Abstract
Increasing land degradation has prompted interest in conservation agriculture which includes growing cover crops. Besides providing soil cover, decaying cover crops may release substantial amounts of nutrients. Decomposition, N and P release from winter cover crops [grazing vetch (Vicia darsycarpa), forage peas (Pisum sativum) and oats (Avena sativa)] were assessed for suitability in a cropping system found in the smallholder irrigation sector of South Africa. Nitrogen and P contribution to maize growth by cover crop residues was also estimated. Decrease in mass of cover crop residues was highest in grazing vetch (7% remaining mass after 124 days) followed by forage peas (16%) and lastly oats (40%). Maximum net mineralized N and P were higher for grazing vetch (84.8 mg N/kg; 3.6 mg P/kg) than for forage peas (66.3 mg N/kg; 2.7 mg P/ha) and oats (13.7 mg N/kg; 2.8 mg P/kg). Grazing vetch and forage pea residues resulted in higher N contribution to maize stover than oat residues. Farmers may use grazing vetch for improvement of soil mineral N while oats may result in enhancement of soil organic matter and reduction land degradation because of their slow decomposition. Terminating legume cover crops a month before planting summer crops synchronizes nutrient release from winter-grown legume cover crops and uptake by summer crops.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Land degradation is one of the major challenges to sustainable agriculture in South Africa (SA). Burning of crop residues and continuous tillage has led to excessive soil erosion (Laker 2004; Mills and Fey 2004). Constraints faced by smallholder farmers also include critically low soil nitrogen and phosphorus levels, heavy weed infestations, lack of tillage and irrigation equipment (Mandiringana et al. 2005; Fanadzo 2007). Programmes promoting conservation tillage (CT) to address land degradation failed (Fowler 1999) due to lack of soil cover and diversified crop rotations (Derpsch 2003). Recently, attention has focused on conservation agriculture (CA) to mitigate the effects of land degradation on smallholder irrigation farms. Different views on what constitutes CA exist and it is often thought to be synonymous with CT. Conservation tillage consists of minimum-tillage practices and maintenance of at least 30% soil cover by plant residues (Fowler and Rockstrom 2001; Baker et al. 2002). Conservation agriculture combines minimal soil disturbance, a permanent soil cover through use of cover crops with crop rotations (Derpsch 2005). Hobbs (2007) argued that CT uses some of the principles of CA, but has more soil disturbance.
For successful uptake of CA technologies by farmers, applicability in their farming systems must be demonstrated. Opinions differ on whether CA benefits can be realized on smallholder farms in Sub-Saharan Africa (Giller et al. 2009). There are about 317 smallholder irrigation schemes in South Africa, accommodating up to 250,000 smallholder irrigators (Bembridge 2000). Water availability at the source for irrigation is not limiting, with some farmers over irrigating due to lack of technical knowledge. In earlier CA studies on farmers’ fields, low biomass production by cover crops did not result in the expected ecological benefits such as improved soil nutrient status for the succeeding maize crop (Derpsch 2003). Growing inappropriate cover crop species not informed by clear objectives to be achieved was blamed for the failure of CA on farmers fields (Derpsch 2003).
A permanent soil cover has been reported to result in reduced soil erosion, soil water conservation, reduction of weed densities as well as improved soil fertility (Derpsch 2003). These other benefits may appeal more to smallholder farmers than control of land degradation. Winter cover crops are planted in autumn, grow through winter under irrigation and are killed in spring prior to planting summer crops, usually maize the staple crop in SA. Nitrogen fixing legumes have been shown to have clear benefits in terms of N supply (Mafongoya et al. 2000; Sainju et al. 2005; Daudu et al. 2006). Cereal cover crops, such as oats and rye grass produce high biomass with large C and N contents and have potential to improve soil organic matter and reduce N loss through leaching, wind and water erosion (Sainju et al. 2005). Organic soil amendments such as cattle manure, pineapple waste, tobacco waste, poultry manure, and pig dung have been shown to improve soil N status in SA (Mkile 2001; Adediran et al. 2003).
