Abstract
Soil organic carbon (SOC) constitutes a large pool within the global carbon cycle. Land use change significantly drives SOC stock variation. In tropical central and eastern Africa, how changes in land use and land cover impact on soil C stocks remains unclear. Variability in the existing data is typically explained by soil and climate factors with little consideration given to land use and management history. To address this knowledge gap, we classified the current and historical land cover and measured SOC stocks under different land cover, soil group and slope type in the humid zone of south-west Rwanda. It was observed that SOC levels were best explained by contemporary land cover types, and not by soil group, conversion history or slope position, although the latter factors explained partly the variation within annual crop land cover type. Lack of the influence of land use history on SOC stocks suggests that after conversion to a new land use/land cover, SOC stocks reached a new equilibrium within the timestep that was observed (25 years). For conversion to annual crops, SOC stocks reach a new equilibrium at about 2.5 % SOC concentration which is below the proposed soil fertility threshold of 3 % SOC content in the Eastern and central African region. SOC stock declined under transitions from banana-coffee to annual crop by 5 % or under transitions from natural forest to degraded forest by 21 % and increased for transitions from annual crops to plantation forest by 193 %. Forest clearing for agricultural use resulted in a loss of 72 %. Assuming steady states, the data can also be used to make inferences about SOC changes as a result of land cover changes. We recommend that SOC stocks should be reported by land cover type rather than by soil groups which masks local land cover and landscape differences. This study addresses a critical issue on sustainable management of SOC in the tropics and global carbon cycle given that it is performed in a part of the world that has high land cover dynamics while at the same time lacks data on land cover changes and SOC dynamics.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The atmospheric concentration of CO2 and other radiatively active gases is steadily increasing in the last century due to anthropogenic activities (Lal 2001; IPCC 2007a). These increased concentrations are considered by the IPCC to pose a threat by causing global warming and a cascade of derived alterations in the earth climate system (IPCC 2007a). To alleviate the threat posed by increasing CO2 emission to the atmosphere, the global community has focused their attention to the management of the carbon (C) cycle (Batjes 1996; Su et al. 2006). A number of studies link land use and land cover changes (LUCC) to approximately 20 % of the global CO2 emissions to the atmosphere (e.g., Houghton 1995, 1999, 2005; Batjes 2004; IPCC 2007b; Van der Werf et al. 2009). Other studies suggest that most of these emissions are coming from the tropics (e.g., Rhoades et al. 2000; IPCC 2007b; Verburg et al. 2011). These emissions are derived from two different sources, above ground biomass and soil organic carbon (SOC) which is a fraction of the total soil organic matter (SOM). The latter has a lag in response to LUCC related to the decomposition rate of SOM. Losses in SOM are not only having repercussions on the global climatic system but also heavily influence more directly local soil processes. The magnitude of SOM stocks in the soil is central to soil quality and productivity because it maintains to a large extent many soil physical properties (e.g., aeration, water retention, formation of stable aggregates; Lal 2004), provides substrate to micro biota (Nye and Greenland 1960; Sanchez 1976) and plays a role in nutrient buffering and supply (Lal 2004; Smaling et al. 1993; Young 1997; Schnitzer 1991; Chapman et al. 2003; Smaling et al. 2012). Levels of SOM have been shown to be particularly important in cultivated tropical soils where soil tillage operations destroy soil aggregates exposing SOM to high temperatures and thus lead to rapid breakdown, while SOM reserves are already low and the use of other inputs for soil fertility management is rare (McDonagh et al. 2001). Most agricultural soils in the tropics have lost 20–83 % of their original SOM over a period of 5 to 50 years of cultivation (Detwiler 1986; Schlesinger 1986; Vagen et al. 2005; Esteban et al. 2000; Lal et al. 2007, Lal 2009). This is especially the case in sub Saharan Africa, affecting plant nutrient supply or soil fertility and consequently food production and security (Detwiler 1986; Schlesinger 1986; Murty et al. 2002; Vagen et al. 2005; Lal 2004, 2009; Smaling et al. 2012).
Policy makers across the tropics propose that carbon finance could provide incentives for forest frontier communities and small holder farmers to move away from poor agricultural land use management to other systems that potentially reduce emissions and/or increase carbon sequestration. However, it is unclear what exactly controls tropical SOC stocks. There is little certainty regarding the carbon outcomes of many key land-use transitions at the center of current policy debates. Besides, it is difficult to obtain reliable estimates of the historic loss of the SOC pool in the tropics (Lal 2002). Data on changes in SOC stocks in tropical land use systems is scarce or fragmented (e.g., Guo and Gifford 2002; Minasny et al. 2010; Powers et al. 2011; Eclesia et al. 2012) and consensus has yet to be reached on how much carbon is lost by changes in tropical land use systems (e.g., Fearnside and Laurance 2003; Ramankutty et al. 2007; Van der Werf et al. 2009; Phelps et al. 2010; Ziegler et al. 2012; Eclesia et al. 2012). A meta-analysis of 74 publications on the influence of land use changes on SOC stocks by Guo and Gifford (2002) showed that most data that could be found were drawn from only four countries (Australia, Brazil, New Zealand, and USA) indicating possible bias in findings on the effects of LUCC on SOC stocks. A more recent but similar study by Powers et al. (2011) covering 27 countries from 80 different studies showed a more even spread over the world, but still with a very small coverage of Africa. No reports were mentioned on the tropical landscapes of central Africa.
