Abstract
In intensive cultivation of coffee (Coffea arabica L.), large N fertilizer inputs are thought to increase nitrate (NO3 −) water contamination and greenhouse gas emissions. This study was carried out (1) to evaluate the nitrogen use efficiency of a highly fertilized C. arabica–Inga densiflora agroforestry system on an Andosol and (2) to determine the control mechanisms of N fluxes and losses. Nitrogen pools and fluxes were monitored for one cropping season in a coffee plantation (density 4,722 plants ha−1, height 2.1 m), shaded by regularly pruned leguminous trees (density 278 trees ha−1; height 8 m), in the Central Valley of Costa Rica. The fate of N fertilizer (250 kg N ha−1 year−1) was traced by adding 15N-urea at 1.61 kg 15N ha−1. The labeled urea was rapidly nitrified or immobilized in soil organic matter with 20.8 % recovered in organic form at the end of the cropping season in the top 2 m of the soil. There was high net N mineralization and nitrification in the top soil (≈200 kg N ha−1 year−1 in 0–10 cm) and up to 257 kg NO3 −–N ha−1 were found in the top 2 m of the soil. Only 25.2 % (63 kg N ha−1) of the applied fertilizer (15N recovery) was taken up by the two plant species (13.5 % in the coffee plants, 9.6 % in the shade trees and 2.1 % in the litter). Total N export in the coffee fruit harvest accounted for 110 kg N ha−1 but only 17.6 kg N ha−1 came from the applied fertilizer (7 % of 15N recovery). During this year of high coffee production, the coffee plant acquired most of its N from mineralized soil N rather than from N fertilizer. High fertilization resulted in a low rate of N2 fixation by I. densiflora, estimated at 22.7 kg N ha−1 year−1 (Ndfa of 16.1 %). As a result of high water drainage (1,745 mm for a total rainfall of 2,678 mm), the main fate of N fertilizer was NO3 − leaching (33–55 % of 15N recovery). The annual NO3 −–N leaching at a depth of 120 cm was 157.2 kg N ha−1 year−1(including 82.8 from applied N) and the N2O–N emission was 5.8 kg N ha−1 year−1. These results clearly showed that the system was N saturated, leading to low use efficiency of the N fertilizer and significant losses of N, principally through NO3 − leaching. This study provided an insight on how to reduce the negative environmental impact of N fertilization in intensive coffee cultivation and increase N use efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Intensively managed coffee (Coffea arabica L.) systems were developed in many Latin American countries such as Costa Rica towards the end of the twentieth century to maximize yield and increase income from exports (Moguel and Toledo 1999). This transformation of traditional coffee agroecosystems included a significant reduction in the shade tree canopy, an increase in planting density of highly productive coffee varieties and the high use of agrochemicals (Picado et al. 2009; Castro-Tanzi et al. 2012). Coffee has increasingly been grown without shade or with reduced shade along with high fertilization, typically ranging from 150 to 350 kg N ha−1 year−1 (Reynolds-Vargas and Richter 1995).
Nitrogen is a key factor for coffee growth and production but the efficiency of N fertilizer use is apparently low in these intensive systems. Using 15N labeled fertilizer, Salas et al. (2002) estimated that only 30–40 % of the applied N was taken up by the coffee plants in Costa Rica. The effect on the environment is that high amounts of N fertilizer increase the available N that ultimately ends up in the aquatic system, leading to eutrophication, and in the atmosphere as greenhouse gas emissions (Vitousek et al. 2009).
In Costa Rica, the combination of high fertilization rates with high rainfall on permeable soil increases the risk of water contamination through NO3 − leaching (Babbar and Zak 1995; Harmand et al. 2007a; Cannavo et al. 2011). Current N fertilization in coffee monoculture or agroforestry systems can easily exceed plant growth and crop yield requirements (Harmand et al. 2007a; Haggar et al. 2011).
N inputs in excess of plant requirements can accumulate in the soil or be lost through leaching, volatilization or runoff. Potential control mechanisms for N use and losses still need to be investigated. Harmand et al. (2007a) reported that the inclusion of eucalyptus trees in a coffee plantation increased N accumulation in the litter and permanent biomass and slightly decreased water drainage and NO3 − leaching as compared to coffee monoculture. Increasing shade tree density (and therefore biomass) may reduce N leaching losses as suggested by Tully et al. (2012). Furthermore, N surplus may be increased when inorganic fertilizer is applied to coffee plantations under leguminous shade trees as a result of biological N2 fixation (Tully and Lawrence 2011). NO3 − retention in the subsoil is increased by the presence of Al and Fe oxi-hydroxides and kaolinite (Harmand et al. 2010) or Al-rich allophane (Ryan et al. 2001) that may delay and mitigate groundwater contamination.
When measuring the nitrogen budget of a cropping system, it is difficult to measure the fate of the applied fertilizer directly, as inorganic N is also provided by soil N mineralization. Numerous studies have used labeled 15N fertilizer to evaluate N fluxes such as denitrification (Rückauf et al. 2004), N uptake by crops (Halitligil et al. 2002; Allen et al. 2004), N leaching (Pu et al. 2001; Lehmann et al. 2004), biological N2 fixation (Nygren et al. 2012) and litter decomposition (Zeller et al. 2000). However, few studies have used this technique to assess the whole N balance (Nannipieri et al. 1999; Lehmann et al. 2004). Some studies have investigated the competition between associated crops using 15N tracers (Allen et al. 2004; Zamora et al. 2009; Hagan et al. 2010), and N transfer from leguminous trees to the main crop (Kurppa et al. 2010). For coffee, the few published studies using 15N labeled fertilizer focused on N use efficiency in monoculture. Apart from Fenilli et al. (2008) who measured N loss by leaching (6 kg N ha−1 year−1), these studies only measured N uptake by crops and considered the rest as “non-measured losses” and subsequent N leaching (Bustamante et al. 1997; Salas et al. 2002).
This study used 15N labeled fertilizer to evaluate N fluxes in a heavily fertilized coffee plantation shaded by Inga densiflora. The specific objectives of this study were (1) to evaluate the N fertilizer use efficiency by measuring N and 15N accumulation in biomass and litter (2) to quantify N2 fixation by the leguminous trees and (3) to quantify soil N dynamics, principally N immobilization in soil organic matter, inorganic N accumulation in soil and N losses through leaching.
Materials and methods
Study site
The study area was at the Research Station of the Coffee Institute of Costa Rica in San Pedro de Barva, 10 km west of San José, Costa Rica (10.03°N, 84.14°O, 1,180 m elevation). The average annual temperature is 21 °C and annual precipitation is about 2,500 mm with a pronounced dry season between January and April. During the study (May 2004–February 2005), the rainfall was 2,678 mm. The slope of the site was uniform and ranged between 3 and 5 %. The soil was an Andosol (FAO-ISRIC-IUSS 2006), derived from the weathering of volcanic ash, classified as Dystric Haplustand (Mata and Ramirez 1999). It was generally well-structured, deep and permeable, with low bulk density and high organic matter content. Selected chemical and physical characteristics of the top 2 m of the soil are given in Table 1. From 30 to 200 cm deep, the soil had high clay content (from 348 to 428 g kg−1) with allophanes accounting only for 2.5 %. The mean soil pH (in water) was 6.3 and the cation exchange capacity (CEC) was 42 cmol+ kg−1.