Nutrient release from decomposing cover crops should be synchronised with crop demand (Ibewiro et al. 2000). The quantification of residue decomposition and nutrient release is thus a pre-requisite for optimising nutrient-use efficiency by maize. The fertilizer value of plant residues left on the soil surface will depend on their ability to decompose and release nutrients (Adediran et al. 2003). Several studies have determined that N, lignin (L) and polyphenol (PP) contents, C/N, L/N, PP/N and (L + PP)/N ratios are useful indices of residue quality that control residue decomposition and N release (Palm 1995; Vanlauwe et al. 1996; Lupwayi et al. 2000). An analysis of these quality parameters aids in explaining any differences in decompositions rates of various organic materials. The quality of residues is also known to affect soil pH, depending on the rate of application and the buffering capacity of the soil (Chintu et al. 2004). Consistent use of cover crop residues may have implications on soil pH management.
Carbon dioxide evolution has been used to indicate microbial activity during decomposition (Mafongoya et al. 2000; Adediran et al. 2003; Kurzatkowski et al. 2004; Chintu et al. 2004). Macro- and meso-fauna are responsible for primary decomposition while soil micro-flora (soil microbes) play a major role in nutrient cycling by releasing constituent nutrients found in organic material (Kurzatkowski et al. 2004). Laboratory incubation studies involve ground organic material mixed with soil at constant temperature and soil moisture. This excludes the activities of soil macro- and meso-fauna. However, the methods are useful for initial screening of organic materials in avoiding detailed and expensive field experiments (Mafongoya et al. 2000) and allowing assessment of the microbial potential of the soil mixed with different organic amendments (Kurzatkowski et al. 2004). The objectives of this study were to (1) assess residue decomposition, N and P release from selected winter cover crops and (2) relate mineralization of residue N and P to uptake by maize.
Materials and methods
Study site
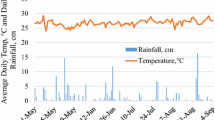
The study was done at the University of Fort Hare Research Farm (32°46′S, 26°50′E) which has a warm-temperate climate, a mean altitude of about 535 m.a.s.l, and mean monthly temperature ranges from 19 to 23°C during the summer season (October to April). The 28-year mean annual rainfall is 575 mm, most of which falls during the summer (Table 1). The soil is of alluvial parent materials and is dominated by micas in the clay fraction, with low amounts of quartz and kaolinite. The soil has 64.2% sand, 16.0% silt and 19.8% clay; pH 6.1, 0.35 g P/kg, 4.04 g K/kg and 4.25 g Ca/kg (Mandiringana et al. 2005).
Source of material used in the study
The study involved a litterbag experiment under field conditions and laboratory incubation experiments. Plant materials were collected from an on-going experiment at the University of Fort Hare Research Farm. Winter cover crops, grazing vetch (Vicia dasycarpa cv. Max), forage peas (Pisum sativum cv. Maple) and oats (Avena sativa cv. Sederberg), were planted on the 20th of June 2008; no fertiliser was applied, however, legumes were inoculated with Rhizobium leguminosarium just before planting. Control plots with no cover crops were included. The three treatments and the controls were arranged in a randomized complete block design with three replications. The gross plot was 7.2 m × 8 m in size. Supplementary irrigation was applied when necessary through sprinkler irrigation. After reaching the flowering stage, all cover crops were killed by rolling them and applying glyphosate (360 g/l) at a rate of 5 l/ha on the 21st October 2008. Maize (cv. PAN 6479) was then planted in all plots without fertilizer on the 3rd December 2008.
Litterbag experiment
Samples of cover crop biomass were collected by cutting at ground-level in each plot prior to cover crop killing, and dried at 65°C to constant weight. Although oven drying may affect mass loss rates (Mafongoya et al. 2000) biomass was dried before putting it in litterbags in order to allow comparison with other studies. A sub-sample of the plant materials was ground (< 1 mm) and analysed for total C and N content using the LECO C/N analyser (LECO Corporation 2003), P content as described by Okalebo et al. (2002) and lignin, cellulose and polyphenols by the acid detergent fibre method (Goering and Van Soest 1970).