Given the high level of land cover change that has happened, and is still happening in this region (Wasige et al. 2013), it seems that studies addressing the effects of land cover change on the SOC in tropical African landscapes are profoundly needed. There have been some attempts to quantify SOC stocks in this region: Batjes (2008) presented SOC estimates per agro-climate zone and soil type for Central Africa (CAF) based on the soil and terrain (SOTER) database at a 1:2.000.000 scale. Gaps in the data were filled using expert rules and taxonomy-based pedotransfer procedures for similar groups of FAO (1988) soil types, providing a first coarse scale approximation of SOC stocks for CAF. This approach has limitations on a smaller scale, particularly in regions with large variations in topography, climate and land use types. Variability in the existing data is typically explained by soil and climate factors with little consideration given to land use and management history. Verdoodt et al. (2010) reported baseline SOC stocks of Rwandan top soils (0–0.3 m) calculated for 121 profiles, representing 99 soil series which spatially cover 47 % of the Rwandan soilscape on soil map sheets at a scale of 1:50,000. Average sampling density was 1 profile for every 14 km2. About 6 % of the profiles were located within a natural reserve. The results roughly correspond to the Central African estimates of Batjes (2008) and faced similar limitations such as coarse resolution of sampling and only one aggregated forest cover classes while there is a lot of variation in forest cover types such as natural forest, degraded forest and plantation forest. Aggregation was also performed for crop cover into one unit instead of the distinctly different and economically important crops such as banana-coffee and tea crop versus annual crops for subsistence. More recently, a study on carbon stocks in the Nyungwe National Forest (Rwanda, part of CAF) focused on a relatively undisturbed area of the forest (Nsabimana 2009). It was unclear from this study how internal forest degradation or land cover conversion influenced SOC stocks. Yet, their study was conducted in an area that experienced considerable LUCC in the recent past, including conversions from primary forest into smallholder farming systems with very limited external inputs (Wasige et al. 2013). This raises questions on whether changes in SOC stocks in this region are occurring under the current land cover, of recent conversions, and what the magnitudes of these changes are. Besides, it could be that alternative explanations exist that explain differences in SOC stocks. Soil types might contribute to differences in SOC stocks as well as positions along slopes (Lal 1986). The aim of this research was therefore to quantify carbon stocks in a data scarce area and to determine which factor (current land use, land use history, soil type or slope) could explain best the variation in SOC stocks. Our hypothesis is that land use and land cover history controls the spatial and temporal heterogeneity of SOC stocks.
Materials and methods
The relation between land use, its change, and soil organic carbon stocks was studied using classified satellite imagery of 1986, 1995 and 2010. The changes between these two time periods (1986–2010 and 1995–2010) were established and land units identified were overlaid with soil classification units taken from secondary information (Verdoodt and Van Ranst 2003). Soil organic carbon was measured, and after correction for clay mineralogy, correlated with current land use, land use change between 1986 and 2010, land use change between 1995 and 2010, slope positions in the landscape and soil classification units.
Location and description of study area
The study site is the 506 km2 river Rukarara catchment (Fig. 1) in the humid hill slopes of South-West Rwanda and in the upper reaches of the Nile Basin (2°28′–2°34′S and 29°23′–29°29′E). The hill slopes exhibit physical variability in terms of elevation, climate, geology, and soils as the river Rukarara drains and descents the catchment. The river has its source at about 3,000 metres above sea level (m.a.s.l) and descends to 1,470 m at the outlet. The steep slopes have lost most of their forest cover and are intensively cultivated by smallholder farmers. Plantation forests are replacing annual crops on highly degraded soils and steep slopes where cultivation is no longer possible. A soil map (1:50,000; FAO 1988 classification) of the area is available (Verdoodt and Van Ranst 2006) and reveals that the soils are variable; Acrisols, Alisols, Ferralsols, Gleysols, Regosols, Histosols and Cambisols occur. The vegetation consists of a fully forested upper part of Nyungwe natural forest while the middle to lower parts of the catchment have agricultural crops. The main cash crops are tea (Camellia spp.) and coffee (Coffea canephora), and food crops are maize (Zea mays), banana (Musa spp.), beans (Phaseolus vulgaris), cassava (Manihot esculenta), sweet potatoes (Ipomoea batatas), solanum potatoes (Solanum tuberocum spp.), sorghum (Sorghum Bicolor (L.) Moench) and wheat (Triticum). Food crops are typically grown on plots of <1 ha (Wasige et al. 2014). Cultivation and tillage by smallerholder farmers is mainly undertaken with the use of simple handheld implements. Both crop rotation and mixed farming are practiced by the majority of the farmers. In most cases, organic manure from livestock pens mixed with crop residues is applied in seedling holes when planting seeds to increase soil fertility which means that SOC will not be uniformly distributed in the agricultural fields. Due to the high economic value of tea, some farmers have replaced their traditional food crops with tea.
Research steps
Land cover classification and change detection
While considering image availability and quality, efforts were made to obtain the images from the same year and where there was difficulty in image availability for a particular targeted year, images from the previous year or the next year (minus one or plus 1 year) were considered. Landsat images of path 173 and row 62 that are predominantly cloud-free for the moments; 1986-July-19th, 1995-January-17th and 2010-August-22nd were selected for land cover classification. The USGS and IGBP land cover classification system (Anderson et al. 1976; Belward 1996) were adopted and modified to include classes that are applicable to the study area and general enough so that they can be readily identified on Landsat imagery. Existing land cover types in this area include; natural forest, degraded forest, plantation forest (which are mostly with fast growing species such as eucalyptus), savannas, annual crops, banana-coffee plantations and tea plantations. Table 1 provides a descriptive summary of the used land cover classes for this study. The acquired Landsat images were geo-referenced to the Rwandan projection (local Transverse Mercator coordinate system) of the 1:50.000 topographic maps, radiometrically normalized (Price 1987) and rectified to each other. Further corrections to the images included atmospheric normalization (Richter 1997) using dark pixel subtraction (Elvidge et al. 1995) to remove haze (Du et al. 2002). This process made it feasible to map LUCC at a medium scale, appropriate for detecting large-area changes and with minimal bias from seasonality. Geo-coded images were then overlaid with vector data (roads, rivers and lakes) where a perfect match was observed and then sub-setted to the extents of the study area. Supervised classification procedures were implemented using Erdas 2010 image processing software to classify the Landsat images into established land cover classes using the Maximum Likelihood Classification (MLC) algorithm. Topographic maps of 1988 with a 1:50.000 scale were used to validate the 1985 Landsat classification map with the assumption that there were no major LUCC between the two periods. The final classification and interpretation of the 2010 land cover map was conducted based on field observation data and 2008 aerial photographs (available at Rwanda’s National Land Centre) as the reference information for validation and accuracy assessment. We used a handheld GPS to locate land cover classes with an accuracy of between 1 and 5 m during the field observations. A total of 150 GPS points covering all land cover types in the basin were collected. Clouds and cloud shadows were masked from the images by manual interpretation and digitizing. The classification accuracy of each type of image was evaluated and a confusion matrix was produced (Table S1 in appendix 1: supplementary materials), from which the overall accuracy and kappa statistics of agreement for each land cover category were computed. It was difficult to carry out accuracy assessment for the 1995 land use/cover maps because of the lack of aerial photographic coverage or any historical reference data for this period. We relied on the quality of the original status of the datasets retrieved. However, we assumed that accuracy assessment for the land use/cover maps of 1986 and 2010 should be sufficient to shed light on the overall accuracy of the land use/cover mapping procedures adopted in this study. To establish the LUCC that occurred during the 1986–2010 period, a post-classification change detection analysis of the land cover maps was performed using ArcGIS10 overlay operations. For all change maps ‘from-to’ LUCC matrices were extracted (Table S2–S3 in appendix 1: supplementary materials) for the period 1986–2010 and for the period 1995–2010. We were interested to know whether the current carbon stocks, were more affected by longer term land cover change history (1986–2010) or only more recent changes (1995–2010).