Experimental design
The coffee agroforestry plantation was established in June 1997 on an area of 1,350 m² previously used for coffee monoculture. Coffea arabica var. Catuaí was planted at 2 × 1 m (4,722 plants ha−1) and the shade tree species I. densiflora was planted at 6 × 6 m (278 trees ha−1) giving a total of 5,000 trees + coffee plants ha−1. Fertilizer was applied at an annual average rate of 250 kg N ha−1, 30 kg P ha−1 (triple superphosphate), 100 kg K ha−1 (KCl) and 80 kg Mg ha−1 (MgO). Urea based fertilizer (NPKMg: 18-3-10-8) at 90 kg N ha−1 was applied in both May and August and NH4NO3 (70 kg N ha−1) was applied in October. For this study, granular fertilizer was spread uniformly by hand on the soil surface. In April 2004 (at the start of the wet season), a 156 m2 (12 m × 13 m) experimental plot, with 6 rows of coffee plants and 4 I. densiflora trees located in the middle of the plantation, was delimited and isolated from the surrounding plantation using boards and plastic sheets sunk vertically into the soil down to a depth of 60 cm. Labeled 15N in the form of urea was applied to the 156 m² plot uniformly at a rate of 90 kg N ha−1 in granular form (with 0.894 atom% excess 15N) on May 24, 2004 and August 03, 2004. The total 15N applied was 1.61 kg N ha−1. No labeled N was used for the last fertilization with NH4NO3 at the rate of 70 kg ha−1 on October 25, 2004.
Soil sampling
Soil samples were taken from the 156 m2 plot using an Edelman auger before the first fertilization on May 7, 2004, before the second fertilization on July 12, 2004 and during the dry season on January 19, 2005, at depths of 0–20, 20–50, 50–80, 80–110, 110–140, 140–170, and 170–200 cm. Four replicates were taken on each sampling date. Soil samples were collected along the coffee rows at 50 cm from each coffee plant (this position provides a representative measurement of the soil properties averaged across the coffee plot), and also at random distances from the base of I. densiflora trees. The samples were taken immediately to the laboratory. Mineral N was determined on field moist subsamples (see section on chemical analysis), and the rest of the samples were air-dried and sieved to less than 2 mm.
N export in coffee berry harvest
The fruit production and N export from the whole of the 15N experimental plot was quantified during the harvest at the end of the rainy season (November 30, 2004, January 5 and 19, 2005). Samples (0.5–0.7 kg) from each coffee fruit harvest were dried at 60 °C to constant weight and weighed. The total N concentration and isotopic composition of the samples were determined as described below.
Biomass and N stocks determination
Aboveground biomass of coffee plants and trees
The coffee biomass was determined for 16 coffee plants randomly selected in January 2005: 8 plants inside the 156 m² plot where 15N was applied and 8 plants outside the plot. The fresh weight of stems, branches, leaves, tap roots and coarse roots was measured. For each plant, sub-samples of each component were taken and oven dried at 60 °C for 72 h to estimate their total dry biomass. Samples of coffee plants were also taken from 6 coffee rows (6 composite samples) from inside and outside the 15N experimental plot to determine the total N and isotopic compositions. Each composite sample consisted of 6 leaves or 6 branches taken on each coffee plant (13 coffee plants per row). Six samples of coffee stems were collected using a Pressler auger. Six primary and secondary roots from the top 10 cm were also sampled.
The method used to measure the biomass of Inga trees was described by Siles et al. (2010a). The trees had a short, single trunk ramifying into two or three vertical stems at a mean height of 75 cm. The trunks and stems were studied separately. The trunk volume was measured and converted into dry biomass using a specific weight of 447 kg DM m−3 established from oven-dried wood samples. The allometric relationships between the stem diameter at 130 cm height (D130) and biomass of all components were determined by Siles et al. (2010a) to provide estimates of leaves, branches, stems and total aboveground biomasses. The relationships for stems and branches were established from a data-set of 17 trees. Ten tree stems were cut in the rainy season (July 2004) and 7 tree stems (2 inside and 5 outside the 15N experimental plot) were cut in the dry season (January 2005). Allometric relationships were also established for leaf and fruit biomass during the rainy season (10 trees) and during the dry season (7 trees). The biomass of coarse tree roots was not measured. At the same experimental site, Hergoualc’h et al. (2012) calculated the coarse root biomass of the Inga trees using the root:shoot ratio of 0.205 ± 0.036 (standard error) for tropical moist plantations with a shoot biomass <125 Mg DM ha−1 as proposed in the literature review of Mokany et al. (2006). Subsamples of leaves, branches and stems from 4 Inga trees (2 inside and 2 outside the 15N experimental plot) were oven dried at 60 °C to constant weight and weighed to estimate the total dry biomass of each component. The total N and 15N content was analyzed. 4 Inga trunk samples (composite of three replicates) were also collected using a Pressler auger as well as primary and secondary roots from the top 10 cm to analyze total N and 15N.
Fine roots
The fine root (diam <2 mm) biomass of coffee plants and Inga trees down to a depth of 1.2 m was estimated after taking soil samples in January 2005 using a radicular auger (10 cm height, 8 cm diameter). Five sample (2 in the 15N plot experiment, 3 outside) were taken along the coffee rows at a distance of 50 cm from coffee plants and 1.5–2 m from the base of Inga trees. The fine roots were collected after washing off the soil with distilled water through a 0.2 mm sieve. The fine roots were oven-dried at 60 °C to constant weight and weighed and the N and 15N contents were analyzed.
Litter layer
The amount of litter present on the ground at the 15N experimental site was estimated in January 2005. Eight composite litter samples of 0.5 m2 (two 50 cm × 50 cm squares) were collected by systematic sampling. One square was at the base of a coffee plant and the other midway between coffee rows. These two squares were representative of the sub-unit formed by 4 coffee plants (2 m × 1 m). All litter above the mineral soil was collected, washed with distilled water, oven-dried at 60 °C to constant weight and weighed and the N and 15N content was analyzed. Litter samples were also collected from the 15N experimental site, using the same method, to measure 15N natural abundance.
Net N mineralization and nitrification in the top soil
Net N mineralization measurements were done by Hergoualc’h et al. (2009). N mineralization was evaluated from July 2004 to February 2005 by “in situ” incubation of undisturbed soil cores (8 cm diam., 10 cm deep). for 28–36 days (Anderson and Ingram, 1993). At the beginning of each period, two paired cores were collected, one pair from the coffee row and the other one between rows. One soil core of each pair was taken to the laboratory to determine NH4 + and NO3 − at the start of incubation. The other core was incubated in a PVC tube in its original position in the soil for 28–36 days before collection and analysis of inorganic N (see below). Mineralization was measured during 3 months of rainy season (July to September), and 3 months of the dry season (December to February). The extrapolation for the rest of the year was done considering 6 months of rainy season, 4 months of dry season and 2 months with intermediate values.
Nitrate leaching
Leachate was sampled during the 2004 rainy season using porous ceramic cups (12 mL volume; SDEC Tensionic, Reignac-sur-Indre, France), filled with distilled water before each sampling period and mounted at the bottom of a rigid tube (2.5 cm diam.). Four ceramic cups were located at two depths (60 cm and 120 cm) at a distance of 40–50 cm from the base of coffee plants and 1.5–2 m from Inga stems. The leachate was collected every 10 days (no suction was applied) assuming equilibrium between the mineral composition of the solution in the ceramic cup and the leachate as demonstrated by Moutonnet et al. (1993). Leachate was sampled 16 times during the study, from May 20, 2004 to December 15, 2004.