For every plot, 10 litterbag bags were filled each with 10 g oven dried biomass material. The litterbags measured 0.20 m × 0.20 m and were made from nylon mesh with 1 mm size pores. The three treatments (type of residue) were superimposed in their respective plots. Plant materials were chopped to <5 cm before they were put into litter bags. Litterbags were placed on the soil surface and plant residues in the plots were rolled on top of the litterbags to create a firm contact between the litterbags and the soil surface to allow maximum influence of meso- and macro-fauna. Litterbags were placed in the field on the 25th October 2008 at the start of the summer season. Temperature, rainfall and irrigation regimes during cover crop decomposition in the field are summarized in Table 1.
Litterbags were sampled at fortnight intervals. For each treatment, three litterbags were removed at each event. Un-decomposed material was carefully separated from the litter bags and roots and soil particles removed. The cleaned samples were put in paper bags and oven dried at 65°C to constant weight to determine mass remaining. The effects of soil adhering to plant materials were discounted by ashing sub-samples of retrieved plant samples in a muffle furnace at 450°C for 5 h. The obtained ash weight contains the ash from the minerals in plant tissue as well as adhering soil. An extra correction factor was used to account for the minerals in the tissue. This factor (plant ash content) was determined using collected plant material free from adhering soil particles. The formula used to determine dry mass remaining in litterbags is as follows:
For the purpose of estimating N and P contribution to maize growth by decaying cover crops, two maize plants were sampled destructively per plot by cutting at their base near the soil surface at 78 days after sowing (DAS), just after flowering. Maize shoot dry weights were measured after oven drying to a constant weight at 65°C. Maize plants were then ground (<1 mm) and analyzed for total N and P using methods described Okalebo et al. (2002). Total N and P uptake by maize was taken as the product N or P concentration and maize dry weights. Nitrogen and P contribution to maize growth by the decaying cover crops was estimated as the difference in N and P uptake by maize growing on cover crop residues and maize in the control plots.
Potential N and P mineralization under laboratory incubation
The soil used for the laboratory incubation studies was taken from the top 20 cm of the soil at the University of Fort Hare Farm. It was air dried and sieved to pass through a 2 mm mesh before it was used in the incubation experiments. Ground samples (<1 mm) of the plant materials were thoroughly mixed with 50 g air dry soil, 0.2 g of grazing vetch, 0.225 g forage peas and 0.35 g oats were mixed separately with the soil. A control with no plant material added was included. These treatments mimicked biomass yields reported in field trials. The plant/soil mixtures were placed in 150 ml plastic bottles; there were 18 bottles for each treatment to allow weekly measurements for up to 6 weeks. The four treatments were arranged in a completely randomised design with three replications.
The plant/soil mixtures were brought to 70% field capacity and incubated at 27°C. Field capacity of the soil was determined as described by Okalebo et al. (2002). Soil water was maintained by periodic addition of water. Three bottles for each treatment were removed from the incubator weekly and analyzed for pH (2.5:1 water to soil suspension), inorganic nitrogen (NH4-N and NO3-N) as described by Okalebo et al. (2002) and extractable P by the Bray-1 method (NASAWC 1990). Net mineralized nutrients were obtained by the difference between values of the control and the treated soil.
CO2-C evolution under laboratory incubation
In a separate study, mineralised C from the treatments described above was estimated using air-tight glass jars containing CO2 traps. Bottles containing the soil mixture and the CO2 traps were placed in jars, sealed and incubated at 27°C. Each treatment was replicated three times. The CO2 produced by the soil in each jar was trapped in 15 ml of 0.1 M NaOH and was removed on days 1, 5, 12, 19, 26, 33, 40, 47 and at 68 days of incubation. At each of the sampling days, the jars were taken out of the incubator and the traps removed and sealed (to avoid CO2 contamination). The jars were left with the lids off for approximately an hour to replenish oxygen. Traps with fresh NaOH solution were placed in the jars which were resealed and placed back into the incubator. Carbon dioxide released was determined by back-titration with 0.1 M HCI after addition of excess BaCl2 to the NaOH to precipitate the carbonates. The net CO2-C evolved was obtained by calculating the difference in the values of the control and the biomass treated soil. Cumulative mineralised CO2-C was calculated as the sum of all previous measurements.