Soil sampling and laboratory analysis
Stratified toposequential randomized sampling scheme was used to select soil sampling sites based on a sampling matrix of contemporary (i.e. 2010) land cover types and three slope positions. Slope positions were categorized as; foot slope, mid slope and upper slope. The upper slope position includes upper and lower interfluves and receives little or no runoff, but contributes runoff to lower slope positions. The middle slope position includes shoulder, upper and lower linear slopes, and receives runoff from the upper slope and contributes runoff to foot slope positions. The foot slope represents the base of the hill (Wang et al. 2001; Kagabo et al. 2013). Slope positions were derived from an ASTER DEM (30 m × 30 m) using the Topographic Position Index (TPI) tool in ArcGIS10. The output raster was overlaid with the land cover map of 2010 (Fig. 2) to identify sample strata in ArcGIS10. Sampling points were created randomly using ArcGIS10 software. A field sampling map with GPS points was then printed to support field identification of selected sampling points. A handheld GPS was used to locate these positions in the field. A total of 644 sites were visited for the collection of soil samples during the month of October 2010, with a soil auger at two depths (0.0–0.2 m and 0.2–0.5 m) in two different fieldwork campaigns. Fixed sampling depths (0.0–0.2 m and 0.2–0.5 m) were used because the soil depth of agricultural soil layer subjected to soil tillage by small holder farmers in Sub-Saharan Africa has been found to vary between 0.2 and 0.3 m soil depth. This allowed us to compare our results with other tropical soil fertility studies (e.g., Stoorvogel et al. 1993; Kanmegne 2004; McCarty et al. 2010; Vågen and Winowiecki 2013). During the field campaign, 87 soil samples were collected from Nyungwe forest and 557 samples were collected from the agricultural part of the catchment. For each site, a plot of 10 m × 10 m was outlined using a tape measure. Four spots along each of the diagonals and one point from the centre per site were sampled. Soil samples from each plot were thoroughly mixed to make a composite sample which was then quota sampled for about 500 g of soil and taken to the laboratory for chemical analysis.
Various direct and indirect methods have been recommended for estimating SOC content. A commonly used direct method is based on the dry combustion of organic matter called the Loss on Ignition (LOI) method and a common indirect method is based on dichromate oxidation [Walkley–Black (WB) method]. Other methods exist such as diffused reflectance infrared spectroscopy or the modern dry-combustion analyzers but the cost of acquiring the necessary equipment for such analyses has been prohibitive for most of the laboratories in Eastern and Central Africa. In our study, the WB (wet oxidation) method recommended by the IPCC was used for laboratory soil analysis of SOM content (IPCC 2003). The method is suitable for use by most resource limited soil analysis laboratories in Eastern and central African. The amount of carbon in SOM varies, but often the “Van Bemmelen Factor” of 1.724 is applied (Schumacher 2002), based on the assumption that 58 % of SOM consist of SOC (Van Reeuwijk 2006). Although higher values have been suggested (up to 2.5; Broadbent 1953) in this study we used the more commonly used value of 1.724. The WB method is commonly used for routine SOC assessments (Bell and Lawrence 2009; Tornquist et al. 2009), and involves a wet combustion of the SOM with a mixture of potassium dichromate and concentrated sulphuric acid at about 125 °C. The residue dichromate is titrated against ferrous sulphate and a correction factor of 1.3 is applied in the calculation of the results to compensate for the incomplete destruction of the organic matter during the process (Van Reeuwijk 2006).
About two soil bulk density samples at 0.0–0.2 m and 0.2–0.5 m soil depth were randomly collected in each of the mini pits using PF-rings located at the middle of the various plot sites, giving a total of 1,288 samples. After drying the samples in an oven at 105 °C, bulk density was calculated using standard procedures described in Anderson and Ingram (1993). Calculation of SOC stocks followed the IPCC procedure (IPCC 2003) as presented in Eq. 1;
where; SOC site is the soil organic carbon stock (Mg C ha−1); SOC content is the concentration of soil organic carbon C (g C kg−1 of soil) for layer j of sampled soil depth; bd is the bulk density of an undisturbed volume of the soil (g cm−3); cf is the coarse fraction, i.e. the >2 mm coarse fragment (weight %) in a single sampled soil depth; and sd is soil depth, the thickness of the soil layer sampled (m).
Quantifying effect of LUCC
To measure changes in SOC as a result of LUCC, repeated sampling of plots before and after a land-use change occurs (chronosequence) would be desirable (Schlesinger 1986; Powers 2004). However, data from long-term monitoring of SOC stocks is often missing in the tropics, as is the case in the Rukarara catchment. This can be circumvented by a paired-comparison approach (biosequence), where sampling occurs at the same time on sites with similar soil and climate types but different land uses for comparison. It involves substituting space-for-time in field sampling of SOC stocks, comparing areas under native and changed land use and land cover. This approach holds the implicit assumptions that (1) the sites were similar in soils and climate apart from land use and (2) were in equilibrium prior to conversion (Rhoades et al. 2000; Powers 2004; Yemefack et al. 2006; Smaling et al. 2012). The latter assumption was implicitly made in this study, but the former was controlled by comparing sites with different land use histories as derived from the LUCC inventory. The percentage of SOC change was estimated according to Eq. 2 (Eclesia et al. 2012);
where; SOC change is the percentage change in SOC after vegetation replacement, SOC current is the SOC content (Mg ha−1) in the soil under the new vegetation, and SOC original is the SOC content (Mg ha−1) of the native ecosystem.