Nitrate leaching at a depth of 60 and 120 cm was calculated using the daily water drainage values obtained by Cannavo et al. (2011) during the study on the same plot. Water flow in the soil was simulated using the HYDRUS 1D model (Simunek et al. 2005). HYDRUS was calibrated and validated using measured values of soil hydraulic properties and measured soil water content every 10 days, daily actual evapotranspiration and surface runoff. The methods used to measure the different water components, were described by Siles et al. (2010b) and Cannavo et al. (2011).
NO3 −–N and NO3 −–15N losses were expressed for a given area by multiplying the calculated amount of drainage water for the sampling period by the NO3 −–N and 15N excess concentrations in the leachate.
N2O gas emissions
N2O emissions were measured in the same plot from October 2004 to September 2005 using the static chamber method as reported by Hergoualc’h et al. (2008). Measurements were done after gas sampling from 12 chambers hermetically sealed during 1 h. The chambers were placed in the soil at a depth of 5 cm, 2 weeks prior to the initial measurements at a distance of 2 m from the Inga trees within the coffee rows and midway between rows. Gas samples were taken regularly, once a month. More intensive sampling was undertaken every day during the 7 days after the application of fertilizer and thereafter on days 10, 13 and 21.
Gas samples were analyzed using a Hewlett Packard 5890 chromatograph, fitted with an electron capture detector for N2O analysis.
Symbiotic N2 fixation by Inga densiflora
As leaves are generally the main sink for recently fixed N (Domenach 1995), only leaves were used in this experiment to evaluate the symbiotic N2 fixation by Inga trees over the year. N2 fixation was evaluated initially in January 2005 (5 replicates) and calculated from foliar % atom excess of 15N (FAE) data for Inga and reference (coffee) plants using the formula given by Fried and Middelboe (1977):
where x refers to Inga trees and r refers to coffee plants. The foliar % atom excess of 15N (FAE) in any species was calculated by subtracting the natural percentage of 15N in the atmosphere, 0.3663 % (Mariotti 1984) from the 15N percentage in the leaf sample. Total N derived from atmosphere (TNdfa) was calculated as the product of %N derived from the atmosphere (%Ndfa) and total foliar N.
N2 fixation by Inga trees was also measured in July 2004 using the natural abundance method (Boddey et al. 2000). Leaf samples were taken from coffee plants and Inga trees (5 replicates) outside the 15N experimental plot. The 15N/14N isotope ratio was calculated as described by Mariotti (1984):
where %15Nx is the percentage of 15N in the sample and %15Na is the percentage of 15N in the air (0.3663 %).
%Ndfa was calculated as described by Shearer and Kohl (1986):
where δ15Nr is the δ15N value from the non-fixing C. arabica as the reference plant, δ15Nx is the value from the N2-fixing I. densiflora in situ and δ15Nfr is the value from I. densiflora grown in N-free solution under greenhouse conditions. Since δ15Nfr has never been determined for I. densiflora, two %Ndfa estimates derived from the δ15Nfr values of 0 and −2 ‰ were used to cover the most probable range (Koponen et al. 2003; Shearer and Kohl 1986).
Chemical analysis
The NH4 + and NO3 − concentrations of soil samples were analyzed. Field-moist subsamples were suspended in a K2SO4 solution (1:10 soil to solution, 0.5 M K2SO4 concentration), shaken for 1 h and centrifuged for 5 min (2,500 rpm). The supernatant was then filtered through a Whatman 42 filter. The nitrate and NH4 + concentrations in the soil extracts were determined photometrically using a flow injection analyzer (Futura 2000, Alliance Instrument, Frépillon, France). Concentrations of NH4 + and NO3 − in the leachate collected in the porous ceramic cups were also determined following the same procedure.
The isotopic composition of NO3 − and NH4 + in the soil extracts or leachate was determined using the microdiffusion method (Brooks et al. 1989; Sorensen and Jensen 1991). Before diffusion, the solution samples were diluted if necessary to give a volume of 30 mL solution containing approximately 200 μg N as NO3 − and as NH4 +.
All vegetation samples (coffee berries, coffee plant and tree components and litter) were oven-dried at 70 °C and milled into fine powder in a rotating ball bearing mill to pass through a 40 mesh screen. The total N concentration and 15N composition of all samples were determined using an elemental analyzer (Carlo Erba NA 1500) connected to an isotope mass spectrometer (Finnigan Delta S) via a split interface.
N balance calculations
15N fluxes and budget were calculated on the basis of 15N in excess of natural abundance (atom% 15N excess). The natural abundance was determined for soil (0.3659–0.3694 atom% 15N), soil solution (0.3664–0.3693 atom% 15N) and coffee plant and shade tree (0.3665–0.3745 atom% 15N) before the 15N tracer was applied (mean of five measurements).
The percentage use of fertilizer N (%UFN), a measure of fertilizer use efficiency, was calculated for the coffee plants and shade trees (Allen et al. 2004):
where S is the N content in the roots, stems, branches, leaves or fruit (kg N ha−1), R is the 15N applied rate (kg N ha−1), a is the atom% 15N abundance in biomass components and b is the natural atom% 15N abundance in biomass components.
The percentage recovery of 15N fertilizer in soil (RFNsoil) and leachate (RFNleachate), a measure of the applied 15N remaining in soil and leachate at 120 cm, respectively, was determined using the following equation (Allen et al. 2004):
where: a is the atom% 15N abundance in the fertilized soil material (or leachate), b is the atom% 15N abundance in the labeled fertilizer, c is the atom% 15N abundance in the non-fertilized soil (or leachate), Np is the total N of soil sample (or leachate) and Nf is the total amount of 15N applied to the soil as labeled fertilizer.
No 15N-labeled fertilizer was applied during the third fertilization but, in accordance with Harmand et al. (2007b), it was assumed that N accumulation in the coffee berries after the last fertilization followed the same pattern as after previous applications.
Statistical analyses
Descriptive statistics (mean, standard error) for each component of the system are presented in order to discuss the relative importance of the different N fluxes. Mean difference and correlations were tested for significance at P < 0.05 and P < 0.1 (R free software). The distribution of each variable was evaluated using the Shapiro–Wilk’s test. The t test was used to compare two means for normally distributed variables and the Mann–Whitney nonparametric test was used to compare two means for non-normally distributed variables. Pearson’s correlations were used to assess the relationships between rainfall and drainage and between dry matter and stem diameter.
Results
Climate and water balance
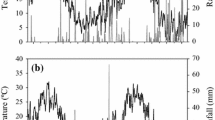
The annual rainfall for 2004 (3,245 mm owing to heavy rainfall of 620 mm in September and 650 mm in October when Costa Rica was affected by the hurricane Ivan) was significantly higher than the historical mean annual rainfall (2,500 mm). Rainfall was unevenly distributed throughout the year with 3,057 mm (94 %) during the wet season from May to November. During the experiment (May 24, 2004–February 15, 2005), the rainfall was 2,678 mm. As reported by Cannavo et al. (2011), the soil water balance showed high drainage at 120 cm (1,745 mm), accounting for 58 % of the rainfall–soil water variation (Table 2). The second most important water loss was evapotranspiration (AET), accounting for 36 % of the rainfall–soil water variation. Little loss by surface runoff (169 mm) was measured during the study (6 % of the rainfall–soil water variation).