Data analyses
The percentage of remaining mass in the litter bags (% RM) was calculated from the remaining mass (RM t ) at each sample period (t) and the initial mass (IM0):
The average annual decomposition rate (k) of leaf litter was estimated using the single exponential function (Olson 1963), represented by the equation:
For % RM and soil pH analysis, an additional factor, time of measurement, was added to type of residue before subjection to analysis of variance. Inorganic N, extractable P and CO2-C measurements from incubation experiments, N and P contributions to maize stover by decaying cover crops in the field were also subjected to analysis of variance using the Genstat 7.1 statistical package.
Results
Decomposition in litterbags
Decrease in mass of plant material was highest in grazing vetch followed by forage peas and lastly oats (Fig. 1). Grazing vetch had the highest k values followed by forage peas with oats having the lowest value (Table 2). Percent remaining mass decreased to 50% of the original value in 20, 42 and 95 days for grazing vetch, forage peas and oats, respectively. Grazing vetch had the highest N concentration (Table 3) while C concentration was similar across the cover crops used in the study. The C/N ratio was lowest for legume cover crops (≤15) whereas oats had the highest C/N ratio of 46. With respect to other quality parameters such as lignin/N and (L + PP)/N, oats had the highest value compared to the other plant materials used in this study (Table 3). The largest proportion of the variation in decomposition rate constants of the residues was explained by the C/N ratio (Table 4).
Stover of maize grown on oat residues had significantly lower (P < 0.05) N content than that of maize grown on grazing vetch and forage peas (Table 5). However, the P content in maize stover was similar across the different cover crops. Grazing vetch contributed about 34.4 kg N/ha to maize stover growth at 78 days after maize sowing, which represented about 41.3% of the total N uptake by the maize stover. On the other hand, oats only contributed about 9.4 kg N/ha, which represented about 15.2% of the total N uptake by maize grown on oat residues.
N, P and CO2-C mineralization in laboratory incubation studies
Grazing vetch and forage peas significantly (P < 0.01) increased mineral N in the soil compared to oats (Fig. 2). Increase in extractable P differed with the type of plant material incubated with the soil. There was not much increase in extractable P up to 2 weeks of incubation, after which, grazing vetch resulted in the greatest increase in extractable P compared to other cover crops (Fig. 3). With increase in incubation time, oats followed by forage peas had the greatest C evolution while grazing vetch had the least (Fig. 4). Mixing the soil with plant materials significantly (P < 0.01) reduced pH by a similar magnitude across the different types of residues (Table 6).
Discussion
The rate of decomposition integrates the effects of the environment (air temperature and precipitation) and the biochemical composition of the plant materials (Ruffo and Bollero 2003). Mass loss over time was in the order grazing vetch > forage pea > oats and was related to the C/N ratio, lignin and polyphenol contents. The fast decay observed immediately after placing plant materials in the field is consistent with faster decomposition of the labile constituents of the plant materials (Ibewiro et al. 2000). The 20% mass loss in oats after about 20 days contrasts sharply with data from a study by Hu et al. (1999), where oats decomposed rapidly resulting in 45.3% mass loss of residues in litterbags in the first 19 days. Placing plant materials at the soil surface (this study) as opposed to incorporating into the soil (Hu et al. 1999) may have led to differences in the surface area of plant materials in contact with soil macro- and micro-fauna, resulting in lower rates of decomposition. Soil temperature and moisture differences may also explain the differences between the two studies. Plant materials were not buried in our study to mimic the practice in conservation agriculture where plant materials are not incorporated but left on the soil surface to provide soil cover.
Grazing vetch and forage peas made contributions of 41.3 and 37.5% of the total N in maize respectively, at 78 DAS. This agrees with other studies that have reported increased N uptake by crops growing on legume residues (Kuo and Jellum 2002; Miguez and Bollero 2005). Nutrient release from decaying plant materials must be synchronized with nutrient uptake by a follow-up crop (Nair 1993). Grazing vetch lost about 70% of its initial weight in the first 60 days while forage peas lost about 60% in the same period. The practice of killing winter-grown cover crops a month before planting the maize crop may allow maize to maximize nutrient uptake from decomposing legume cover crops. At 60 days after cover crop termination, maize growth would be at about 4–5 weeks, farmers usually top dress at this time.