Data processing and statistical analyses
R software (R Development Core Team 2012) was used during data processing and analysis to detect significant differences between SOC stocks under different land covers, spoiled types and change histories. An Analysis of Variance (ANOVA) with a Games-Howell post hoc test was used to detect significant differences among land cover types, land cover conversion types, slope positions and soil groups. The Games Howell test (Games and Howell 1976) was selected for the Post Hoc comparison because it is robust for small unequal sample sizes and it is robust for differences in variances across the groups, which was the case with our data set. We tested differences in SOC concentrations (in %) over the various explaining factors for both the top soil (0–0.2 m) and sub soil (0.2–0.5 m) layer separately, and for the SOC density (Mg C ha−1) for the two layers combined (0–0.5 m). Because not all combinations of soil type and land cover type are resent in the dataset, we also made comparisons between SOC stocks in different soil types under agricultural land cover and differences in SOC stocks in different soils under forested land cover.
Results
Soil organic carbon by contemporary land cover types, soil types and slope position
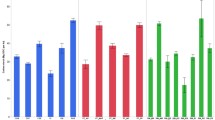
Total carbon stocks for the top 0.5 m layer is depicted are Fig. 3a, b showing that the highest SOC stocks can be found under forest cover types, while low SOC stocks can be found under agricultural cover types. The exception is plantation forests, which are in essence man-made, and often are covered by exotic (but fast growing) species, which have the highest SOC stocks. For all soil groups, SOC stocks under all forest land cover types (natural forest, plantation forest and degraded forest) was significantly different (P < 0.05) from that of agricultural land cover types (tea, banana-coffee and annual crops; Fig. 3a). The fact that for different soil types similar SOC stock values are found, while for similar soil types under different land cover types, different SOC stocks are found (Fig. 3b), suggests that land cover causes the largest difference rather than soil type. Nevertheless there were some significant differences also between soil types, when keeping the land cover type fixed, suggesting that also soil type has a moderate effect on the SOC stock.
aSOC stocks for land cover type for 0–50 soil depth. AC annual crops; BC banana coffee; PF plantation forest; T tea; DF degraded forest; NF natural forest. Different letters indicate significant differences (P < 0.05) according to the Games–Howell. b SOC stocks for soil types per land cover type for 0–50 soil depth. Different letters indicate significant differences (P < 0.05) according to the Games–Howell test for SOC stocks in soils under agriculture (AC, BC and T) and for SOC stocks in soils under forest (DF, NF and PF) land cover types
Among forest cover types, SOC stocks were significantly higher for plantations compared to degraded forest cover types. There were no significant differences between natural and degraded forest SOC stocks. The highest SOC stocks in soils under forest cover were observed in Acrisols and Histosols which were significantly different from lower stocks in Cambisols and Alisols (Fig. 3b). In the agricultural land cover type, high SOC stocks were observed in Ferralsols, under tea and in Lixisols under banana-coffee and low SOC stocks were observed in annual crops on the Acrisols (Fig. 3b). Tea indeed has the highest SOC content for any of the agriculture-soil type combinations. On Cambisols, Alisols and Acrisols both forest and agricultural land cover types were observed, and for these soils also the largest spread in SOC values was found. Within Cambisols, SOC stock variability followed a similar trend where SOC in agricultural cover types was lower than in forest cover types. Figure 4 shows the variation of SOC stocks along slope positions for the different land cover types. Although differences were found between land cover types, marginal significant differences (P < 0.04) in SOC stocks were only observed between the upper and lower slope positions in annual crops.
Variation of SOC stocks along three slope positions per land cover types. The first box of each series is down slope, the second mid slope and the last upslope. Different letters indicate significant differences (P < 0.05) between slope positions within each land cover type according to the Games–Howell test
Soil organic carbon by land cover conversions
The different SOC concentrations associated with land cover conversion types between 1986 to 2000, and 1995 to 2010 are presented in Fig. 5a, b, respectively. Similar to the above results, SOC concentrations are generally higher for the land cover types related to forestry, compared to land cover types related to agriculture. Between land cover transitions, areas that are currently under plantation forest, but originated from agriculture in 1986 have a significantly higher level of SOC concentrations compared to land cover conversion types from banana-coffee to annual crop (Fig. 5a). There were significant differences between land cover types that remained annual crop and that changed to annual crop from banana-Coffee. No significant difference was observed between land cover changes that changed from banana-coffee to annual crop and annual crop to banana-coffee. SOC stocks increased for land cover transitions from annual crop to plantation forest by 193 % while forest clearing for agricultural use resulted in a loss of 72 %. Figure 5a shows that SOC concentrations declined for the land cover transition from banana-coffee to annual crop by 5 %. Between 1995 and 2010 (see Fig. 5b), land cover change from plantation forest to annual crop had significantly higher SOC concentrations in the top soil compared to land cover that remained annual crop.
SOC content (%) for the top soil (0.0–0.2 m) and sub soil (0.2–0.5 cm) for land cover conversion types between 1986 and 2010 (a) and between 1995 and 2010 (b). The cases where no land cover change occurred are indicated with one land cover type, while for the other cases, the first land cover type refers to the situation in 1986 (a) or 1995 (b) and the second refers to 2010. AC annual crops; BC banana coffee; PF plantation forest; T tea; DF degraded forest; NF natural forest. Different letters indicate significant differences (P < 0.05) according to the Games–Howell test for individual land cover types. Conversion types were compared amongst each other within one soil layer
Discussion
Impact of contemporary land cover type on spatial distribution of SOC stocks
Generally, under all soil types, higher SOC stocks were associated with forest land uses and lower SOC stocks were associated with agricultural land uses. Natural forest and plantation forest had an average SOC stock of 310 Mg C ha−1 and 372 Mg C ha−1, respectively, across soil types which is higher compared to similar studies for the same soil depth (0–0.5 m) in Cameroon (85 Mg C ha−1; Kanmegne 2004) and the Ethiopian highlands (190 Mg C ha−1; Lemma et al. 2006). The actual value for each forest in different regions most likely varies with soil type, local land cover history and degree of deforestation. The soil samples were taken to a fixed depth.
