Abstract
Soil net nitrogen mineralization (NNM) of four grasslands across the elevation and precipitation gradients was studied in situ in the upper 0–10 cm soil layer using the resin-core technique in Xilin River basin, Inner Mongolia, China during the growing season of 2006. The primary objectives were to examine variations of NNM among grassland types and the main influencing factors. These grasslands included Stipa baicalensis (SB), Aneulolepidum Chinense (AC), Stipa grandis (SG), and Stipa krylovii (SK) grassland. The results showed that the seasonal variation patterns of NNM were similar among the four grasslands, the rates of NNM and nitrification were highest from June to August, and lowest in September and October during the growing season. The rates of NNM and nitrification were affected significantly by the incubation time, and they were positively correlated with soil organic carbon content, total soil nitrogen (TN) content, soil temperature, and soil water content, but the rates of NNM and nitrification were negatively correlated with available N, and weakly correlated with soil pH and C:N ratio. The sequences of the daily mean rates of NNM and nitrification in the four grasslands during the growing season were AC > SG > SB > SK, and TN content maybe the main affecting factors which can be attributed to the land use type.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil nitrogen mineralization, the transformation process from organic N to inorganic N is one of the major processes of nitrogen cycle (Davidson et al. 1992; Ledgard et al. 1998; Cobo et al. 2002). Net N mineralization (NNM) is the outcome of two concurrent and oppositely directional processes: gross N mineralization and gross N immobilization turnover (Luxhøi et al. 2006). The actual availability of inorganic N depends on the rate of NNM and its transport through the soil (Fenn et al. 2005). Soil NNM primarily determines soil N availability and net primary productivity (Vitousek and Howarth 1991; Hooper and Johnson 1999); on the other hand, it plays an important role in determining N losses to the environment, affecting N2O production in terrestrial ecosystems, and alters N cycling routes (Davidson et al. 1993; Fenn et al. 2005).
Many recent relevant studies focused on various ecosystems (Hatch et al. 1998; