Abstract
A randomized field experiment with three replicates was conducted in the subtropical region of China to investigate the effects of integrated rice-duck system (RD) on methane (CH4) emission, active soil organic carbon fractions and their relationships in 2007 and 2008, compared with conventional rice system (CK). Methane emissions were measured at 7–9 days intervals using a closed static chamber technique, and two fractions of active soil organic carbon, namely, dissolved organic carbon (DOC) and microbial biomass carbon (MBC), were analyzed simultaneously. Soil DOC and MBC in RD and CK had similarly distinct seasonal variation patterns within the 2 years. During this time DOC and MBC concentrations were low at the early growth stage, increased during panicle differentiation and heading period, and dropped during grain filling period of rice. CH4 emission fluxes from RD and CK followed a similar seasonal variation pattern both in 2007 and 2008. Two peaks of CH4 emission were observed, the first at the tillering stage, second at panicle differentiation and heading stage. The CH4 cumulative emission was reduced in RD by 19.3 and 19.6% in 2007 and 2008, respectively, compared with CK. Seasonal variation pattern of CH4 emission was regulated by soil DOC, MBC and soil temperature, all of which were significantly positively correlated with methane emissions. Improvement in soil redox status was the predominant reason for significant reduction of CH4 emission in RD. These results clearly indicate that integrated rice-duck system could be an effective mode of rice farming for decrease in methane emission in southern China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice paddy has been identified as one of the major anthropogenic sources contributing about 10–15% to global CH4 emissions with an estimated annual emission of 50–100 Tg of methane (Reiner and Milkha 2000). So it is urgent to control methane emission from rice paddy for mitigating the global warming. Methane emission is the result of its production and oxidation in the soil most of which is transported from soil to atmosphere through rice plants (Krüger et al. 2001). CH4 emission fluxes from different wetland ecosystems usually range from 0.47 to 171.12 mg m−2 h−1 (Wei 2000). Spatial and temporal variation of CH4 emission is regulated by a variety of agronomic and environmental factors and the complex interactions of the whole system of rice plant, soil and atmosphere (Jean and Pierre 2001; Wang and Li 2002). Soil organic carbon (SOC) is the major substrate for methanogens (Penning and Conrad 2007), thus closely relating to methane production. Early studies proved that soil organic carbon content was significantly correlated with methane production (Yao et al. 1999). However, SOC consists of different C fractions, and the nature of different soil organic carbon fractions affects the rate of both CH4 production and consumption (Mitra et al. 2002). Soil dissolved organic carbon (DOC) and microbial biomass carbon (MBC) have been identified as the important fractions in active soil organic carbon pool (Lutzow et al. 2007). Recent researches revealed that the root-derived CH4 production and emission were positively related to DOC concentration in the rhizosphere (Lu et al. 2000a), because the root-derived DOC served as a major C source for methanogenic activity (Reiner and Milkha 2000). However the relationship between active soil organic carbon fractions and methane emission has not been studied thoroughly in different rice-based production system.
Rice fields in China have been considered as an important contributor to the budget of CH4 emission (Wang and Li 2002). Paddy field is an anthropogenic regulated ecosystem, where any cultural practices controlling either methane production, oxidation, or transport processes could be the possible strategies for inhibiting CH4 emission (Yagi et al. 1997). Wetland rice-duck integrated system has a long history in China and is a type of rice-based production model combining duck breeding in south China. Some published studies have documented that this system has preferable ecological benefits and relatively high economic benefits because of its integrated utilization of resources in paddies (Wang 2000; Wang et al. 2003b; Yu et al. 2005). The advantages of rice-duck integrated system is to utilize the habits of ducks, such as moving and looking for food industriously, to help control plant diseases, pests, and weeds, thus reducing pesticide application. Raising ducks in the paddy has not decreased or increased the rice yield (Yu et al. 2005), but did increase the income from the resulting duck products. The decline of methane emission from rice-duck integrated system had been preliminarily observed by some researchers (Huang et al. 2005; Xiang et al. 2006), who considered it the result of increased dissolved oxygen concentration in floodwater and soil which inhibited the activities of methanogens and thus enhanced CH4 oxidation rate (Zheng et al. 1997; Kumaraswamy et al. 2000; Wang 2000). In recent years, the rice-duck system has been extended as one of eco-agriculture models for environmental friendly rice production in China by Ministry of Agriculture. For example, the extension area of the rice-duck system in Hubei province reached to 140,620 ha in 2007, about 7% to the total rice planting area in this region (Hubei Provincial Bureau of Statistics 2008). However, higher labor costs and water demand restrict its promotion area to some extent. In the future, new operation mode should be explored to overcome its constraints and maximize its benefit.
However, so far the effects on methane emission and its dynamics of rice-duck systems have not been investigated thoroughly. Therefore, the main objectives of this study were:(1) to obtain information on the dynamics and quantification of methane emission from the rice-duck system, and to evaluate the potential impact of the system on methane emission; (2) to examine the relationships of active soil organic carbon fractions and methane emission to provide management strategies for soil carbon management for CH4 mitigation.
Materials and methods
Site description
The experiment was carried out in 2007 and 2008 at the Experimental Farm of Huazhong Agricultural University, Hubei Province, China. The experimental site is located at 30°37′-N latitude and 114°21′-E longitude at an altitude of about 23 m above sea level. This region has a humid mid-subtropical monsoon climate with an average annual temperature of 16.7°C, a mean annual precipitation of 1,220 mm, and most of the rainfall occurring between April and August. The subsurface water table is about 1.5 m. The main soil properties (0–20 cm depth) of this study site were described as follows: pH, 6.8; total N, 1.05 g kg−1; total P, 1.17 g kg−1; organic matter, 16.01 g kg−1; NO3 −-N, 12.1 m g kg−1; and NH4 +-N, 0.52 mg kg−1. Rapeseed was planted from October to May every year before rice transplanting. Residues of rapeseed were cleared away in 2007, but returned to field and buried when the field was ploughed in 2008.