Our results suggests that afforestation with fast-growing (exotic) tree species leads to increases in SOC storage. Average SOC content in plantation forest was found to be higher compared to SOC stocks in natural forest. As pointed out by Mann (1986) and Lugo and Brown (1993) the differences in SOC accumulation under different tropical forest is due to differences in; tree species, litter production, litter quality, and microclimate.
The SOC concentrations observed in annual crops was at the lower end of the spectrum at about 2.5 % SOC content which is below the soil fertility threshold of 3 % SOC content that is proposed for east and central African region (Okalebo et al. 2002), implying that more additions of soil organic matter and thereby possible returns of organic matter to the soil. The SOC stocks observed in annual crops (127 Mg C ha−1) is higher but in the same order of magnitude compared to values for the humid forest zone of southern Cameroon, where for the top 0.5 m under different land uses along a slash-and-burn chronosequence, values between 61 and 100 Mg C ha−1 were reported (Kanmegne 2004). The differences between the two study sites could be associated with differences in soil type and land use management. We do not have bulk density values from the Kanmegne (2004) study (a parameter which is included in the calculation when computing SOC stocks see Eq. 1) but the slightly higher values of SOC in our study could be due to high bulk densities. The soils in the south west of Rwanda have been continuously cultivated for over 30 years (Wasige et al. 2013) and have poor soil structure (Verdoodt and Van Ranst 2003) (Table 2). Soils with poor soil structure will have high bulk density compared to soils with aggregate soil structure. Continuous cultivation without compensating for lost SOC and nutrients has been shown to be detrimental to soil structure (Lal 2004).
Interactive impacts of land cover type, soil type and slope position on SOC stocks
In our analysis, SOC levels were best explained by contemporary land cover types, and only in second instance by soil group, conversion history or slope position (Fig. 3a, b). This is suggesting that under the same climate, SOC stocks are mainly land use specific rather than soil type specific (Fig. 3). We found that historical land cover had a marginal influence on the current SOC stocks (Fig. 5a). This has been described before for plantation and pasture establishment in native forests and grasslands of South America (Eclesia et al. 2012) where SOC changes (0–0.2 m of soil depth) were independent of the initial native vegetation (forest, grassland, or savanna) but strongly depended on the characteristics of the new vegetation (tree plantations or pastures), its age, and precipitation. Detwiler (1986) in the tropics and Esteban et al. (2000) in a global study, showed that the carbon stock reduced to approximately 60 % of the level under forest cover after 5 year of cultivation and to approximately 25 % after 20–50 years. Generally, it is reported that conversion of forest to farmland in tropical regions may result in a 20–83 % loss of SOC stocks (Detwiler 1986; Schlesinger 1986; Vagen et al. 2005; Lal 2009). Likewise, over a period of 25 years, soils in our study seem to have lost 72 % after conversion from forest to annual crops. Histosols under forest cover had the highest SOC stocks, which concurs with the fact that by definition these soils should have far more SOC being derived from incompletely decomposed plant remains. Under agricultural land cover types, the highest SOC stocks were observed in Ferralsols, but this observation is compromised with the fact that we only had tea plantations on this soil type. The high SOC stocks in Ferralsols under tea crop could be explained by a higher mineral fertilizer application enhance high biomass production and the practice of pruning tea and leaving the residues on the top soils as mulch, a common practice for this land cover type in Rwanda, which adds SOM to the soil (MINAGRI 2008).
Although Poels (1989) and Stoorvogel et al. (1997) have reported that even under natural conditions SOC losses occur in natural forest, the carbon cycle in natural forest may be almost closed and the soil–plant system is often in a steady-state condition i.e. any physical losses by erosion, leaching or losses by mineralization to CO2 are compensated by photosynthesis and litter input. This probably explains in part the luxuriance of SOC stocks in soils under forest cover (Yemefack et al. 2006). Low SOC stocks observed under agricultural soils could be explained by poor soil fertility management and a long history of tillage that breaks soil aggregates and exposes SOC to high temperatures and therefore high decomposition rates. In most cases farmers in Rwanda do not compensate for the carbon losses (Clay et al. 1998; Ansoms and McKay 2010). They may not have, or have limited access to manure and fertilizers and burn crop residues prior to seed bed preparation (Ansoms and McKay 2010). Besides, some studies have shown that the decomposition of tropical crop residues is normally faster than forest litter which would suggest that the potential they have to contribute to long-term SOC is limited (Jenkinson and Ayanaba 1977). Crop organic resources contain more of the labile SOC pools (light fraction C and particulate organic carbon) which are sensitive to management practices (Janzen et al. 1992; Solomon et al. 2000) and consequently highly influenced by the cultivation history of the soil (Nye and Greenland 1960; Davidson and Ackerman 1993; Murty et al. 2002). Studies have found that the light fraction and particulate organic carbon, are relatively easily decomposable and are greatly depleted upon cultivation (Cambardella and Elliott 1992; Six et al. 1999; Solomon et al. 2000) explaining why a new, lower equilibrium SOC stock is reached upon conversion from forest to agriculture. Very large additions of organic matter are generally required to compensate for the high decomposition rates and with that to increase the soil’s carbon content (Hartemink 1997).
We also present a comparison between our results with coarse resolution data on SOC stocks at 0–0.5 m soil depth as reported by Batjes (2008) for his study in Central Africa (CAF), which includes Rwanda (Table 3). Generally, mean SOC stocks of soils for the humid zone of central Africa are comparable with those found in soils in our study, with marginal differences, except for Histosols, for which we only had data from forested land cover types. SOC values for soils under forest land cover were higher and under agricultural land cover were lower than the figure reported by Batjes (2008). The differences could accrue from the fact that for our study we corrected for land use/cover impacts on SOC stocks which was not the case with Batjes study. We therefore point out that there is a need to correct for land use effects on SOC stocks at a scale relevant for local management as well as national carbon inventories and when results are to be compared with studies elsewhere.
