 derived from poultry litter mineralization is formed more slowly with greater opportunity for plant uptake. This explanation is consistent with the observed seasonal dynamics of NO3 in the shallow soil water of the fertilizer treatment in each year except 2004 (Fig. 5a). As water flux through the root zone was not measured, it is not possible to conclude whether the observed differences in NO3-N concentrations between treatments would result in significant differences in N loss via leaching. Nonetheless, spikes in groundwater NO3 observed in spring and summer, especially for the fertilizer treatment, indicates that some rapid movement of fertilizer NO3 through the soil profile was occurring. The relatively low NO3-N concentrations do suggest, however, that NO3-N leaching was not a significant pathway of N loss for either treatment.
Nutrient balance
An average of 5.0 Mg ha−1 (as-is basis or 3.9 Mg ha−1 on dry-weight basis) of poultry litter was applied per year from 2001 to 2006 with average annual inputs of 154, 57, 78, and 175 kg ha−1 of N, P, K, and Ca, respectively. Over the same years the fertilizer-treated area received an average annual N addition of 73 kg ha−1 and a single P and K application of 67 and 34 kg ha−1 of P and K in 2000. The amount of each nutrient in the poultry litter that is plant-available depends on litter mineralization rates, adsorption equilibrium in the soil, and for N, the amount of NH3 volatilization, NO3 leaching, and biological denitrification. Laboratory incubations of poultry litter in soil have produced estimates of net N mineralization from 31 to 67 % over incubation lengths from 35 to 150 days (Cabrera and Gordillo 1995). Sauer et al. (1999) reported that poultry litter from a poultry house under similar management as the house used as the litter source in this study contained 85.1 % organic N, 8.7 % NH3-N, and 6.2 % NO3-N. Ammonia volatilization from surface-applied litter to tall fescue pastures in Georgia averaged 28.7 and 41.7 % of the applied N in NH3 form with most of the loss occurring within 10 days of application (Marshall et al. 1998). The annual N inputs for the litter- and fertilizer-treated areas in this study would result in equivalent amounts of plant-available N if, on average, ~50 % of the organic N in the applied litter was plant-available. This estimate is reasonable considering the literature values for N mineralization and NH3-N volatilization losses, and if N losses via surface runoff and NO3 leaching and denitrification are not significant.
Measured concentrations of N, P, K, and Ca in the applied poultry litter and harvested orchardgrass were used with litter application rate and forage yield data to estimate nutrient balances. This approach excludes losses via surface runoff or leaching, which were not measured. Excluding 2003 when data are missing for one of the forage harvests, there were average annual surpluses of 27 and 93 kg ha−1 for P and Ca and an average annual deficit of −87 kg ha−1 for K. The net P surplus is consistent with soil P data, however, soil Ca did not increase appreciably nor did soil K decrease with time in the litter-treated area. Netthisinghe et al. (2011) estimated that it would take at least 5 year of only inorganic fertilizer application to orchardgrass soils to reduce soil P, Cu, and Zn concentration back to normal ranges following buildup of these nutrients following 4 years of broiler litter application. Regarding N, the poultry litter- and fertilizer-treated areas had average annual deficits of −26 and −74 kg ha−1, respectively. Presumably, this shortfall was at least partially compensated for through mineralization of soil organic N, mineralization of previous years’ poultry litter, and N mineralized from unharvested forage and tree leaves. As only N was added to the fertilizer-treated area after 2000, concentrations of K, Mg, and S decreased appreciably over the duration of the study (from 88.7 to 72.9 kg ha−1 for K, from 63.4 to 53.2 kg ha−1 for Mg, and from 18.4 to 7.2 kg ha−1 for S). Soil N and P in the fertilizer-treated area had smaller declines from 34.1 to 32.2 kg ha−1 for P and 0.143 to 0.140 % for N, but soil Ca actually increased from 1,229 to 1,692 kg ha−1. Again, the increase in soil Ca may be a legacy of the 6.7 Mg ha−1 lime application in 2000.
Conclusions
Soil fertility management in much of the Ozark Highlands is driven by the desire to utilize poultry litter as a nutrient source while avoiding significant impacts on local surface and subsurface water quality. Established local markets for forest products and a consistent demand for high quality forage make alley cropping systems well-suited for this region. Several years of data on nutrient dynamics in soil, forage, tree tissue, soil water, and groundwater following establishment of a hardwood-orchardgrass alley cropping system indicate that the recommended annual poultry litter application rate generally supplied adequate nutrition for forage and tree growth. Litter-treated orchardgrass consistently exhibited an increased risk of grass tetany and both litter- and fertilizer-treated forage had Na and Zn content below nutritional recommendations for beef cattle. Annual tree growth was influenced more by climatic factors, especially below-normal growing season rainfall. Lack of guidelines for interpreting tree leaf tissue nutrient concentrations for eastern black walnut and northern red oak and for pecan under Ozark Highland conditions makes any detailed assessment of tree nutritional status difficult.