Materials
Rice variety was Liangyoupeijiu (Oryza sativa L.), which is a predominant local middle-rice variety and provided by Crop Production, Physiology and Ecology Center, Huazhong Agricultural University. Duck variety was native Sheldrake (Tadorna, provided by the stock farm of Huazhong Agricultural University).
Experimental design and field management
The experimental plots were allotted to two treatments: (1) conventional rice fields with ducks reared in them, viz. integrated rice-duck ecosystem (RD), and (2) conventional rice fields without ducks rearing (CK). The two treatments were arranged in a randomized complete block design with three replicates. The area of each experimental plot was 140 m2.
After the experimental paddy field was ploughed, N (urea), P (CaHPO4) and K (K2SO4) fertilizer were applied as basal fertilizers at the standard rate of 225 kg N ha−1, 135 kg P2O5 ha−1 and 135 kg K2O ha−1 on May 25 in 2007 and May 22 in 2008. Rice was transplanted and harvested on May 31 and September 8 in 2007, and on May 27 and September 10 in 2008. Six 20-day-old ducklings were introduced into the paddy fields (RD) with a density of 450 ducks per hectare on June 15 in 2007 and June 14 in 2008. These paddy fields were surrounded with nylon nets (50 cm in height) and a shed for the ducks was also built in the corner of each field. These ducks were retrieved on August 20 in 2007 and 2008. A standing water depth of about 5 cm was maintained in the field of the two treatments during the period of raising ducks. After ducks retrieval, no irrigation was done. All management and agronomic practices were conducted the same for the two experimental treatments.
CH4 sampling and measurements
CH4 measurements were taken during rice growth stage at 7–9 days intervals on three sampling locations in each plot in 2007 and 2008. The static chamber technique was adopted for sampling CH4 (Crill et al. 1988) using 60 cm × 60 cm × 110 cm chests made from transparent plexiglass and the joints were sealed with silicone grease to make them leak proof. The day before gas sampling, a bamboo springboard between the paddy field ridge and the chamber was put to reduce disturbance to the soil by walking at each sampling location. Then a stainless chamber base (60 cm × 60 cm × 10 cm, with the water trough 1.5 cm wide to accommodate the chambers surrounding the top of it) was inserted into the soil at each sampling location to ensure a minimum disturbance to the soil at the time of gas collection in the chambers. Water was added into the water trough of the chamber base after inserting the chamber in it to prevent gas leakage. The gas sampling was carried out between 0900 hours and 1100 hours. Six hills of rice seedlings were covered in each sampling chamber. Each sampling was a composite of 4 individual measurements taken at 10-min intervals. The gases in the chest were at first mixed by a fan on the top of the box, then drawn off by using a syringe, and immediately transferred into a 20 ml vacuum glass container. Samples of three replications were taken from each plot and the average was taken as representative value for that plot.
The gas samples were analyzed by gas chromatography by using the gas chromatograph meter (Shimadzu GC–14B), fitted with a 6–1/8-ft stainless-steel column (Porapack N) and a flame ionization detector (FID). For determination of methane, N2 (flow rate: 330 ml min−1), H2 (flow rate: 30 ml min−1), and zero air (flow rate: 400 ml min−1) were used as the carrier, fuel, and supporting gas, respectively. Column, injector, and detector temperature were set at 75, 50, and 180°C, respectively. The gas emission flux was calculated from the difference in gas concentration according to the equation of Zheng et al. (1998):
where F is the gas emission flux (mg m−1 h−1), ρ is the gas density at the standard state, h is the height of chamber above the soil (m), C is the gas mixing ratio concentration (mg m−3), and T is the mean air temperature inside the chamber during sampling. Average flux and standard error of CH4 emissions were calculated from three replicates. Total amount of CH4 emissions was calculated from the summation of methane emission in several growth stages of rice.
Measurements of active soil organic carbon fractions
When gas samples were collected, soil samples were collected synchronously. Soil (0–20 cm soil depth) was sampled at five random positions in each plot. Some of fresh soil samples were drawn out and mixed to measure soil dissolved organic carbon (DOC), soil microbial biomass carbon (MBC).Then other soil samples were air-dried and sieved through a 0.25-mm sieve for measurement of total soil organic carbon (TOC) Soil TOC was determined by the potassium dichromate oxidation method, with using 0.8 mol l−l K2Cr2O4-H2SO4 solution at 170–180°C (oil bath).
Soil DOC was extracted from 15 g fresh soil with 30 ml distilled water on a shaker for 30 min, followed by centrifugation at 4,000 rpm for 15 min and vacuum filtration through 0.45 mm filters (Wright et al. 2005). Then the filtrate was measured by the potassium dichromate oxidation method to determine soil DOC.
Soil MBC was measured by fumigation extraction method (Vance et al. 1987). Two 10 g portions of fresh soil were weighed into 100 ml capacity beakers. One portion served as control and was extracted immediately with 0.5 mol l−1 K2SO4. The other portion was fumigated for 24 h in a vacuum container. The fumigated soil samples were extracted with 30 ml 0.5 mol l−1 K2SO4 for 30 min. Organic carbon in the soil extracts was measured by dichromate oxidation and soil MBC was calculated as followed: MBC = K EC × EC, where, EC was organic C extracted from fumigated soil minus organic C extracted from unfumigated soil, K EC was the extraction efficiency factor of 2.22.
Soil Eh at a depth of 5 cm was measured with a portable Eh meter (FJA-3, Nanjing Chuan-Di Instrument & Equipment CO., LTD.Nanjing, China). A thermometer (Delta-T Logger) was installed to each plot to record soil temperature (5 cm below soil surface).
Statistical analysis of data
Statistical analyses were carried out by using SPSS 11.5 for Windows and the results were expressed as mean values of three replicates. The means were compared by least significant difference tests (LSD) method for each treatment in the same year. Then, linear correlation analyses were performed to determine relationships between CH4 fluxes and these parameters, and the significance probability levels of the results were given at the P < 0.05 (*) and P < 0.01 (**), respectively.