Biomass production and N accumulation
In coffee plants, the total biomass accumulation (fruit + permanent biomass) was 26.4 t ha−1 dry matter (DM) and fruit accounted for 19 % (4.97 t ha−1DM) of the total biomass (Table 3). The nitrogen concentration was highest in the leaves (30.7 g N kg−1 DM) and fruit (22.1 g N kg−1 DM. During the year, 110 kg N ha−1 in coffee fruit was exported and the annual N accumulation in the permanent biomass of coffee plants was 31.5 kg N ha−1. The applied N (from the 15N-labeled N) was incorporated mainly into leaves and fruits. Coffee berries were the main biomass component accumulating the mineral fertilizer (7 % of applied N). According to the 15N-labeled N recovered in green leaves and litter in January 2005, leaves accounted for 3.4 % of the applied N. Little applied N was found in coffee plant stems (0.85 %), coarse roots (0.36 %) and total fine roots (0.60 %). According to Crouzet et al. (2007), 80 % of these fine roots were coffee roots, indicating that 13.5 % of the applied N was incorporated into the coffee plants, accounting for a total amount of 33.7 kg N ha−1.
Based on destructive measurements, the allometric relationships between D130 and biomasses (total, stems, branches and leaves) can be described as exponential functions (Table 4). The stems and branches represented the main biomass component of the aboveground biomass followed by the trunk, leaves and fruit (Table 3). Foliar biomass during the dry season was half (1.7 t ha−1 DM) that during the rainy season (3.7 t ha−1 DM in May 2004) as reported by Siles et al. (2010a). The coarse root biomass was estimated at 5.9 t ha−1 DM. The total Inga biomass amounted to 32.9 t ha−1 DM. This was 1.25 times higher than the coffee plant biomass (26.4 t ha−1 DM). The nitrogen concentrations in the leguminous tree components were also higher than in the coffee plant components. The annual N accumulation in permanent tree biomass, without fruit (57.2 kg N ha−1 year−1) was much higher than in permanent biomass of coffee plants (31.4 kg N ha−1 year−1). Although these values of mean annual N accumulation in the permanent biomass after 7 years could overestimate the current annual N accumulation, fruit accounted for only 5.2 % of the total annual N accumulation of the Inga trees (3.15 kg N ha−1 year−1) compared to 78 % in coffee plants (110 kg N ha−1 year−1). 9.45 % of the N fertilizer was recovered by the shade trees, amounting to 23.6 kg N ha−1.
Fine root (<2 mm) biomass accounted for 25 % of the total root biomass according to data presented in Table 3. A large amount of litter was present on the ground by the end of the study, representing 9.2 % of the total aerial biomass (excluding coffee fruit). At the end of the study, 0.6 % of the applied N was recovered in fine roots and 2.1 % in the litter layer. It was calculated that the combined vegetative biomass took up 25.2 % of the applied N fertilizer, representing an overall recovery of 62.9 kg N ha−1.
The two methods used in this study gave comparable results for N2 symbiotic fixation by I. densiflora (Table 5). The 15N labeling method estimated that 16.1 % of N was derived from the air (%Ndfa), whereas the natural abundance method estimated %Ndfa at between 15 % (δ15Nfree-N = −2 ‰) and 21.3 % (δ15Nfree-N = 0 ‰). However, other δ15Nfree-N reported values for tropical leguminous trees: −1.09 ‰ for Inga edulis (Nygren and Leblanc 2009), −2.07‰ for Gliricidia sepium (Nygren et al. 2000) and −2.52 ‰ for Acacia senegal (Isaac et al. 2011) give a lower estimated range of %Ndfa of between 14 and 17 % which is close to the value obtained with the 15N labeling method. Therefore, considering that (1) I. densiflora acquired 16.1 % of its foliar N from fixation (labeling method) and (2) Inga leaf biomass was 3.74 t ha−1 DM in July 2004 (Siles et al. 2010a), the annual I. densiflora N2 fixation amounted to 22.7 kg N ha−1. However, this could be underestimated as some of the fixed N may have been transported to plant components other than the leaves.
Nitrogen dynamics in soil
Low ammonium concentrations were found in the soil (less than 4 mg N kg−1 of soil) (Fig. 1a). At the beginning of the experiment (before the first N amendment on 7 May), the soil NO3 − content decreased with depth from 22 mg N kg−1 in the top 30 cm layer to 1.7 mg N kg−1 2 m below the surface (Fig. 1b). The first N input (90 kg N ha−1) on May 24 significantly increased the soil NO3 − content at a depth down to 50 cm (P < 0.01) to 68 mg N kg−1 in the 0–20 cm layer and 27 mg N kg−1 in the 20–50 cm layer on July 12 before the second application of fertilizer. On this date, 60 % of the N applied during the first application of fertilizer (54 kg N) was found in inorganic (NO3 −) form in the top 50 cm of the soil (Fig. 2a). Between 50 cm and 200 cm, there was also a small amount of 15N–NO3 −, accounting for 11 % of the applied N (9.9 kg N). In January 2005, 3 months after the third application of fertilizer at the end of October, the soil NO3 −–N content profile was not significantly different from that measured before the first annual application of fertilizer in early May 2004. The soil NO3 −–N stock in the 0–2 m soil layer was 112 kg N ha−1 on May 7, increasing to 257 kg N ha−1 in July after the first application of fertilizer and then decreasing to 180 kg N ha−1 at the end of the rainy season, three months after the third application of fertilizer (Table 6).
a Soil NH4 +–N content (mg N kg−1), b soil NO3 −–N content (mg N kg−1) at three dates, before the first application of fertilizer (input of 90 kg N ha−1, May 7, 2004), before the second application of fertilizer (input of 90 kg N ha−1, July 12, 2004) and 3 months after the third application of fertilizer (input of 70 kg N ha−1, January 19, 2005) under coffee plants shaded by Inga densiflora
In January 2005, no 15NO3 or 15NH4 was detected in the soil. However, 20.8 % of the applied N (52 kg/ha) was present in organic form in the 0–200 cm soil layer, mainly concentrated in the top 120 cm (39 kg ha−1: 15.7 % of applied N) (Fig. 2b).
Nitrate leaching
The nitrate concentration patterns in the leachate were similar at depths of 60 and 120 cm during the study (Fig. 3b), although concentrations were often lower at 120 cm than at 60 cm. The nitrate concentration in the leachate increased rapidly at 60 and 120 cm after each N fertilizer application. Maximum NO3 −-N concentrations at a depth of 60 cm, were 16.3 on June 18, 22.8 on August 30 and 20.4 mg N L−1 on December 10, 2004. The maximum values at a depth of 120 cm were 10.2 mg N L−1 on June 7, 18.9 mg N L−1 on September 10 and 8.6 mg N L−1 on November 15. The 15N atom excess in the leachate also increased rapidly after the two first 15N-urea applications with similar patterns to the NO3 − concentrations at depths of 60 and 120 cm (Fig. 3c). From September 10, the 15N atom excess decreased rapidly and reached very low values on October 22 just before the third application of fertilizer without 15N addition. Three main N leaching periods occurred during the study (Fig. 4b). Each was due to the combination of N fertilizer application and significant water drainage (Fig. 4a). The high drainage variations observed were correlated with the daily rainfall (Fig. 3a, R2 = 0.55, P < 0.1). The three highest drainage values, ranging between 50 and 56 mm day−1, occurred during the hurricane season (September–October). At 120 cm depth, the maximum N leaching values for each 10-day period were 13.5 kg N ha−1 after the first application of fertilizer, 28.7 kg N ha−1 after the second and 19.2 kg N ha−1 after the third application of fertilizer (Fig. 4b). As a result of two high rainfall events after the third application of fertilizer (October 22), a large amount of NO3 − leaching occurred within 20 days (Fig. 4b), which accounted for 64 % and 40 % of the third N input (70 kg N ha−1) at 60 and 120 cm depths, respectively. This high flux contained very low residual NO3 − from the two first applications of fertilizer (less than 1 kg at 120 cm depth, as shown in Fig. 4d). At the end of the rainy period, the total N leaching was 214.1 kg N ha−1 at a depth of 60 cm and 157.2 kg N ha−1 at 120 cm, equivalent to 86 % and 63 % of the total annual N input, respectively (Fig. 4c). The recovery of the total applied N in leaching losses at depths of 60 and 120 cm was less and was estimated at 54.6 % (136.5 kg N ha−1) at 60 cm and 33.1 % (82.8 kg N ha−1) at 120 cm.