Steadily increasing soil P in the litter-treated area may necessitate a lower litter application rate or alternate year applications in the future unless P removal by the forage and uptake by the trees increases. Previous studies have shown a direct correlation between soil P and P concentration in runoff (e.g. Sauer et al. 2000) and current recommendations for cool season forage production are that no additional P is necessary if the soil P concentration is >50 mg kg−1. A much smaller increase in soil N was observed with poultry litter application but soil water and groundwater NO3-N concentrations were consistently lower under the litter treatment. This result suggests that the mineralization of organic N in poultry litter was sufficiently slow to optimize plant uptake and prevent excessive NO3-N movement out of the root zone. It is expected that, as the trees grow, this efficient capture of N will likely continue or perhaps even improve so that accumulation of P in the surface soil layer may be the limiting factor in determining the long-term sustainability of this alley cropping system.
Notes
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
References
Ankumah RO, Kpomblekou AK, Ajwa HA (2002) Trace and nontrace element content of broiler litter. Commun Soil Sci Plant Anal 33(11–12):1799–1811
Bolan NS, Szogi AA, Chuasavathi T, Seshadri B, Rothrock MJ Jr, Panneerselvam P (2010) Uses and management of poultry litter. Worlds Poult Sci J 66(4):673–698
Bransby DI, Matches AG, Krause GF (1977) Disk meter for rapid estimation of herbage yield in grazing trials. Agron J 69(3):393–396
Brauer D, Ares A, Reid W, Thomas A, Slusher JP (2006) Nut-yield variations and yield-diameter relationships in open-canopy black walnut trees in southern USA. Agrofor Syst 67(1):63–72
Burner DM (2003) Influence of alley crop environment on orchardgrass and tall fescue herbage. Agron J 95(5):1163–1171
Cabrera ML, Gordillo RM (1995) Nitrogen release from land-applied animal manures. In: Steele K (ed) Animal waste and the land-water interface. Lewis Publishers, New York, pp 393–403
Chapman SL (1996) Soil and solid poultry waste nutrient management and water quality. Poult Sci 75(7):862–866
Cooperative Extension Service (1998) Soil test recommendations. AGR9. University of Arkansas, Fayetteville, p 120
Dey D, Conway MR, Garrett HE, Hinckley TS, Cox GS (1987) Plant-water relationships and growth of black walnut in a walnut-forage multicropping regime. Forest Sci 33(1):70–80
Garrett HE, Harper LS (1999) The science and practice of black walnut agroforestry in Missouri, U.S.A.: A temperate zone assessment. In: Buck LE et al (eds) Agroforestry in sustainable agricultural systems. CRC Press, Boca Raton, pp 97–110
Garrett HE, Kerley MS, Ladyman KP, Walter WD, Godsey LD, Van Sambeek JW, Brauer DK (2004) Hardwood silvopasture management in North America. Agrofor Syst 61–62(1–3):21–33
Gray D, Garrett HE (1999) Nitrogen fertilization and aspects of fruit yield in a Missouri black walnut alley cropping practice. Agrofor Syst 44(2–3):333–344
Harper MD, Phillips WW, Haley GJ (1969) Soil survey of Washington County, Arkansas. USDA, Soil Conservation Service, US Gov Print Office, Washington
Hileman LH (1973) Response of orchardgrass to broiler litter and commercial fertilizer. Report Series 207. Agricultural Experiment Station, University of Arkansas, Fayetteville
Jose S, Gillespie AR, Seifert JR, Mengel DB, Pope PE (2000) Defining competition vectors in a temperate alley cropping system in the Midwestern USA 3. Competition for nitrogen and litter decomposition dynamics. Agrofor Syst 48(1):61–77
Kang BT, Reynolds L, Atta-Krah AN (1990) Alley farming. Adv Agron 43:315–359
Kurtz WB, Garrett HE (1990) Economic aspects of eastern black walnut management. Acta Hortic 284:319–325
Marshall SB, Wood CW, Braun LC, Cabrera ML, Mullen MD, Guertal EA (1998) Ammonia volatilization from tall fescue pastures fertilized with broiler litter. J Environ Qual 27(5):1125–1129
McClain WE II, Blevins DG (2014) Poultry litter application caused low leaf calcium and magnesium, increasing the grass tetany potential of stockpiled tall fescue. Forage Grazinglands. doi:10.1094/FG-2009-1022-01-RS
McGinley BC, Coffey KP, Sauer TJ, Goodwin HL, Humphry JB, Coblentz WK, McBeth LJ (2004) Mineral content of forages grown on poultry litter-amended soils. Prof Anim Sci 20(2):136–145