Results
Soil temperature and soil redox potential (Eh)
Variations of soil temperature(ST) in the plots are given in Fig. 1. The temperature varied slightly from 22 to 32°C,with the features of lower ST at the early and later growth stage of rice, and higher at the middle growth stage of rice (July and August). During rice growth, mean ST in RD were 26.75°C in 2007 and 28.20°C in 2008, and mean ST in CK were 26.95 and 28.07°C in 2007 and 2008. There was no significant difference between RD and CK within the 2 years, however the mean ST in 2008 were 1.45°C (P < 0.01) in RD and 1.12°C (P < 0.05) in CK higher than those in 2007.
Table 1 shows soil redox potential (Eh) in paddy fields. In 2007 and 2008, the soil Eh followed a similar trend of declining with rice growth before duck retrieved. After ducks were reared, compared to CK, soil Eh in RD was significantly higher (P < 0.01). Especially in the early period of duck rearing, soil Eh in RD increased rapidly, but the rate slowed down with the time, then in the late stage of duck-rearing, there was no significant difference in soil Eh between RD and CK. During duck rearing, the mean soil Eh in RD were −126.8 and −144.2 mV in 2007 and 2008, respectively, and the mean soil Eh in CK were −143.5 and −166.3 mV in 2007 and 2008, respectively, which were significantly lower than those in RD (P < 0.01). For both of the treatments, soil Eh in 2008 was significantly lower than that in 2007 (P < 0.01).
Seasonal variations and amounts of CH4 emission
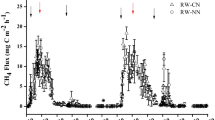
As shown in Fig. 2, CH4 emission fluxes in CK and RD were relatively low just after flooding, subsequently increased, and remained relatively high during duck rearing, but decreased rapidly to the lowest values at harvesting. Two peaks appeared in the early tillering stage and the late heading stage of rice in 2007. In 2008, one occurred in the late tillering stage and the other in the early heading stage similarly for both of RD and CK. During rice growth, the mean CH4 emission flux in RD was 7.68 mg m−2 h−1 in 2007and 19.62 mg m−2 h−1in 2008, which were 21.3% (P < 0.05) and 17.0% (P < 0.01) lower than that in CK respectively. Especially during the period of rice-duck coexisting, rearing ducks in paddy field had a positive effect on mitigation of CH4 emission. However, there was no significant difference in CH4 emission flux before ducks introduced and after ducks were retrieved.
Table 2 shows CH4 emission during different growth periods of rice in different treatments. Most of the methane was emitted during the tillering stage and heading stage of rice for both RD and CK. During rice growth in 2007 and 2008, the amount of CH4 emission from CK were 1.24 times (P < 0.01) of those from RD within the 2 years, respectively.
Dynamics of active soil organic carbon fractions
Variations of dissolved soil organic carbon (DOC) in the plots are shown in Fig. 3. The pattern of DOC dynamics in RD was similar as that in CK both in 2007 and 2008. The concentration of DOC in CK and RD reached to the highest peaks at the heading stage of rice, and afterwards decreased dramatically to the lowest values after drainage of the paddy field in 2007 and 2008. However variations of DOC during tillering stage and panicle differentiation stage (from transplanting of rice to July 20) in 2007 was quite different from that in the same period of 2008. During this period in 2007, the concentration of DOC dropped slightly after transplanting of rice, then increased slowly until after maximum tillering stage. While in the same period in 2008, the concentration of DOC increased slowly, and then decreased soon after maximum tillering stage, and then increased to a maximum at the heading stage. As shown in Table 3, there was no significant difference in seasonally averaged amount of DOC in 2007 between the two treatments (P > 0.05). However, seasonally averaged concentration of DOC in RD significantly higher than that of CK in 2008 (P < 0.01). Seasonally averaged concentration of DOC in 2008 was higher than that in 2007 for both of RD and CK. Return of rape residues to the experimental field in 2008 might be the one of major reasons for the interannual difference.
As can be seen in Fig. 4, similar dynamics of soil microbial biomass carbon (MBC) occurred both in 2007 and 2008. After rice transplanting, the concentration of MBC increased steadily, and then reached a maximum value at the panicle differentiation, and then decreased gradually up to the rice ripening stage. In July and early August of 2007 and 2008, the concentration of MBC was higher than that in June and the period of rice ripening. As shown in Table 3, seasonally averaged concentration of MBC in RD was slightly higher than that of CK in 2007 and 2008 (P > 0.05). However, it was markedly higher in concentration of MBC in 2008 than that in 2007 for both of RD and CK.
Growth stages and grain yield of rice
As shown in Table 4, RD had similar growth stages of rice as compared to the CK. From transplanting to harvesting date of rice, both of the treatments took 100 days in 2007 and 105 days in 2008. In RD, rice and ducks coexisted in the field for 66 days in 2007 and 67 days in 2008. There was also no significant difference in grain yields between the two treatments. The presence of ducks did not impact rice growth and reduce the total yields of rice grain. Moreover, incomes could be obtained from duck products. Similar results have been reported in earlier studies (Wang 2000; Xiang et al. 2006). However, the rice grain yield of RD in 2008 was 18.5% higher than that in 2007 (P < 0.01); similarly, the yield of CK in 2008 was 17.0% higher than that in 2007 (P < 0.01).
Discussion
Seasonal variations of CH4 emission from middle-rice field
Different patterns of seasonal variations in methane emission flux from rice fields have been found in different rice cropping systems, with normally two or three emission peaks observed during the rice growing season (Wang and Li 2002). In the present study, two pronounced peaks were detected in 2007 and 2008 (Fig. 2). Initial peak of CH4 efflux was observed at active vegetative growth stage of rice (complete tillering stage) at about 30 days after transplanting, which may be associated with (1) microbial decomposition of left over plant residues (Cai 1997; Xu et al. 2000); (2) vigorous respiration of rice plant in tillering stage. This is conducive to promote CH4 emission, because most of CH4 is emitted through plants (Butterbach-Bahl et al. 1997). The second CH4 flux maxima occurred in panicle differentiation and heading period of the rice plants, which were mainly attributed to (1) the higher availability of substrates through root exudation or decaying plant residues for methanogenic bacteria in the rice rhizosphere (Adhya et al. 1994; Mitra et al. 2005); (2) highly reduced conditions in the rice rhizosphere (Adhya et al. 1994; Towprayoona et al. 2000). Soil temperature also is an important influencing factor for occurrence of this CH4 emission pattern (Jean and Pierre 2001). As can be seen from Fig. 1, at the full tillering and heading stage of rice, soil temperature was higher than that in early tillering stage and ripening stage, making the full to heading stage more suitable for microbiological reactions thus promoting methane production. As shown in Table 5, soil temperature positively correlated with CH4 emission flux both in RD (r = 0.5263, P < 0.01) and CK(r = 0.4998, P < 0.01). Therefore, seasonal variation pattern of CH4 emission in the experiment were possibly the combined effect of soil carbon substrates supply, rice plant growth and soil temperature.