Slope position was only important in annual crops land cover types and partly explained the variation in SOC stocks between upper and lower slope positions. Our finding is similar to Hancock et al. (2007) who reported that there was little relationship between hill slope position and SOC. Lal (1986) explained that soil erosion on hill slopes of agricultural catchments is one of the driving factors of SOC variability.
Possible sources of error in SOC estimates
Possible sources of error in our results could be due to the following; (a) WB method used in laboratory soil analysis runs the risk of underestimating SOC content compared to the modern dry-combustion analyzers and, (b) the soil samples were taken to a fixed soil depth. A fixed mass would have been more appropriate (Ellert and Bettany 1995). WB method was used in our study because we do not have access to such automated systems (modern dry-combustion methods) for more accurate estimation of SOC content. WB method is still a much used technique despite its limitations (Bell and Lawrence 2009; Van Reeuwijk 2006; Tornquist et al. 2009). Also, Sleutel et al. (2007) has reported very close linear relationships between the SOC content measured with modern dry-combustion methods and traditional wet oxidation methods (WB method), enabling the comparison of SOC data across methods, which is crucial for the measurement of SOC stock changes. In the future, determining suitable correction factors for incomplete WB combustion to the modern dry-combustion analyzers needs attention because that would allow to convert historical data and also efforts that are implemented these days and that use WB (like our study) to be corrected and make comparisons between locations more reliable.
A fixed mass would have been more appropriate for comparison of SOC content across land cover types (Ellert and Bettany 1995). This makes comparison with for example the (undisturbed) forest soils less accurate. However, given the overall large differences between forest soils and agricultural soils, we do not believe that a comparison of results from fix soil depth sampling caused a problem to the conclusions drawn on the differences between these two groups of land cover types.
Conclusion
We evaluated the effects of land cover changes on SOC stocks in south-west Rwanda in tropical central Africa. It was observed that SOC levels were best explained by contemporary land cover types, and not by soil group, conversion history or slope position, although between the latter factors still some minor but significant differences existed. Generally, SOC stocks in the current agricultural land cover are at lower end of the spectrum at about 2.5 % SOC which is below the soil fertility threshold of 3 % SOC content proposed for east and central African region, implying that additions of SOM are required to increase the soil’s fertility. The low levels in SOC stocks in agricultural land use suggest a high potential for SOC sequestration, when more attention would be paid to this in agricultural management. The higher SOC levels under tea cover suggest indeed that different management can partly induce this. We recommend that SOC stocks should be reported by land cover type rather than by soil groups because our results are better explained by the latter classification.
References
Anderson JM, Ingram JSI (1993) Tropical soil biology and fertility: a handbook of methods. CAB International, Wallingford
Anderson JR, Harvey EE, Roach JT, Witmer RE (1976) A land use and land cover classification system for use with remotely sensed data. United States Geological Survey Professional Paper No. 964. Washington, DC
Ansoms A, McKay A (2010) A quantitative analysis of poverty and livelihood profiles: the case of rural Rwanda. Food Policy 35(6):584–598
Batjes NH (1996) Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 47:151–163
Batjes NH (2004) Soil carbon stocks and projected changes according to land use and management: a case study for Kenya. Soil Use Manag 20:350–356
Batjes NH (2008) Mapping soil carbon stocks of Central Africa using SOTER. Geoderma 146:58–65
Bell M, Lawrence D (2009) Soil carbon sequestration—myths and mysteries. Trop Grassl 43:227–231
Belward AS (1996) The IGBP-DIS global 1 km land cover data set (DISCover): proposal and implementation plans. IGBP-DIS Working Paper 13, International Geosphere–Biosphere Programme Data and Information System Office, Toulouse, France
Broadbent FE (1953) The soil organic fraction. Adv Agron 5:153–183
Cambardella CA, Elliott ET (1992) Particulate soil organic matter across a grassland cultivation sequence. Soil Sci Soc Am J 56:777–783
Chapman SJ, Campbell CD, Puri G (2003) Native woodland expansion: soil chemical and microbiological indicators of change. Soil Biol Biochem 35:753–764
Clay D, Reardon T, Kangasniemi J (1998) Sustainable intensification in the highland tropics: Rwandan farmers’ investments in land conservation and soil fertility. Econ Dev Cult Change 46:351–377
Davidson EA, Ackerman IL (1993) Changes in soil carbon inventories following cultivation of previously untilled soils. Biogeochemistry 20:161–193
Detwiler RP (1986) Land use change and the global carbon cycle: the role of tropical soils. Biogeochemistry 2:67–93
Du Y, Guindon B, Cihlar J (2002) Haze detection and removal in high resolution satellite image with wavelet analysis. IEEE Trans Geosci Remote Sens 40:210–217
Eclesia PR, Jobbagy GE, Jackson BR, Biganzoli F, Pineiro G (2012) Shifts in soil organic carbon for plantation and pasture establishment in native forests and grasslands of South America. Glob Change Biol 18:3237–3251
Ellert BH, Bettany JR (1995) Calculation of organic matter and nutrients stored in soils under contrasting management regimes. Can J Soil Sci 75:529–538
Elvidge CD, Yuan D, Weerackoon RD, Lunetta RS (1995) Relative radiometric normalization of landsat multispectral scanner (MSS) data using an automatic scattergram controlled regression. Photogramm Eng Remote Sens 61:1255–1260