a Daily rainfall (mm) during the study, b NO3 −–N concentrations in leachate at depths of 60 and 120 cm, c 15NO3 −–N concentrations (isotopic excess). The arrows I, II and III show the dates when the fertilizer was applied. Labeled N was applied only on May 24, 2004 and August 03, 2004. The third N application (October 25) with NO3NH4 was not 15N labeled
N balance and recovery of applied N in the coffee agroecosystem
A tentative N budget was calculated to compare N input through mineral fertilizer, atmospheric N deposition and biological N2 fixation, N accumulation in the system (accumulation in plant and litter and immobilization in soil) and N outputs through the coffee fruit harvest, NO3 − leaching, gaseous emissions and water runoff (Fig. 5). Some components of the N balance were estimated from published data from the same experimental site. Nitrogen loss by runoff was estimated from the study by Harmand et al. (2007a). Nitrogen gas emissions in N2O and NH3 forms were estimated from the studies by Hergoualc’h et al. (2008) and Fenilli et al. (2008) respectively (see discussion). Atmospheric N deposition was estimated from Clark et al. (1998) to be 7 kg N ha−1 year−1 in the Monte Verde region in Costa Rica. The balance between the measured input N fluxes (fertilizer + N2 fixation + atmospheric N deposition: 279.7 kg N ha−1) and the output N fluxes (N in coffee fruit + NO3 − leaching + N2O emissions + N in runoff: 274.5 kg N ha−1) was fairly well balanced during this year of high coffee production.
Annual N fluxes in kg N ha−1 year−1. N2O emissions and N mineralization were obtained from Hergoualc’h et al. (2008) and Hergoualc’h et al. (2009), respectively. Nitrogen runoff was estimated from Harmand et al. (2007a), nitrogen volatilization from Fenilli et al. (2008) and atmospheric N deposition from Clark et al. (1998)
The total recovery of the applied N in plants, soil and leaching water at 120 cm depth amounted to 74.9 % (Table 7). Only 13.5 % of the applied N was taken up by coffee plants and 12 % by shade trees. In the soil (0–120 cm), most of the applied N (15.7 %) was found in organic form at the end of the rainy season. More than 33 % of the applied N was leached to a depth below 120 cm. Comparing N fluxes and recovery of applied N provided some unexpected results. In particular, coffee berries exported 110 kg N ha−1, of which only 17.6 kg N ha−1 originated from the high fertilizer input (250 kg N ha−1).
Discussion
Recovery of applied N
This study measured, as accurately as possible, the various components of the N balance of the system and the recovery of the applied N in plants (coffee plants and associated shade trees), soil and leaching water at a depth of 120 cm. The percentage recovery of the tracer at the end of the experiment (75 %) was in the same range as that obtained by Lehmann et al. (2004) in a maize cropping system (69.4 %). However, these authors also measured a total recovery of 93 % for a soybean cropping system at the same site. Fenilli et al. (2008) measured a total recovery of applied N of 90 % in a coffee monoculture, with little N loss through leaching (when fertilizer was applied at a rate of almost 300 kg N ha−1) owing to low water drainage.
Several reasons may explain why our total recovery was not closer to 100 %. Firstly, large variations (and hence standard errors) are inherently associated with N flux measurements, particularly when dealing with a multilayered agroforestry system. The standard error for the total recovery was 14.4 % which was mainly attributed to standard errors of leaching at 120 cm (8.5 %) and secondarily to soil N immobilization (3.2 %) (Fig. 5). This variability in the N content of the soil and the leachate is not surprising as such a high variability does exist for chemical elements in most soils. Secondly, NO3 − leaching may have been underestimated. The NO3 − concentration was assessed every 10 days based on an equilibrium between the mineral composition of the solution in the ceramic cup and the leachate after 3–5 days (Moutonnet et al. 1993) and some large flushes may not have been detected in between sampling dates. Calculations based on leaching at 60 cm gave a higher total recovery of the applied N (90.2 %) which suggests that the NO3 − leached at 120 cm was underestimated. The difference in 15NO3 − leaching between 60 cm (55 % of applied N) and 120 cm (33 % of applied N) was similar to the recovery of N fertilizer from biomass and litter. A third of this difference (almost 6 %) could be explained by N immobilization in organic form in the 60–120 cm soil layer. Plant uptake could also contribute to lower NO3 − leaching at 120 cm but not in such high quantities. In this system, 75 % of the total fine root biomass of the top 100 cm was concentrated in the top 60 cm of soil (Siles et al. 2010a, b), suggesting that plant N uptake occurred mainly in the top soil. 15NO3 − leaching at 120 cm may, therefore, have been clearly underestimated.
Unmeasured fluxes may also contribute to this imbalance. However, this was unlikely to be the case for surface runoff which was relatively low (169 mm, 6 % of rainfall in Table 2). in another study, Harmand et al. (2007a) reported low NO3 − + NH4 +–N loss in runoff in a C. arabica shaded by Eucalyptus deglupta on a Costa Rican Acrisol, accounting for 0.5 % of the annual N fertilizer input (180 kg N ha−1 year−1). Based on these results, N losses in surface runoff would account for only 1.25 kg N ha−1 year−1 in our study.
Possible N gaseous emissions such as NH3 through volatilization and N2O and N2 through denitrification may have occurred. Recovery of 15N fertilizer in NH3, N2O and N2 forms was not measured during the experiment. However, Hergoualc’h et al. (2008) measured N2O emission in the same experimental field over 1 year, from October 2004 to November 2005. They measured an annual N2O production of 5.8 kg N ha−1 and evaluated the contribution of N fertilizer to this emission at 2.5 kg N ha−1 year−1 (1 % of the annual N fertilizer input). Furthermore, this soil showed a low potential to reduce N2O into N2 (Hergoualc’h et al. 2009). This study did not measure NH3 emissions. Fenilli et al. (2008) estimated that volatilization losses amounted to 1.6 % of the applied N in coffee monoculture in Brazil when N fertilizer was applied as ammonium sulfate at 300 kg N ha−1 and the soil pH was 5.3. Moreover, they estimated significant re-absorption of the volatilized ammonia, up to 43 % of the 1.6 % lost, when the coffee plant density was 7,620 plants ha−1 (Bergamo Fenilli et al. 2007). Rochette et al. (2009) reported 9 % of urea N volatilized in a soil with a pH (in water) of 5.4. In this study, therefore, where urea was applied, ammonia losses may have been at least 1.6 % if not higher.