As shown in Fig. 2 and Table 2, the seasonally averaged CH4 emission flux and amounts of RD and CK in 2008 were about 2.5–2.7 times of those in 2007. Similar interannual differences of CH4 emission have been found by others (Wang and Li 2002). Though it is very difficult to explain the interannual differences of emission, differences in climatic conditions, residue management of pre-crops are closely related to this without doubt. Residues of rapeseed were only returned and buried into the experimental field in 2008, which might be one important reason for significant higher CH4 emission in 2008. It has been documented that incorporation of fresh crop straw into rice paddy soils enhanced CH4 emission (Singh et al. 1996; Cai 1997; Zou et al. 2005), usually triggering the fastest response at 2 or 4 weeks after incorporation (Lu et al. 2000b). Higher average soil temperature during tillering, panicle differentiation and heading stage of rice in 2008 than that in 2007 might be another important influencing factor (Fig. 1).
Seasonal variations of DOC and MBC in middle-rice fields
As soil organic carbon is a heterogeneous mixture of organic substances, the different forms or fractions of SOC might have different sensitivity on the changes of external environment, as well as different effects on soil ecological process. Dissolved organic carbon (DOC) is one of the important fractions in active soil carbon pool. Although its proportion in total soil organic carbon pool is quite small (Haynes 2005), and ranged from 4 to 5.5% in our study (Table 3), DOC plays an important role in the biogeochemical cycling of C, N, P and S (Kalbitz et al. 2000). Sources of DOC are complex, mainly including the degradation products of exogenous organic matter and humus, microbial biomass and rhizosphere exudates (Kalbitz et al. 2000; Harrison et al. 2008). DOC concentration in soil solution may be controlled by the interactive effect of biotic and abiotic factors, particularly by biotic factors (Kalbitz et al. 2000; Luo et al. 2009). Some previous studies indicated that rising temperature might increase DOC concentration in soil (Freeman et al. 2004; Harrison et al. 2008). The significantly positive correlations between DOC and soil temperature also were detected both for RD (r = 0.5752, P < 0.01) and CK (r = 0.4521, P < 0.01) in our study (Table 5). The reason may be that higher temperature activates biological activity, enhances decomposition of litter and organic matter in soil (Robinson 2002; Aerts 2006), thus increases sources of DOC. Exogenous organic matter, such as crop residues, feces, usually containing high soluble and potentially soluble biodegradable organic fractions, and is considered an most important source of DOC (Kalbitz et al. 2000). Incorporation of rape residues in 2008 might be one of the major reasons for higher DOC concentration than that in 2007 (Fig. 3; Table 3), and higher soil temperature in 2008 might be another contributor. One possible conclusion is that input of duck feces and remnants to the paddy soil during rice-duck cooperation period might be the reason for increase of soil DOC in rice-duck integrated system (Table 3), however, it was inconsistent between the 2 years, and further research is needed to understand the mechanisms of DOC dynamics in rice-duck system. Inputs of carbon to soil were mainly governed by the processes of root growth and root exudates (Cleveland et al. 2004), for example, about 200 kg-C ha−1 in root rhizosphere obtained from rice plant during rice growth period (Lu et al. 2002a; Watanabe et al. 2004), and this may be the main source of the DOC in soil. By using the method 13C labeling, Lu et al. (2004) estimated that around 34% of DOC came from photosynthesis during rice growth. The root exudation rate increases with the advancing rice growth until heading stage but decreases at maturity stage (Aulakh et al. 2001). Hence higher DOC concentration in soil was measured from the late July to the early August in our study (Fig. 3), probably as a result from higher soil temperature combined with larger amount of root exudates and rice senesced litter as well as vigorous activity of microbes. Towards the end of the rice growth, a dramatic decline in dissolved organic carbon content occurred in our experiment (Fig. 3), and similar result was recorded by Lu et al. (2000a), which might be attributed to decline in root exudation, decomposition of dissolved organic carbon pool and decrease in soil temperature.
MBC represents the quantity of microbes in the soil, and is regarded as one of the most labile fractions of SOC which is turned over very rapidly (Lutzow et al. 2007). Pool size of MBC in our study was detected as the result of 2.3–2.5% of TOC (Table 3), which was comparable to 2–4% reported by Reichardt et al. (1997). Though its percentage of the TOC is low, MBC is very susceptible to soil management practices and environmental change (Hargreaves et al. 2003) and plays an important role in nutrient cycling in rice soil (Conrad 1996). Previous studies indicated that MBC had obvious seasonal variations during rice growth (Reichardt et al. 1997; Lu et al. 2002b), which was related to the rice growth process, field management practices, and seasonal changes of environmental factors. Our research showed that MBC changed slightly at first and then significantly increased during the period of maximum tillering to heading of rice, then a rapid decrease at grain filling stage (Fig. 4). Slow change of MBC during the early tillering stage was likely due to a decrease in aerobic populations probably with a concurrent off-setting increase in anaerobes (Reichardt et al. 1997; Bai et al. 2000; Lu et al. 2002b). Less carbon substrate and lower temperature in this period might be other reasons for the steady change of MBC. The increase in MBC during the period of maximum tillering to heading of rice could be the effect of the enhanced quantity of root exudates that acted as C-substrates for microbes (Nayak et al. 2007) and the rise in soil temperature. A significantly positive correlation also was detected between MBC and soil temperature for both RD (r = 0.6273, P < 0.01) and CK (r = 0.6150, P < 0.01) in our experiment (Table 5). In contrast in other studies, low root exudation at grain filling stage (Aulakh et al. 2001) possibly resulted in lower MBC. More studies have shown that application of organic amendments, such as compost or crop residues, could increase MBC in paddy soil (Murase et al. 2006; Hao et al. 2008). Therefore, return of rape residuals to the paddy field likely increased the content of MBC in 2008 compared with that in 2007 (Fig. 4). Soil DOC and MBC are important parts of the soil carbon pool, which inevitably interacts in the process of soil organic carbon transformation. Some studies revealed that DOC played an important role in the delivery of labile C and nutrients to microbial communities (Cleveland et al. 2004). Positive correlation between DOC and MBC was found both in RD (r = 0.2823, P < 0.05) and CK (r = 0.3382, P < 0.01) (Table 5). Rearing ducks in our study did not significantly increase the content of MBC in soils, however changes in microbial community structure has been reported by (Fu et al. 2008), and the diversity of soil microbes was revealed in our previous study (Zhan et al. 2008).