Oats as a cover crop may not contribute much in terms of N and P to the succeeding crop, as seen by the slower decomposition rate and lower N and P contributions to maize. This has soil fertility management implications; obviously crops growing on oat residues would require higher fertilizer amounts than crops growing on legume cover crops. Fertilizer response studies may shed more light on appropriate fertilizer applications on crops growing on cover crop residues.The persistence of oats may make it particularly attractive for reducing land degradation through reducing soil erosion, improving soil organic matter and other soil quality parameters (Ruffo and Bollero 2003). Besides reducing land degradation, oat residues may also offer other benefits such as smothering of weeds as well as soil moisture conservation (Berry et al. 1987).
Nitrogen mineralization in laboratory incubation studies closely reflected field mass loss trends and chemical composition. Grazing vetch was highly degraded by soil microbes as indicated by the rapid release of N. Carbon dioxide evolution reached a maximum much earlier in grazing vetch and forage peas compared to oats. This may suggest that soil microbes were able to complete decomposition of grazing vetch and forage peas much earlier compared to oats. A C/N ratio above 25 is known to increase potential for N immobilisation in the soil (Nair 1993; Sainju et al. 2005). Oats residues in this study had a much higher C/N ratio (46), suggesting a high probability of N Immobilization.
In this study net P mineralization was in the order grazing vetch > forage peas = oats with grazing vetch mineralizing about 3.6 mg P/kg. Whether net mineralization of P occurs depends on the P content of the material. Residues with P values < 0.2% show little or no net mineralization (Floate 1970). All the materials used in this study had P contents greater than 0.2% except for oats (Table 3). Horst et al. (2001) observed that while herbaceous cover crops can contribute to increased P availability to crops, these measures cannot substitute for maintenance P fertilizer application. In similar studies, Adediran et al. (2003) reported P mineralization of 13 mg P/kg for tobacco waste and about 4 mg P/kg for pineapple waste with P values of 0.22% and 0.12%, respectively. Mafongoya et al. (2000) reported net P immobilisation when leaves of agroforestry tree species (Gliricidia sepium, Acacia nilotica), with total P content of <0.2%, were incubated with soil.
In this study, the incorporated plant residues tended to reduce soil pH. Changes in soil pH due to plant residue incorporation depend on the quality of residues, rate of application of the residues and the initial soil pH (Wong et al. 1998; Paul et al. 2001; Xu and Coventry 2003). Soils with pH values greater than those of the residues generally suffer a decrease in soil pH after treatment, while plant materials generally result in an increase in soil pH in acid soils (pH < 5). The soil pH at the start of the experiment was mildly acidic (pH = 6.1) and this may explain the decrease in soil pH observed in this study. It may be envisaged that with long-term use of cover crops, soil acidification may result.
Farmers in low external input production systems, such as those in South Africa’s smallholder irrigation schemes, will demand multiple benefits from cover crops. Reduction of land degradation may not necessarily be their overriding concern. The contribution of cover crop residues to overall crop productivity is of particular importance to these farmers. In a separate study, grazing vetch fixed approximately 111.5 kg N/ha which may translate to about 400 kg lime ammonium nitrate (28% N) with a current market value of about US$220.00. The combined effect of grazing vetch residues on soil N improvement and weed suppression resulted in the highest benefit to cost ratio of 1.9 when maize was planted without fertilization (Murungu 2010). This may make grazing vetch particularly more attractive than oats since oats require a significant investment in fertilizers while grazing vetch require less fertilization for its growth. However, smallholder irrigation farmers maybe reluctant to plant any crop that will not yield a food, feed or cash harvest. One alternative would be to graze these cover crops, leaving some residue on the surface and leaving roots in the soil—which would give a good part of the N benefit after a legume. Performance of cover crops in integrated crop/livestock systems may require further study.