Esteban G, Jobba GY, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10(2):423–436
FAO (1988) FAO-UNESCO soil map of the world, revised legend (with corrections and updates). FAO World Soil Resources Report 60 (reprinted with updates as ISRIC Technical Paper 20 in 1997). ISRIC, Wageningen
Fearnside PM, Laurance WF (2003) Comment on ‘determination of deforestation rates of the world’s humid tropical forests’. Science 299(5609):1015
Games PA, Howell JF (1976) Pairwise multiple comparison procedures with unequal n‘s and/or variances: a Monte Carlo Study. J Educ Stat 1:113–125
Guo LB, Gifford RM (2002) Soil carbon stocks and land use change: a meta analysis. Glob Change Biol 8:345–360
Hancock GR, Martinez C, Wells T (2007) Modelling and assessment of soil carbon variability at the point and hillslope scale. In: Oxley L, Kulasiri D (eds) MODSIM 2007 International Congress on Modelling and Simulation. Modelling and Simulation Society of Australia and New Zealand, December 2007, pp 1743–1748
Hartemink AE (1997) Soil fertility decline in some major soil groupings under permanent cropping in Tanga Region, Tanzania. Geoderma 75:215–229
Houghton RA (1995) Land-use change and the carbon cycle. Glob Change Biol 1:275–287
Houghton RA (1999) The annual net flux of carbon to the atmosphere from changes in land use: 1850–1990. Tellus B 51:298–313
Houghton RA (2005) Aboveground forest biomass and the global carbon balance. Glob Change Biol 11:945–958
IPCC (2003) Good practice guidance for land use, land-use change and forestry. IPCC National Greenhouse Gas Inventories Programme, Hayama (JP)
IPCC (2007a) Climate change 2007: the physical science basis. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL (eds) Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge
IPCC (2007b) Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. In: Parry ML, Canziani OF, Palutikof PJ, van der Linden JP, Hanson CE (eds) Climate change 2007: impacts, adaptation and vulnerability. Cambridge University Press, Cambridge
Janzen HH, Campbell CA, Brandt SA, Lafond GP, Townley-Smith L (1992) Light fraction organic matter in soils from longterm crop rotations. Soil Sci Soc Am J 56:1799–1806
Jenkinson DS, Ayanaba A (1977) Decomposition of carbon-14 labelled plant material under tropical conditions 1. Soil Sci Soc Am J 41:912–915
Kagabo DM, Stroosnijder L, Visser SM, Moore D (2013) Soil erosion, soil fertility and crop yield on slow-forming terraces in the highlands of Buberuka, Rwanda. Soil Tillage Res 128:23–29
Kanmegne J (2004) Slash and burn agriculture in the humid forest zone of Southern Cameroon: soil quality dynamics, improved fallow management and farmers’ perceptions. Ph.D. Thesis. Wageningen University and Research Centre, The Netherlands, pp 180
Lal R (1986) Conversion of tropical rainforest: agronomic potential and ecological consequences. Adv Agron 39:173–264
Lal R (2001) World cropland soils as a source or sink for atmospheric carbon. Adv Agron 71:145–191
Lal R (2002) The potential of soils of the tropics to sequester carbon and mitigate the greenhouse effect. Adv Agron 76:1–30
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304:1623–1627
Lal R (2009) Soil degradation as a reason for inadequate human nutrition. Food Secur 1:45–57
Lal R, Follett RF, Stewart BA, Kimble JM (2007) Soil carbon sequestration to mitigate climate change and advance food security. Soil Sci 172(12):943–956
Lemma B, Berggren Kleja D, Nilsson I, Olsson M (2006) Soil carbon sequestration under different exotic tree species in the southwestern highlands of Ethiopia. Geoderma 136(3–4):886–898
Lugo AE, Brown S (1993) Management of tropical soils as sinks or sources of atmospheric carbon. Plant Soil 149:27–41
Mann LK (1986) Changes in soil carbon storage after cultivation. Soil Sci 142(5):279–288
McCarty WG, Reeves BJ III, Yost R, Doraiswamy CP, Doumbia M (2010) Evaluation of methods for measuring soil organic carbon in West African soils. Afr J Agric Res 5(16):2169–2177
McDonagh JF, Birch T, Magi J (2001) Soil organic matter decline and compositional change associated with cereal cropping in Southern Tanzania. Land Degrad Dev 12:13–26
MINAGRI (2008) A revised tea strategy for Rwanda: transforming Rwanda’s tea industry. Available from www.minagri.gov.rw/…/revised-tea-strategy-rwanda-2008-transfoming-Rwanda’s-tae-industry. Accesses 10 Aug 2012
Minasny B, Sulaeman Y, McBratney AB (2010) Is soil carbon disappearing? The dynamics of soil organic carbon in Java. Glob Change Biol 17:1917–1924
Murty D, Kirschbaum MUF, Mcgilvray H (2002) Does conversion of forest to agricultural land change soil carbon and nitrogen? A review of the literature. Glob Change Biol 8:105–123
Nsabimana D (2009) Carbon stock and fluxes in Nyungwe forest and Ruhande Arboretum in Rwanda. Ph.D. Thesis, University of Gothenburg, Sweden
Nye PH, Greenland DJ (1960) The soil under shifting cultivation. Technical communication no. 51. Commonwealth Bureau of Soils, Harpenden
Okalebo JR, Gathua KW, Woomer PL (2002) Laboratory methods of soil and plant analysis: a working manual of soil. TSBF/UNESCO/ROSTA, Nairobi
Phelps J, Webb EL, Agrawal A (2010) Does REDD+ threaten to recentralize forest governance? Science 328:312–313
Poels RLH (1989) Nutrient input and output in undisturbed and silviculturally treated tropical rain forest in Suriname. Neth J Agric Sci 37:383–386
Powers JS (2004) Changes in soil carbon and nitrogen after contrasting land-use transitions in Northeastern Costa Rica. Ecosystems 7:134–146
Powers JS, Corre MD, Twine TE, Veldkamp E (2011) Geographic bias of field observations of soil carbon stocks with tropical land-use changes precludes spatial extrapolation. Proc Natl Acad Sci 108:6318–6322
Price JC (1987) Special issue on radiometric calibration of satellite data. Remote Sens Environ 22:1–158