Relationships between CH4 emission and active soil organic carbon fractions
CH4 production and emission is an essential link of carbon cycling in paddy field. CH4 is the dominant terminal degradation product of soil organic materials by microbes in submerged rice fields (Krüger et al. 2001), therefore the amount and quality of soil organic carbon inevitably affects the process of CH4 production (Wassmann et al. 1998; Penning and Conrad 2007). Some research indicates that regulation of seasonal variation of CH4 flux is under the control of organic carbon available in the soils (Lu et al. 2000b; Das and Baruah 2008). As shown in Table 5, the content of total soil organic carbon significantly correlated with CH4 emission fluxes both in RD (r = 0.3448, P < 0.01) and CK (r = 0.2680, P < 0.05). Similar findings had been reported by Wassmann et al. (1998). Further, the labile carbon pool may indicate the substrate-supplying capacity to soil microorganisms (Wang et al. 2003a). Such positive correlations were observed between CH4 emission flux and DOC concentration (Lu et al. 2000a). Significantly positive correlation between CH4 emission flux and DOC concentration was detected in RD (r = 0.5635, P < 0.01), as well as in CK (r = 0.5139, P < 0.01). Furthermore, MBC concentration was found to positively correlate with CH4 emission flux both in RD (r = 0.4749, P < 0.01) and CK (r = 0.5211, P < 0.01), possibly because higher MBC indicated larger population of methanogenic bacteria in flooded paddy soil. Few studies have been previously reported relationships between MBC and CH4 emission. Therefore, higher concentration of DOC and MBC were important reasons for higher CH4 emission flux during panicle differentiation and heading period, while lower concentration of DOC and MBC contributed to lower CH4 emission during late grain filling stage of rice in our experiment. The results suggested control DOC in paddy field could be an effective option for mitigation of CH4.
Mitigation of CH4 emission from rice-duck ecosystem
Rice-duck integrated system mitigated methane emission significantly from paddy soils, especially at the higher CH4 emission stages (Fig. 2; Table 2), which was in agreement with the findings reported by some researchers (Deng et al. 2004; Huang et al. 2005; Xiang et al. 2006). There are several reasons for the change of CH4 emission from paddy field after introducing ducks into the field. Duck activities in the paddy field inevitably brought about alteration in soil environment and rice growth, and then lead to soil microbial activity and carbon cycle changes. The marked effects of duck activities on soil environment were likely the result of increasing the dissolved oxygen content in the water body (Fu et al. 2008; Li et al. 2009 ) and, thus, improved soil redox status (Table 1). This would constrain the activity of methanogenic bacteria, thus decreasing the methane production rate, as well as promoting the activity of methane-oxidizing bacteria to induce more CH4 oxidization. On the one hand, walk and stir of ducks in the field accelerated the rate of oxygen dissolved in the water body. On the other hand, our previous study (Wang et al. 2006) and other scientists (Wang et al. 2003b) have demonstrated that ducks can suppress weeds, planktons, and other aerobic organisms in paddy fields, therefore, the consumption of dissolved oxygen by those organisms were decreased. These two aspects possibly enhanced the movement of oxygen into the soil. Moreover, the habit of ducks such as feeding, disturb the soil, enhance the gas exchange between the soil and the atmosphere, increased the contact opportunity of soil with oxygen, and ameliorated the soil redox status (Wang et al. 2003b). Redox potential (Eh) is used as an index for the oxidation–reduction conditions in paddy soil, which is considered as one of the most important factor for CH4 emission. Generally, CH4 emission occurs at lower than -150 mV and emissions increase with continued decreases in the soil Eh (Wang et al. 1993; Gao et al. 2002). Our study (Table 1) and some previous reports (Huang et al. 2003; Wang et al. 2003b; Fu et al. 2008) indicated that the paddy redox status was improved distinctly in the rice-duck system, which created an unfavorable environment for the activity of methanogenic bacteria. Fu et al. (2008) found population of methanogenic bacteria was decreased in the rice-duck ecosystem. Another important contribution to reduction of CH4 emission in rice-duck integrated ecosystem might be the increase of CH4 oxidation. Methane can be oxidized in the water–soil interface and in rhizosphere during the flooding period by aerobic methane-oxidizing bacteria, whose activity needs oxygen and this activity can remain vigorous even at low oxygen level (Wang and Li 2002). Methane oxidation in the rice duck system could also have been strengthened in the rhizosphere due to higher secretion of oxygen by rice root. This would be dependent on the rearing ducks in the paddy field promoting the growth of rice (Yu et al. 2005). However, there is no conclusive evidence of enhanced CH4 oxidation in rice-duck integrated system, and further research should be carried out in the future research. At last, food intake of ducks decreased the number of weeds and unproductive tillers of rice), and accordingly reduced the amount of methane production resulting from their decay and decomposition under strictly anaerobic conditions (Huang et al. 2005).