Some organic N might also have been leached away. In a Brazilian Oxisol Lehmann et al. (2004) found that soluble organic N, accounted for 39 %, in the topsoil, and 63 %, in the subsoil, of the total soluble N. In our study, it was found that 5.1 % of the N fertilizer was immobilized in organic matter in the 120–200 cm layer. This accumulation may be due partly to the percolation of soluble organic N.
Soil N dynamics and N leaching
This Andisol was found to have significant soil net N mineralization potential and was able to produce a large amount of 207 kg mineral N ha−1 year−1 in the top 10 cm (Hergoualc’h et al. 2009). However, this measured mineralized N may have been derived from previous microbial immobilization of N fertilizer. Positive mineralization-fertilizer interactions were reported by Kolberg et al. (1999). During the study, an average of 96 % of the total mineralized N in the 0-20 cm soil layer was in the form of NO3 −, while the NH4 + concentration was very low. This study showed that NH4 + from applied fertilizer or from mineralization of organic N was rapidly transformed into NO3 − as in other tropical ecosystems. Nitrogen fertilizer and soil net N mineralization by far exceeded annual N accumulation by the plants (coffee plants and associated shade trees) and N export in coffee fruit, indicating a high potential for NO3 − leaching and a significant risk of groundwater contamination by NO3 −.
The soil NO3 −–N content in the top 2 m of the soil on July 12, before the second application of fertilizer, amounted to 257 kg N ha−1 of which only 54 kg originated from the first application of fertilizer (Fig. 2a). A comparison with the amount measured on May 7 (112 kg N ha−1,) indicates the very high risk of N leaching during the rest of the rainy season, particularly between August and October when rainfall was very high and 70 % of the annual NO3 − leaching occurred (Fig. 4b). In this soil with high hydraulic conductivity and high annual rainfall, water drainage amounted to 65 % of the rainfall (Cannavo et al. 2011). Thus, the combination of high soil water flow with high mineral N concentration generated significant NO3 − losses by leaching. Despite the large amount of NO3 − leaching just after the third application of fertilizer (Fig. 4b) which accounted for a significant part of the third N input, the soil NO3 − content was still high in January 2005 at the end of the rainy season (180 kg N ha−1 in the top 2 m of the soil). This NO3 − accumulation was not significantly different from the NO3 − content on May 2004 but it is possible that residual NO3 − from the last application of fertilizer was still present in the soil. As no significant NO3 − accumulation (15NO3 −) was detected in the subsoil and high NO3 − leaching occurred, NO3 − retention seems to be low effective in this Andosol, with low allophane content and apparently low anion sorption capacity.
N use efficiency and soil N saturation
This study demonstrated the low N fertilizer use efficiency of the agroecosystem. Almost 20 % of the N fertilizer applied in May was taken up by coffee plants and 18 % by the Inga trees (data not shown) and 60 % was still in the top 60 cm of the soil on July 12 (Fig. 2a). This indicates that 98 % of the N applied in the first application was recovered in the system 15 days before the second application of fertilizer. In accordance with the increased NO3 − leaching rate during September and October, the subsequent applications of fertilizer were less efficient and, by the end of the experiment, the total recovery of applied N by the coffee plants and trees was only 25.2 % (63 kg N ha−1) of the N fertilizer, including 7 % (17.6 kg N ha−1) exported in coffee fruit. Despite a high coffee production in 2004–2005 (4.97 Mg DM ha−1 year−1) representing a high N export of 110 kg N ha−1 equivalent to 44 % of the annual N input, only 7 % of the annual N input actually contributed to this export (15N tracer results). This indicates that coffee plants acquired their N preferentially from the mineralized soil N pool and also reallocated N from vegetative parts (branches and leaves) to coffee fruit. The high production in 2004–2005 followed a very low yield (1.97 Mg DM ha−1 year−1) during the 2003–2004 season (Siles et al. 2010a). This low yield may have contributed to a high N accumulation in the vegetative parts of the coffee plants for the benefit of the coffee production during the following year.
The considerable soil mineral N accumulation throughout the rainy season, under the present fertilization regime, apparently resulted in an absence of N competition between the shade trees and the coffee crop and a low N2 fixation rate by the I. densiflora shade trees (%Ndfa = 16 % accounting for 22.7 kg N ha−1). Our results contrast with N2 fixation rates reported by Nygren and Leblanc (2009) and Nygren et al. (2012) to be in the range of 50–63 % for I. edulis in a low input cacao agroforestry system. Our results agree with Barron et al. (2011), who showed that, for Inga spp. in lowland tropical forests, individual mature trees can decrease nodulation and N2 fixation in response to high soil NO3 − levels and confirm that N2-fixing plants tend to down-regulate fixation in N-rich environments (Andrews et al. 2011).
Therefore, the addition of large amounts of N fertilizer every year in this system led to a soil N saturation, a low use of the annual N input by the plants, an under utilization of the N2 fixation potential of the leguminous trees and a high potential of N losses through NO3 − leaching and gaseous emissions. In Costa Rica, Salas et al. (2002) studied 15N and N dynamics in C. arabica monoculture and reported that less than 50 % of N remained in the agroecosystem, underlining the high risk of groundwater contamination. Castro-Tanzi et al. (2012) also documented N saturation in coffee farms in Tarrazú, Costa Rica, receiving an average of 212 kg N ha−1 year−1, suggesting soil acidification, depletion of soil exchangeable Ca and reduction in N use efficiency by the coffee plants as a result of excessive N input. In different climatic conditions, particularly with annual rainfall and drainage 2 and 7 times lower than in the present study, Fenilli et al. (2008) found limited N losses by leaching and a better N use efficiency. Seventy percent of the fertilizer (almost 300 kg N ha−1 year−1) remained in the shoots and roots of coffee plants.
Conclusion
This study investigated the N balance in a coffee agroecosystem with I. densiflora as associated shade trees (density 278 trees ha−1; age 7 year; height 8 m), using a 15N tracer. N fluxes were calculated for the top 120 cm of soil and N accumulation in plants and litter was evaluated over an annual coffee cycle. The results showed that the high annual N fertilizer input (250 kg N ha−1 year−1) over 7 consecutive years led to an N saturation of the system resulting in a low use of the annual N input (25 %) by the coffee plants and trees, even in a year of high coffee production. Furthermore, this excessive fertilizer input over the years led to reduced N2 fixation by the leguminous trees and generated a high potential of N losses through NO3 − leaching (from 33 to 55 % originating from the annual agrochemical fertilizer input). Nitrate was apparently rapidly leached in this Andosol with low allophane content and apparently low anion sorption capacity. The over application of N increases the potential of water contamination and eutrophication through NO3 − leaching and of greenhouse gas effect through N2O emissions.
In order to manage soil nutrients and especially N in a more environmentally friendly manner, fertilizer practices in coffee plantations should be refined to take advantage of the potential of N2 fixation by leguminous shade trees and increase the synchrony of N input with seasonal plant needs. During heavy rainfall, where N input is liable to be leached rapidly, it is highly recommended to reduce and fraction the amount of N fertilizer applied during the plant growing and fruiting cycle. Furthermore, the use of foliar fertilizer and slow–release N fertilizer formulations should be encouraged. Additionally, the use of models coupling water balance and nitrogen cycling should help to determine the most critical periods when high losses are expected and hence help advisory services to provide sound recommendations to farmers.