Ramankutty N, Gibbs HK, Achard F, Defries R, Foley JA, Houghton RA (2007) Challenges to estimating carbon emissions from tropical deforestation. Glob Change Biol 13:51–66
R Development Core Team (2012) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/. Accesses 12 Aug 2012
Rhoades CC, Eckert GE, Coleman DC (2000) Soil carbon differences among forest, agriculture, and secondary vegetation in lower montane Ecuador. Ecol Appl 10:497–505
Richter R (1997) Correction of atmospheric and topographic effects for high spatial resolution satellite imagery. Int J Remote Sens 18:1099–1111
Sanchez PA (1976) Properties and management of soils in the tropics. Wiley, New York
Schlesinger WG (1986) Changes in soil carbon storage and associated properties with disturbance and recovery. In: Trabalka JR, Reichle DE (eds) The changing carbon cycle: a global analysis. Springer, New York, pp 194–220
Schnitzer M (1991) Soil organic matter—the next 75 years. Soil Sci 151:41–58
Schumacher BA (2002) Methods for the determination of total organic carbon (TOC) in soils and sediments: Las Vegas, United States Environmental Protection Agency
Six J, Elliott ET, Paustian K (1999) Aggregate and soil organic matter dynamics under conventional and no-tillage systems. Soil Sci Soc Am J 63:1350–1358
Sleutel S, De Neve S, Singier B, Hofman G (2007) Quantification of organic carbon in soils: a comparison of methodologies and assessment of the carbon content of organic matter. Commun Soil Sci Plant Anal 38:2647–2657
Smaling EMA, Stoorvogel JJ, Windmeijer PN (1993) Calculating soil nutrient balances in Africa at different scales. II. District scale. Fertil Res 35:237–250
Smaling EMA, Lesschen JP, van Beek CL, de Jager A, Stoorvogel JJ, Batjes NH, Fresco LO (2012) Where do we stand, twenty years after the assessment of soil nutrient balances in Sub-Saharan Africa? In: Lal R, Stewart BA (eds) World soil resources and food security. CRC Press, Boca Raton
Solomon D, Lehmann J, Zech W (2000) Land use effects on soil organic matter properties of chromic Luvisols in semi-arid Northern Tanzania: carbon, nitrogen, lignin and carbohydrates. Agric Ecosyst Environ 78:203–213
Stoorvogel JJ, Smaling EMA, Janssen BH (1993) Calculating soil nutrient balances in Africa at different scales. I. Supra-national scale. Fertil Res 35:27–235
Stoorvogel JJ, Janssen BH, van Breemen N (1997) The nutrient budgets of a watershed and its forest ecosystems in the Taï National Park in Côte d’Ivoire. Biogeochemistry 37:159–172
Su ZY, Xiong YM, Zhu JY, Ye YC, Ye M (2006) Soil organic carbon content and distribution in a small landscape of Dongguan, South China. Pedosphere 16:10–17
Tornquist CG, Giasson E, Mielniczuk J, Cerri CEP, Bernoux M (2009) Soil organic carbon stocks of Rio Grande do Sul, Brazil. Soil Sci Soc Am 73:975–982
Vågen T, Winowiecki AL (2013) Mapping of soil organic carbon stocks for spatially explicit assessments of climate change mitigation potential. Environ Res Lett 8:015011
Vagen TG, Lal R, Singh BR (2005) Soil carbon sequestration in Sub-Saharan Africa: a review. Land Degrad Dev 16:53–71
Van der Werf GR, Morton DC, Defries RS, Olivier JGJ, Kasibhatla PS, Jackson RB, Collatz GJ, Randerson JT (2009) CO2 emissions from forest loss. Nat Geosci 2:737–738
Van Reeuwijk LP (2006) Procedures for soil analysis. 7th edition. Technical Report 9. ISRIC—World Soil Information. Wageningen, Netherlands
Verburg PH, Neumann K, Nol L (2011) Challenges in using land use and land cover data for global change studies. Glob Change Biol 17:974–989
Verdoodt A, Van Ranst E (2003) Land evaluation for agricultural production in the tropics: a large-scale land suitability classification for Rwanda. Universiteit Gent, Belgium
Verdoodt A, Van Ranst E (2006) The soil information system of Rwanda: a useful tool to identify guidelines towards sustainable land management. Afrika Focus 19(1–2):69–92
Verdoodt A, Baert G, Van Ranst E (2010) Baseline organic carbon stocks of Rwandan top soils. 19th World Congress of soil science, soil solutions for a changing world 1–6 August 2010, Brisbane, Australia
Wang J, Fu B, Qiu Y, Chen L (2001) Soil nutrients in relation to land use and landscape positions in the semi-arid small catchments on the loess plateau in China. J Arid Environ 48:537–550
Wasige EJ, Groen TA, Smaling EMA, Jetten V (2013) Monitoring basin-scale land cover changes in Kagera Basin of Lake Victoria using ancillary data and remote sensing. Int J Appl Earth Obs Geoinf 21:32–42
Wasige EJ, Groen T, Smaling EMA, Jetten V (2014) Soil fertility and nutrient balances of low input land use systems of South-West Rwanda, Upstream of Lake Victoria Basin. J Agric Ecosyst Environ (under review)
Yemefack M, Rossiter DG, Jetten VG (2006) Empirical modelling of soil dynamics along a chronosequence of shifting cultivation systems in southern Cameroon. Geoderma 133(3–4):380–397
Young A (1997) Agroforestry for soil management. CAB International, Wallingford
Ziegler AD, Phelps J, Yuen JQ, Webb EL, Lawrence D, Fox JM, Bruun TB, Leiszk SJ, Ryan CM, Dressler W, Mertz O, Pascual U, Padochkk C, Koh LP (2012) Carbon outcomes of major land-cover transitions in SE Asia: great uncertainties and REDD+ policy implications. Glob Change Biol 18(10):3087–3099
Acknowledgments
The authors would like to thank Mr. Safari Patrick of Ministry of Environment and Lands (MINELA), Rwanda and CGIS -NUR for field support, all the farmers in Rukarara watershed for their collaboration, and we want to acknowledge Boudewijn de Smeth and Frank van Ruitenbeel for assistance in the laboratory measurements of SOM and David Rossiter for assistance in statistical analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wasige, J.E., Groen, T.A., Rwamukwaya, B.M. et al. Contemporary land use/land cover types determine soil organic carbon stocks in south-west Rwanda. Nutr Cycl Agroecosyst 100, 19–33 (2014). https://doi.org/10.1007/s10705-014-9623-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-014-9623-z