Feeding activities of ducks might enhance CH4 emission. First, duck feces and remnants could be decomposed in the water flooding, resulting in oxygen consumption and decreased soil redox status. Second, input of duck feces and remnants to the paddy soil might be the reason for increase of soil DOC in rice-duck integrated system. Our and other results indicated that soil DOC had positive correlation with CH4 emission flux (Lu et al. 2000a; Ding and Cai 2002; Song et al. 2004), thus promoted CH4 emission. Third, promotion effect of duck activities on vitality on rice root (Yu et al. 2005) might increase root exudates, which provided more favorable degradable organic matter for methanogenic bacteria, thus enhanced CH4 production. Lastly, duck feces in the paddy also produced methane. Methane emissions from enteric fermentation of the duck tend to be smaller than feces, and 0.03 kg CH4 could be generated from feces decomposition of one duck per year (IPCC 2006). Estimated according to this data, the amount of methane emission from ducks’ feces was 0.39 g m−2 in RD. This was a very small portion of the total methane emission from paddy, and had been included in the result of methane emission detected because feces were evacuated to the field as ducks moved about in the paddy. Nevertheless, the inhibitory effect on methane emissions of duck activities exceeded the promotive effect in rice-duck integrated system. Thereby, the amount of CH4 emission was reduced, and the result of reduced methane in the rice-duck integrated system depended on increasing the dissolved oxygen content in soil and water and thus, improving soil redox status.
Conclusions
In middle-rice soil in southern China, DOC and MBC were only 4–5.5% and 2–2.5% of total soil organic carbon respectively. However, they had similarly distinct seasonal variation patterns within different years, with the feature of lower concentration at the early growth stage, increasing during panicle differentiation and heading period, and dropping during grain filling period of rice. The variation of DOC and MBC were related to changes of rice growth and seasonal fluctuation of soil temperature.
Two peaks of CH4 emission from middle-rice soil occurred both in the 2 years, first at the tillering stage, second at the panicle differentiation and heading stage. CH4 emission fluxes had significantly positive correlations with soil DOC, MBC and soil temperature. Soil DOC could be managed by agricultural measures, which indicated that control of DOC concentration in soil might be an effective option for mitigation of CH4 emission. But our current findings about influence of DOC on CH4 emission are still insufficient, and further research is warranted in order to fully understand the mechanism of their interaction, to determine management strategies to control DOC concentration.
Significant reduction in CH4 emission was observed in rice-duck integrated system by 19.3% in 2007 and 19.6% in 2008, compared with that in conventional rice system, for which improvement in soil redox status might be predominant reason. Coupled with its relatively high economic benefits and preferable ecological benefits, wetland rice-duck integrated model could be a desirable integrated utilization method of paddy fields. This model could also potentially play an important role reducing methane emissions and creating conditions for more sustainable development of agricultural systems in China.
References
Adhya TK, Rath AK, Gupta PK, Rao VR, Das SN, Parida KM, Parashar DC, Sethunathan N (1994) Methane emission from flooded rice fields under irrigated conditions. Biol Fertil Soils 18:245–248. doi:10.1007/BF00647675
Aerts R (2006) The freezer defrosting: global warming and litter decomposition rates in cold biomes. J Ecol 94:713–724
Aulakh MS, Wassmann R, Bueno C, Kreuzwieser J, Rennenberg H (2001) Characterization of root exudates at different growth stages of ten rice (Oryza sativa L.) cultivars. Plant Soil 3:139–148. doi:10.1055/s-2001-12905
Bai Q, Gattinger A, Zelles L (2000) Characterization of microbial consortia in paddy rice soil by phospholipid analysis. Microb Ecol 39:273–281. doi:10.1007/s002480000020
Butterbach-Bahl K, Papen H, Rennenberg H (1997) Impact of gas transport through rice cultivars on methane emission from rice paddy fields. Plant Cell Environ 20:175–1183. doi:10.1111/j.1365-3040.1997.tb00693.x
Cai ZC (1997) A category for estimate of CH4 emission from rice paddy fields in China. Nutr Cycl Agro Ecosyst 49:171–179. doi:10.1023/A:1009729800707
Cleveland CC, Neff JC, Townsend AR, Hood E (2004) Composition, dynamics and fate of leached dissolved organic matter in terrestrial ecosystems: results from a decomposition experiment. Ecosystems 7:275–285. doi:10.1007/s10021-003-0236-7
Conrad R (1996) Soil microorganisms as controllers of atmospheric trace gases (H2, CO, CH4, OCS, N2O and NO). Microbiol Rev 60:609–640
Crill PM, Bartlett KB, Harriss RC, Gorham E, Verry ES, Sebacher DI, Madzar L, Sanner W (1988) Methane flux from Minnesota peatlands. Glob Biogeochem Cycl 2:371–384
Das K, Baruah KK (2008) A comparison of growth and photosynthetic characteristics of two improved rice cultivars on methane emission from rainfed agroecosystem of northeast India. Agr Ecosyst Environ 124:105–113. doi:10.1016/j.agee.2007.09.007
Deng X, Liao XL, Huang H (2004) Studies on amount of methanogens in the rice-duck agroecosystem. Acta Ecol Sinica 24(8):1696–1700
Ding WX, Cai ZC (2002) Effects of soil organic matter and exogenous organic materials on methane production in and emission from wetlands. Acta Ecol Sinica 22(10):1672–1677