McMullen RL, Brye KR, Miller DM, Mason RE, Daigh AL, Menjoulet BC, Pirani AL, Gbur EE, Evans-White MA (2014) Long-term runoff water quality as affected by broiler-litter application to a Udult in the Ozark Highlands. Soil Sci Soc Am J 78(6):2017–2031
Mehlich A (1984) Mehlich 3 soil test extractant: a modification of Mehlich 2 extractant. Commun Soil Sci Plant Anal 15(12):1409–1416
Menjoulet BC, Brye KR, Pirani AL, Haggard BE, Gbur EE (2009) Runoff water quality from broiler litter-amended tall fescue in response to natural precipitation in the Ozark Highlands. J Environ Qual 38(3):1005–1017
National Research Council (1996) Nutrient requirements of beef cattle, 7th edn. National Academy Press, Washington
Netthisinghe AMP, Gilfillen B, Willian TW, Rowland NS, Sistani KR (2011) Inorganic fertilizers after broiler litter amendment reduce surplus nutrients in orchardgrass soils. Agron J 103(2):536–543
Netthisinghe AMP, Woosley PB, Gilfillen B, Cook KL, Sistani KR (2014) Nutrient-source and tillage impacts on tall fescue production and soil properties. Agron J 106(4):1427–1437
Peterson EW, Davis RK, Brahana J, Orndorff HA (2002) Movement of nitrate through regolith covered karst terrane, northwest Arkansas. J Hydrol 256(1–2):35–47
Ponder F Jr (1985) Soil-water variations and black walnut growth. N Nut Growers Assoc Ann Rept 76:149–154
Ponder F Jr, Jones JE (2001) Annual applications of N, P, and K interrupt alternate-year nut crops in black walnut. J Plant Nutr 24(4–5):661–670
Ponder F Jr, Jones JE, Haines J (1998) Annual applications of N, P, and K for four years moderately increase nut production in black walnut. HortScience 33(6):1011–1013
Sainju UM, Senwo ZN, Nyakatawa EZ, Tazisong IA, Reddy KC (2010) Poultry litter application increases nitrogen cycling compared with inorganic nitrogen fertilization. Agron J 102(3):917–925
Sandal PC, Staten RD, Davis AM (1953) Pasture experiments in north Arkansas 1946–1952. Bulletin 537. Agricultural Experiment Station, University of Arkansas, Fayetteville
Sauer TJ, Daniel TC, Moore PA Jr, Coffey KP, Nichols DJ, West CP (1999) Poultry litter and grazing animal waste effects on runoff water quality. J Environ Qual 28(3):860–865
Sauer TJ, Daniel TC, Nichols DJ, West CP, Moore PA Jr, Wheeler GL (2000) Runoff water quality from poultry litter-treated pasture and forest sites. J Environ Qual 29(2):515–521
Slaton NA, Brye KR, Daniels MB, Daniel TC, Norman RJ, Miller DM (2004) Nutrient input and removal trends for agricultural soils in nine geographic regions of Arkansas. J Environ Qual 33(5):1606–1615
Steel RGD, Torrie JH (1980) Principles and procedures of statistics. McGraw-Hill Book Co, New York
Stephenson AH, McCaskey TA, Ruffin BG (1990) A survey of broiler litter composition and potential value as a nutrient resource. Biol Wastes 34(1):1–9
Tewolde H, Adeli A, Sistani KR, Rowe DE (2011) Mineral nutrition of cotton fertilized with poultry litter or ammonium nitrate. Agron J 103(6):1704–1711
Thomas AL, Brauer DK, Sauer TJ, Coggeshall MV, Ellersieck MR (2008) Cultivar influences rootstock and scion survival of grafted black walnut. J Am Pomol Soc 62(1):3–12
Van Es HM, Sogbedji JM, Schindelbeck RR (2006) Effect of manure application timing, crop, and soil type on nitrate leaching. J Environ Qual 35(2):670–679
Acknowledgments
The authors are grateful to Paul Doi, Amy Morrow, Anna Myhre, and Kevin Jensen (National Laboratory for Agriculture and the Environment), Nancy Wolf (Univ. of Arkansas Agricultural Diagnostics Lab), Tammy Horton (Dale Bumpers Small Farms Research Center), Amanda Pirani (Univ. of Arkansas, Dept. of Crop, Soil and Environmental Sciences) and Robert Rhein, Dr. Keith Lusby, and Dr. Dirk Philipp (Univ. of Arkansas, Dept. of Animal Science) for their assistance in completion of this study. Financial support from the U.S. Forest Service and Natural Resources Conservation Service is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sauer, T.J., Coblentz, W.K., Thomas, A.L. et al. Nutrient cycling in an agroforestry alley cropping system receiving poultry litter or nitrogen fertilizer. Nutr Cycl Agroecosyst 101, 167–179 (2015). https://doi.org/10.1007/s10705-014-9667-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-014-9667-0