References
Allen SC, Jose S, Nair PKR, Brecke BJ, Ramsey CL (2004) Competition for 15N-labeled fertilizer in a pecan (Carya illinoensis K. Koch)-cotton (Gossypium hirsutum L.) alley cropping system in the southern United States. Plant Soil 263:151–164
Anderson JM, Ingram J (1993) Tropical soil biology and fertility. A handbook of methods. CAB International, Oxford, p 221
Andrews M, James EK, Sprent JI, Boddey RM, Gross E, dos Reis Bueno, Jr F (2011) Nitrogen fixation in legumes and actinorhizal plants in natural ecosystems: values obtained using 15N natural abundance. Plant Ecol Divers 4(2–3):131–140
Babbar LI, Zak DR (1995) Nitrogen loss from coffee agroecosystems in Costa Rica - leaching and denitrification in the presence and absence of shade trees. J Environ Qual 24:227–233
Barron AR, Purves DW, Hedin LO (2011) Facultative nitrogen fixation by canopy legumes in a lowland tropical forest. Oecologia 165:511–520
Bergamo Fenilli TA, Reichardt K, Ocheuze T, Triveli, PCO, Favarin JL (2007) Volatilization of ammonia derived from fertilizer and its reabsorption by coffee plants. Commun Soil Sci Plant Anal 38:1741–1751
Boddey RM, Peoples MB, Palmer B, Dart PJ (2000) Use of the N-15 natural abundance technique to quantify biological nitrogen fixation by woody perennials. Nutr Cycl Agroecosyst 57:235–270
Brooks PD, Stark JM, McInteer BB, Preston T (1989) Diffusion method to prepare soil extracts for automated nitrogen-15 analysis. Soil Sci Soc Am J 53:1701–1711
Bustamante C, Ochoa M, Rodriguez-Maritza I (1997) Balance of tree 15 N nitrogen fertilizers in a Cuban Oxisol cultivated for Coffea arabica L. Tropicultura 15:169–172
Cannavo P, Sansoulet J, Harmand JM, Siles P, Dreyer E, Vaast P (2011) Agroforestry associating coffee and Inga densiflora results in complementarity for water uptake and decreases deep drainage in Costa Rica. Agric Ecosyst Environ 140:1–13
Castro-Tanzi S, Dietsch T, Urena N, Vindas L, Chandler M (2012) Analysis of management and site factors to improve the sustainability of smallholder coffee production in Tarrazu, Costa Rica. Agric. Ecosyst, Environ 155:172–181
Clark KL, Nadkarni NM, Schaefer D, Gholz HL (1998) Atmospheric deposition and net retention of ions by the canopy in a tropical montane forest, Monteverde, Costa Rica. J Trop Ecol 14:27–45
Crouzet G, Harmand JM, Joffre R, Siles P, Dambrine E (2007) Quantifying fine root biomass distribution of coffee and tree in a Coffea arabica–Inga densiflora association using near infrared spectroscopy (NIRS). In: Proceedings of the second international symposium on multi-strata agroforestry systems with perennial crops: making ecosystem services count for farmers, consumers and the environment. 17–21 Sept 2007, Turrialba, Costa Rica
Domenach AM (1995) Approche de l’estimation de la fixation symbiotique des arbres par l’utilisation des abondances isotopiques naturelles de l’azote. In: Maillard P, Bonhomme R (eds) Utilisation des isotopes stables pour l’étude du fonctionnement des plantes, Paris 16–17 décembre 1993. Les Colloques 70, INRA Editions, Versailles, France, pp 159–172
FAO-ISRIC-IUSS Working Group WRB (2006) World reference base for soil resources 2006. In: World soil resources reports, No. 103, FAO, Rome
Fenilli TAB, Reichardt K, Favarin JL, Bacchi OOS, Silva AL, Timm LC (2008) Fertilizer N-15 balance in a coffee cropping system: a case study in Brazil. Revista Brasileira de Ciencia do Solo 32:1459–1469
Forsythe W (1985) Fısica de Suelos: Manual de Laboratorio. San Jose, Costa Rica, IICA
Fried M, Middelboe V (1977) Measurement of amount of nitrogen fixed by a legume crop. Plant Soil 47:713–715
Hagan DL, Jose S, Thetford M, Bohn K (2010) Partitioning of applied N-15 fertilizer in a longleaf pine and native woody ornamental intercropping system. Agrofor Syst 79:47–57
Haggar J, Barrios M, Bolaños M, Merlo M, Moraga P, Munguia R, Ponce A, Romero S, Soto G, Staver C, Virginio EMF (2011) Coffee agroecosystem performance under full sun, shade, conventional and organic management regimes in Central America. Agrofor Syst 82:285–301
Halitligil M, Akin A, Ylbeyi A (2002) Nitrogen balance of nitrogen-15 applied as ammonium sulphate to irrigated potatoes in sandy textured soils. Biol Fertil Soils 35:369–378
Harmand JM, Avila H, Dambrine E, Skiba U, De Miguel S, Renderos Duran RV, Oliver R, Jimenez F, Beer J (2007a) Nitrogen dynamics and soil nitrate retention in a Coffea arabica—Eucalyptus deglupta agroforestry system in Southern Costa Rica. Biogeochem 85:125–139
Harmand JM, Chaves V, Avila H, Cannavo P, Dionisio L, Crouzet G, Zeller B, Vaast P, Oliver R, Dambrine E (2007b) Nitrogen dynamics and nitrate leaching in Coffea arabica systems in Costa Rica. According to site conditions, fertilization and shade management. In: 21st international conference on coffee science, Montpellier, France, 11–15 Sept 2006. [Cd-Rom]. ASIC, Montpellier, p 1045–1052. Colloque Scientifique International sur le Café. 21, 2006-09-11/2006-09-15, Montpellier, France
Harmand JM, Avila H, Oliver R, Saint-André L, Dambrine E (2010) The impact of kaolinite and oxi-hydroxides on nitrate adsorption in deep layers of a Costarican Acrisol under coffee cultivation. Geoderma 158:216–224
Hergoualc’h K, Harmand JM, Cannavo P, Skiba U, Oliver R, Hénault C (2009) The utility of process-based models for simulating N2O emissions from soils: A case study based on Costa Rican coffee plantations. Soil Biol Biochem 41:2343–2355
Hergoualc’h K, Skiba U, Harmand JM, Henault C (2008) Fluxes of greenhouse gases from Andosols under coffee in monoculture or shaded by Inga densiflora in Costa Rica. Biogeochem 89:329–345
Hergoualc’h K, Blanchart E, Skiba U, Henault C, Harmand JM (2012) Changes in carbon stock and greenhouse gas balance in a coffee (Coffea arabica) monoculture versus an agroforestry system with Inga densiflora, in Costa Rica. Agric Ecosyst Environ 148:102–110
Henríquez C, Cabalceta G (1999) Guía práctica para el estudio introductoria de los suelos con un enfoque agrícola. Asociación Costarricense de la Ciencia del Suelo, p 111
Isaac ME, Harmand JM, Drevon JJ (2011) Growth and nitrogen acquisition strategies of Acacia Senegal seedlings under exponential phosphorus additions. J Plant Physiol 168:776–781
Kolberg RL, Westfall DG, Peterson GA (1999) Influence of cropping intensity and nitrogen fertilizer rates on in situ nitrogen mineralization. Soil Sci Soc Am J 63:129–134
Koponen P, Nygren P, Domenach AM, Rox CL, Saur E, Roggy LC (2003) Nodulation and dinitrogen fixation of legume trees in a tropical freshwater swamp forest in French Guiana. J Trop Ecol 19:655–666