Freeman C, Fenner N, Ostle NJ, Kang H, Dowrick DJ, Reynolds B, Lock MA, Sleep D, Hughes S, Hudson J (2004) Export of dissolved organic carbon from peatlands under elevated carbon dioxide levels. Nature 430:195–198. doi:10.1038/nature02707
Fu ZQ, Huang H, Liao XL, Hu Y, Xie W, He BL (2008) Effect of ducks on CH4 emission from paddy soils and its mechanism research in the rice-duck ecosystem. Acta Ecol Sinica 28(5):2107–2114
Gao S, Tanji KK, Scardaci SC, Chow AT (2002) Comparison of redox indicators in a paddy soil during rice-growing season. Soil Sci Soc Am J 66:805–817
Hao XH, Liu SL, Wu JS, Hu RG, Tong CL, Su YY (2008) Effect of long-term application of inorganic fertilizer and organic amendments on soil organic matter and microbial biomass in three subtropical paddy soils. Nutr Cycl Agroecosyst 81:17–24. doi:10.1007/s10705-007-9145-z
Hargreaves PR, Brookes PC, Ross GJS, Poulton PR (2003) Evaluating soil microbial biomass carbon as an indicator of long-term environmental change. Soil Biol Biochem 35:401–407. doi:10.1016/S0038-0717(02)00291-2
Harrison AF, Taylor K, Scott A, Poskitt J, Benham D, Grace J, Chaplow J, Rowland P (2008) Potential effects of climate change on DOC release from three different soil types on the Northern Pennines UK: examination using field manipulation experiments. Glob Chang Biol 14:687–702. doi:10.1111/j.1365-2486.2007.01504.x
Haynes RJ (2005) Labile organic matter fractions as central components of the quality of agricultural soils: an overview. Adv Agron 85:221–268
Huang H, Yang ZH, Wang H, Hu ZY, Cheng SG, Cheng C (2003) A study on the pattern of methane emission in wetland rice-duck complex ecosystems. Acta Ecol Sinica 23(5):929–934
Huang Y, Wang H, Huang H, Feng ZW, Yang ZH, Luo YC (2005) Characteristics of methane emission from wetland rice-duck complex ecosystem. Agric Ecosyst Environ 105:181–193. doi:10.1016/j.agee.2004.04.004
Hubei Provincial Bureau of Statistics (2008) Rural statistical yearbook 2007 of Hubei province. China Statistics Press, Beijing
IPCC (2006) 2006 IPCC Guidelines for national greenhouse gas inventories—volume 4: agriculture, forestry and other land use. NGGIP Publications, Japan. http://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/4_Volume4/V4_10_Ch10_Livestock.pdf
Jean LM, Pierre R (2001) Production, oxidation, emission and consumption of methane by soils: a review. Eur J Soil Biol 37:25–50. doi:10.1016/S1164-5563(01)01067-6
Kalbitz K, Solinger S, Park JH, Michalzik B, Matzner E (2000) Controls on the dynamics of dissolved organic matter in soils: a review. Soil Sci 165:277–304
Krüger M, Frenzel P, Conrad R (2001) Microbial processes influencing methane emission from rice fields. Glob Chang Biol 7:49–63. doi:10.1046/j.1365-2486.2001.00395.x
Kumaraswamy S, Rath AK, Ramakrishnan B, Sethunathan N (2000) Wetland rice soils as sources and sinks of methane: a review and prospects for research. Biol Fert Soils 31:449–461. doi:10.1007/s003740000214
Li CF, Cao CG, Wang JP, Zhan M, Yuan WL, Ahmad S (2009) Nitrous oxide emissions from wetland rice—duck cultivation systems in Southern China. Arch Environ Contam Toxicol 56:21–29. doi:10.1007/s00244-008-9159-9
Lu Y, Watanabe A, Neue HU, Huang C, Bueno CS (2000a) Methanogenic responses to exogenous substrates in anaerobic rice soils. Soil Biol Biochem 32:1683–1690. doi:10.1016/S0038-0717(00)00085-7
Lu Y, Wassmann R, Neue HU, Huang C (2000b) Dynamics of dissolved organic carbon and methane emissions in a flooded rice soil. Soil Sci Soc Am J 64:2011–2017
Lu Y, Watanabe A, Kimura M (2002a) Input and distribution of photosynthesized carbon in a flooded rice soil. Glob Biogeochem Cycl 16(4):1085. doi:10.1029/2002GB001864
Lu Y, Watanabe A, Kimura M (2002b) Contribution of plant-derived carbon to soil microbial biomass dynamics in a paddy rice microcosm. Biol Fertil Soils 36:136–142. doi:10.1007/s00374-002-0504-2
Lu Y, Watanabe A, Kimura M (2004) Contribution of plant photosynthates to dissolved organic carbon in a flooded rice soil. Biogeochemistry 71:1–15. doi:10.1007/s10533-005-3258-8
Luo GY, Xu GP, Wang YF, Wang SP, Lin XW, Hu YG, Zhang ZH, Chang XF, Duan JC, Su AL, Zhao YG (2009) Effects of grazing and experimental warming on DOC concentrations in the soil solution on the Qinghai-Tibet plateau. Soil Biol Biochem 41:2493–2500. doi:10.1016/j.soilbio.2009.09.006
Lutzow M, Kogel-Knabner I, Ekschmitt K, Flessa H, Guggenberger G, Matzner E, Marschner B (2007) SOM fractionation methods: relevance to functional pools and to stabilization mechanisms. Soil Biol Biochem 39:2183–2207. doi:10.1016/j.soilbio.2007.03.007
Mitra S, Wassmann R, Jain MC, Pathak H (2002) Properties of rice soils affecting methane production potentials: 2. Differences in topsoil and subsoil. Nutr Cycl Agroecosyst 64:183–191. doi:10.1023/A:1021175404418
Mitra S, Aulakh MS, Wassmann R, Olk DC (2005) Triggering of methane production in rice soils by root exudates: effects of soil properties and crop management. Soil Sci Soc Am J 69:563–570
Murase J, Matsui Y, Katoh M, Sugimoto A, Kimura M (2006) Incorporation of 13C-labeled rice-straw-derived carbon into microbial communities in submerged rice field soil and percolating water. Soil Biol Biochem 38:3483–3491. doi:10.1016/j.soilbio.2006.06.005
Nayak DR, Babu YJ, Adhya TK (2007) Long-term application of compost influences microbial biomass and enzyme activities in a tropical Aeric Endoaquept planted to rice under flooded condition. Soil Biol Biochem 39:1897–1906. doi:10.1016/j.soilbio.2007.02.003