Conclusions
Grazing vetch, and to a lesser extent, forage peas decompose faster increasing soil mineral N and extractable P compared to oat residues. Grazing vetch makes a significant N contribution to a succeeding maize crop. Killing legume cover crops a month before planting summer crops would synchronize nutrient release from winter-grown legume cover crops and uptake by summer cash crops. Smallholder irrigation farmers may grow grazing vetch to improve soil mineral N while the slow decomposition rates for oats are useful in improving soil organic matter and reducing land degradation.
References
Adediran JA, De Baets N, Mnkeni PNS, Kiekens L, Muyima NYO, Thys A (2003) Organic waste materials for soil fertility improvement in the border region of the Eastern Cape, South Africa. Bio Agric Hort 20:283–300
Baker CJ, Saxton KE, Ritchie WR (2002) No-tillage seeding: science and practice, 2nd edn. CAB International, Oxford
Bembridge TJ (2000) Guideline for rehabilitation of small-scale irrigation schemes in South Africa. Water Research Commission, Report no. 891/00, Pretoria, South Africa
Berry WAJ, Mallett JB, Greenfield PL (1987) Water storage, soil temperatures and maize (Zea mays L.) growth for various tillage practices. S Afr J Plant Soil 4:26–30
Chintu R, Zaharah AR, Wan Rasidah AK (2004) Decomposition and nitrogen release patterns of Paraserianthes falcataria tree residues under controlled incubation. Agrofor Syst 63:45–52
Daudu CK, Uyovbisere E, Amapu YI, Onyibe JE (2006) Qualitative and quantitative evaluation of four organic materials as nutrient resources for maize in the Nigerian Savanna. J Agron 5:220–227
Derpsch R (2003) South Africa Report, promotion of conservation agriculture. Project Number TCP/SAF/2902. National Department of Agriculture Final Report, South Africa
Derpsch R (2005) The extent of conservation agriculture adoption worldwide: implications and impact. Paper presented to III world congress on conservation agriculture, Nairobi, Kenya, October 2005. http://www.fao.org/ag/ca/en/www.rolf-derpsch.com. Accessed on 25 May 2007
Fanadzo M (2007) Weed management by small-scale irrigation farmers—–the story of Zanyokwe. SA Irrigation 29:20–24
Floate MJS (1970) Decomposition of organic materials from hill soils and pastures. II. Comparative studies on the mineralization of carbon, nitrogen and phosphorus from soil. Soil Bio Biochem 2:173–185
Fowler R (1999) Conservation tillage research and development in South Africa. In: Kaumbutho PG, Simalenga TE (eds) Conservation tillage with animal traction. ATNESA, Harare, pp 51–60
Fowler R, Rockstrom J (2001) Conservation tillage for sustainable agriculture: an agrarian revolution gathers momentum in Africa. Soil Till Res 61:93–108
Giller KE, Witter E, Corbeels M, Tittonell P (2009) Conservation agriculture and smallholder farming in Africa: the heretics’ view. Field Crop Res 114:23–34
Goering HK, Van Soest PJ (1970) Forage fiber analysis (apparatus, reagents, procedures and some applications). USDA Agriculture Handbook No. 379
Hobbs PR (2007) Conservation agriculture: what is it and why is it important for future sustainable food production? J Agric Sci 145:127–137
Horst WJ, Kamh M, Jibrin JM, Chude VO (2001) Agronomic measures for increasing P availability to crops. Plant Soil 237:211–223
Hu SJ, van Bruggen AHC, Grunwald NJ (1999) Dynamics of bacterial populations in relation to carbon availability in a residue-amended soil. Appl Soil Eco 13:21–30
Ibewiro B, Sanginga N, Vanlauwe B, Merckx R (2000) Nitrogen contributions from decomposing cover crop residues to maize in a tropical derived savanna. Nutr Cycl Agroecosyst 57:131–140
Kuo S, Jellum EJ (2002) Influence of winter cover crop residue management on soil nitrogen availability and corn. Agron J 94:501–508
Kurzatkowski D, Martius C, Höfer H, Garcia M, Förster B, Beck L, Vlek P (2004) Litter decomposition, microbial biomass and activity of soil organisms in three agroforestry sites in central Amazonia. Nutr Cycling Agroecosyst 69:257–267