Kurppa M, Leblanc HA, Nygren P (2010) Detection of nitrogen transfer from N2-fixing shade trees to cacao saplings in 15 N labelled soil: ecological and experimental considerations. Agrofor Syst 80:223–239
Lehmann J, Lilienfein J, Rebel K, Carmo Lima SD, Wilcke W (2004) Subsoil retention of organic and inorganic nitrogen in a Brazilian savanna Oxisol. Soil Use Manag 20:163–172
Mariotti A (1984) Natural 15N abundance measurements and atmospheric nitrogen standard calibration. Nature 311:251–252
Mata RA, Ramirez JE (1999) Estudio de caracterizacion de suelos y su relacion con el manejo delcultivo de café la provincia de Heredia. ICAFE, San José, Costa Rica, pp 92
Moguel P, Toledo V (1999) Biodiversity conservation in traditional coffee systems of Mexico. Conserv Biol 13:1–12
Mokany K, Raison RJ, Prokushkin AS (2006) Critical analysis of root: shoot ratios in terrestrial biomes. Glob Change Biol 12:84–96
Moutonnet P, Pagenel JF, Fardeau JC (1993) Simultaneous field measurement of nitrate-nitrogen and matric pressure-head. Soil Sci Soc Am J 57:1458–1462
Nannipieri P, Falchini L, Landi L, Benedetti A, Canali S, Tittarelli F, Ferri D, Convertini G, Badalucco L, Grego S, Vittori-Antisari L, Raglione M, Barraclough D (1999) Nitrogen uptake by crops, soil distribution and recovery of urea-N in a sorghum-wheat rotation in different soils under Mediterranean conditions. Plant Soil 208:43–56
Nygren P, Leblanc H (2009) Natural abundance of 15N in two cacao plantations with legume and non-legume shade trees. Agrofor Syst 76:303–315
Nygren P, Cruz P, Domenach AM, Vaillant V, Sierra J (2000) Influence of forage harvesting regimes on dynamics of biological dinitrogen fixation of a tropical woody legume. Tree Physiol 20:41–48
Nygren P, Fernández MP, Harmand JM, Leblanc HA (2012) Symbiotic dinitrogen fixation by trees: an underestimated resource in agroforestry systems? Nutr Cycl Agroecosyst 94:123–160
Picado W, Ledezma R, Granados R (2009) Territorio de coyotes, agroecosistemas y cambio tecnológico en una región cafetalera de Costa Rica. Revista de Historia, 119–165, UNA-UCR 59-60
Pu G, Strong WM, Saffigna PG, Doughton J (2001) Denitrification, leaching and immobilisation of applied 15 N following legume and grass pastures in a semi-arid climate in Australia. Nutr. Cyc. Agroecosys. 59:199–207
Reynolds-Vargas JS, Richter DD (1995) Nitrate in groundwaters of the Central Valley, Costa Rica. Environ Intern 21:71–79
Rochette P, MacDonald JD, Angers DA, Chantigny MH, Gasser MO, Bertrand N (2009) Banding of urea increased ammonia volatilization in a dry acidic soil. J Environ Qual 38:1383–1390
Rückauf U, Augustin J, Russow R, Merbach W (2004) Nitrate removal from drained and reflooded fen in soils affected by soil N transformation processes and plant uptake. Soil Biol Biochem 36:77–90
Ryan MC, Graham GR, Rudolph DL (2001) Contrasting nitrate adsorption in Andisols of two coffee plantations in Costa Rica. J Environ Qual 30:1848–1852
Salas R, Bornemiza E, Zapata F, Chaves V, Rivera A (2002) Absorcion del fertilizante nitrogenado por la planta de café y su influencia sobre la contaminacion de las aguas subterraneas. In: Euned (ed) Manejo integrado de aguas subterraneas, pp 89–103
Shearer G, Kohl DH (1986) N2-fixation in field setting: estimations based on natural 15N abundance. Aust J Plant Physiol 13:699–756
Siles P, Harmand JM, Vaast P (2010a) Effects of Inga densiflora on the microclimate of coffee (Coffea arabica L.) and overall biomass under optimal growing conditions in Costa Rica. Agrofor Syst 78:269–286
Siles P, Vaast P, Dreyer E, Harmand JM (2010b) Rainfall partitioning into throughfall, stemflow and interception loss in a coffee (Coffea arabica L.) monoculture compared to an agroforestry system with Inga densiflora. J Hydrol 395:39–48
Simunek J, Van Genuchten MT, Sejna M (2005) The HYDRUS-1D software package for simulating the one-dimensional movement of water, heat and multiple solutes in variable-saturated media. Version 3.0, HYDRUS Software Series 1, Department of Environmental Sciences, University of California Riverside, Riverside, California, p 270
Sorensen P, Jensen E (1991) Sequential diffusion of ammonium and nitrate from soil extracts to polytetrafluoroethylene trap for 15N determination. Anal Chim Acta 252:201–203
Summer ME, Miller WP (1996) Cation exchange capacity and exchange coefficient. In: Sparks DL (ed) Methods of soil analysis. SSSSA Book Series 5, Madison, WI, USA, pp 1205–1230
Tully KL, Lawrence D (2011) Closing the loop: nutrient balances in organic and conventional coffee agroforests. J Sustain Agric 35:671–695
Tully KL, Lawrence D, Scanlon TM (2012) More trees less loss: nitrogen leaching losses decrease with increasing biomass in coffee agroforests. Agric Ecosyst Environ 161:137–144
Vitousek PM, Matson PA (1988) Nitrogen transformations in a range of tropical forest soils. Soil Biol Biochem 20:361–367
Vitousek PM, Naylor R, Crews T, David MB, Drinkwater LE, Holland E, Johnes PJ, Katzenberger J, Martinelli LA, Matson PA, Nziguheba G, Ojima D, Palm CA, Robertson GP, Sanchez PA, Townsend AR, Zhang FS (2009) Nutrient imbalances in agricultural development. Science 324:1519–1520
Zamora DZ, Jose S, Napolitano K (2009) Competition for 15N labeled nitrogen in a loblolly pine-cotton alley cropping system in the southeastern United States. Agric Ecosyst Environ 131:40–50
Zeller B, Colin-Belgrand M, Dambrine E, Martin F, Bottner P (2000) Decomposition of 15 N-labelled beech litter and fate of nitrogen derived from litter in a beech forest. Oecologia 123:550–559
Acknowledgments
The authors are grateful to C. Fonseca from ICAFE (Instituto del café de Costa Rica) for giving permission to work on the study site, L. Dionisio (ICAFE) for assistance in collecting samples and P. Leandro from CATIE (Centro Agronómico Tropical de Investigación y Enseñanza), S. Bienaimé and B. Pollier from INRA (Institut National de la Recherche Agronomique) for laboratory analyses. The European Commission (INCO project CASCA, ICA4-CT-2001-10071) partially funded this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cannavo, P., Harmand, JM., Zeller, B. et al. Low nitrogen use efficiency and high nitrate leaching in a highly fertilized Coffea arabica–Inga densiflora agroforestry system: a 15N labeled fertilizer study. Nutr Cycl Agroecosyst 95, 377–394 (2013). https://doi.org/10.1007/s10705-013-9571-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-013-9571-z