Penning H, Conrad R (2007) Quantification of carbon flow from stable isotope fractionation in rice field soils with different organic matter content. Org Geochem 38:2058–2069. doi:10.1016/j.orggeochem.2007.08.004
Reichardt W, Mascarina G, Padre B, Doll J (1997) Microbial communities of continuously cropped irrigated rice fields. Appl Environ Microb 63:233–238
Reiner W, Milkha SA (2000) The role of rice plants in regulating mechanisms of methane missions. Biol Fertil Soils 31:20–29. doi:10.1007/s003740050619
Robinson CH (2002) Controls on decomposition and soil nitrogen availability at high latitudes. Plant Soil 242:65–81. doi:10.1023/A:1019681606112
Singh JS, Singh S, Raghubanshi AS, Singh S, Kashyap AK (1996) Methane flux from rice/wheat agroecosystem as affected by crop phenology, fertilization and water level. Plant Soil 183:323–327. doi:10.1007/BF00011448
Song CC, Yang WY, Xu XF, Lou YJ, Zhang JB (2004) Dynamics of CO2 and CH4 concentration in the mire soil and its impact factors. Environ Sci 25(4):1–6
Towprayoona S, Harvey NW, Jittasatra O, Kerdchuchean O (2000) Influence of rice variety and soil type on production and emission of methane from rice fields. Asian J Energy Environ 1:251–262
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass carbon. Soil Biol Biochem 19:703–704
Wang Y (2000) Studies on ecological benefits of planting and breeding model in rice fields. Acta Ecol Sinica 20(2):311–316
Wang MX, Li J (2002) CH4 emission and oxidation in Chinese rice paddies. Nutr Cycl Agroecosyst 64:43–55
Wang ZP, DeLaune RD, Masscheleyn PH, Patrick WH Jr (1993) Soil redox and pH effects on methane production in a flooded rice soil. Soil Sci Soc Am J 57:382–385
Wang WJ, Dalal RC, Moody PW, Smith CJ (2003a) Relationships of soil respiration to microbial biomass, substrate availability and clay content. Soil Biol Biochem 35:273–284. doi:10.1016/S0038-0717(02)00274-2
Wang H, Huang H, Yang ZH, Liao XL (2003b) Integrated benefits of rice-duck complex ecosystem. Rural Eco Environ 19:23–26
Wang JP, Cao CG, Jin H, Wang CF, Liu FH (2006) Effects of rice-duck farming on aquatic community in rice fields. Sci Agric Sinica 39(10):2001–2008
Wassmann R, Neue HU, Bueno C, Lantin RS, Alberto MCR, Buendia LV, Bronson K, Papen H, Rennenberg H (1998) Methane production capacities of different rice soils derived from inherent and exogenous substrates. Plant Soil 203:227–237. doi:10.1023/A:1004357411814
Watanabe A, Machida N, Takahashi K, Kitamura H, Kimura M (2004) Flow of photosynthesized carbon from rice plants into the paddy soil ecosystem at different stages of rice growth. Plant Soil 258:151–160
Wei CF (2000) The effects of cultivation system on methane emission from winter flooded paddy fields in southwest area. Acta Pedol Sin 37(2):157–164
Wright AL, Provin TL, Hons FM, Zuberer DA, White RH (2005) Dissolved organic C in compost-amended bermudagrass turf. Hortscience 40:830–835
Xiang PA, Huang H, Huang M, Gan DX, Zhou Y, Fu ZQ (2006) Studies on technique of reducing methane emission in a rice-duck ecological system and the evaluation of its economic significance. Sci Agric Sinica 5(10):758–766
Xu H, Cai ZC, Jia ZJ, Tsuruta H (2000) Effect of land management in winter crop season on CH4 emission during the following flooded and rice growing period. Nutr Cycl Agroecosyst 58:12–18. doi:10.1023/A:1009823425806
Yagi K, Tsuruta H, Minami K (1997) Possible options for mitigating methane emission from rice cultivation. Nutr Cycl Agroecosyst 49:213–220. doi:10.1023/A:1009743909716
Yao H, Conrad R, Wassmann R, Neue HU (1999) Effect of soil characteristics on sequential reduction and methane production in sixteen rice paddy soils from China, the Philippines, and Italy. Biogeochemistry 47:269–295
Yu SM, Ouyang YN, Zhang QY, Peng G, Xu DH, Jin QY (2005) Effects of rice-duck farming system on Oryza sativa growth and its yield. Chin Appl Ecol 16(7):1252–1256
Zhan M, Cao CG, Wang JP, Dai GZ (2008) Analysis of diversity of soil microbial community of the wetland rice-duck and rice-fish complex ecosystem. Acta Pedologica Sinica 45(6):1179–1183
Zheng YH, Deng GB, Lu GM (1997) Eco-economic benefits of rice-fish-duck complex ecosystem. Chin Appl Ecol 8(4):431–434
Zheng XH, Wang MX, Wang YS, Shen RX, Li J (1998) Comparison of manual and automatic methods for measurement of methane emission from rice paddy fields. Adv Atmos Sci 15(4):569–579. doi:10.1007/s00376-998-0033-5
Zou JW, Huang Y, Jiang JY, Zheng XH, Sass RL (2005) A 3-year field measurement of methane and nitrous oxide emissions from rice paddies in China: effects of water regime, crop residue, and fertilizer application. Glob Biogeochem Cycles 19:GB2021. doi:10.1029/2004GB002401
Acknowledgments
This study was financially supported by National Foundation of Key Science of China (No. 2006BA520A02). The authors would like to thank Mr. Zheng Liesheng for helping with the gas sample analyses, Professor Hu Ronggui for his suggestions in our research work. We also thank Professor Donald D. Tyler, Biosystem Engineering and Environmental Science Department of University of Tennessee USA, for his volunteer revision. We would like to extend our appreciation to the two anonymous reviewers for their painstaking revisions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhan, M., Cao, C., Wang, J. et al. Dynamics of methane emission, active soil organic carbon and their relationships in wetland integrated rice-duck systems in Southern China. Nutr Cycl Agroecosyst 89, 1–13 (2011). https://doi.org/10.1007/s10705-010-9371-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-010-9371-7