Laker MC (2004) Advances in soil erosion, soil conservation, land suitability evaluation and land use planning research in South Africa, 1978–2003. S Afr J Plant Soil 21:345–368
LECO Cooperation (2003) Truspec carbon/nitrogen determinator. Leco Cooperation 3000. Lakeview Avenue St Joseph, M149085-2396, USA
Lupwayi NZ, Girma M, Haque M (2000) Plant nutrient contents of cattle manures from small-scale farms and experimental stations in the Ethiopian highlands. Agric Ecosyst Environ 78:57–63
Mafongoya PL, Barak P, Reed JD (2000) Carbon, nitrogen and phosphorus mineralization of tree leaves and manure. Bio Fert Soils 30:298–305
Mandiringana OT, Mnkeni PNS, Mkile Z, van Averbeke W, Van Ranst E, Verplancke H (2005) Mineralogy and fertility status of selected soils of the Eastern Cape Province, South Africa. Comm Soil Sci Plant Anal 36:2431–2446
Miguez FE, Bollero GA (2005) A review of corn yield response under winter cover cropping systems using meta-analytic methods. Crop Sci 45:2318–2329
Mills AJ, Fey MV (2004) Declining soil quality in South Africa: effects of land use on soil organic matter and surface crusting. S Afr J Plant Soil 21:388–398
Mkile Z (2001) The use and agronomic effectiveness of kraalmanure in the Transkei region of the Eastern Cape, South Africa. MSc. Dissertation, University of Fort Hare, Alice, South Africa
Murungu FS (2010) Evaluation and management of cover crop species and their effects on weed dynamics, soil fertility and maize (Zea mays L.) productivity under irrigation in the Eastern Cape Province, South Africa. PhD thesis. University of Fort Hare, South Africa
Nair PKR (1993) An introduction to agroforestry. Kluwer, Dordrecht
Non-Affiliated Soil Analysis Work Committee (NASAWC) (1990) Hadbook of soil standard soil testing methods for advisory purposes. Soil Science Society of South Africa, Pretoria
Okalebo JR, Gathua KW, Woomer PL (2002) Laboratory methods of soil and plant analysis. A working manual (2nd edn). SACRED Africa, Nairobi Office, P. O. Box 79, The Village Market, Nairobi, Kenya
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Palm CA (1995) Contributions of agroforestry trees to nutrient requirements of intercropped plants. Agrofor Syst 30:105–124
Paul KI, Black AS, Conyers MK (2001) Effect of plant residue return on the development of surface soil pH gradients. Biol Fertil Soils 33:75–82
Ruffo ML, Bollero GA (2003) Modeling rye and hairy vetch residue decomposition as a function of degree days and decomposition days. Agron J 95:900–907
Sainju UM, Whitehead WF, Singh BP (2005) Bi-culture legume-cereal cover crops for enhanced biomass yield and carbon and nitrogen. Agron J 97:1403–1412
Vanlauwe B, Swift MJ, Merckx R (1996) Soil litter dynamics and N use in Leucaena alley cropping systems in Southwestern Nigeria. Soil Biol Biochem 28:739–749
Wong MTF, Nortcliff S, Swift RS (1998) Method for determining the acid ameliorating capacity of plant residue compost, urban waste compost, farmyard manure, and peat applied to tropical soils. Commun Soil Sci Plant Anal 29:2927–2937
Xu RK, Coventry DR (2003) Soil pH changes associated with lupin and wheat plant materials incorporated in a red–brown earth soil. Plant Soil 250:113–119
Acknowledgments
This document is an output from a project funded by the Govan Mbeki Research and Development Centre (GMRDC), University of Fort Hare. The views expressed are not necessarily those of GMRDC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murungu, F.S., Chiduza, C., Muchaonyerwa, P. et al. Decomposition, nitrogen and phosphorus mineralization from winter-grown cover crop residues and suitability for a smallholder farming system in South Africa. Nutr Cycl Agroecosyst 89, 115–123 (2011). https://doi.org/10.1007/s10705-010-9381-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-010-9381-5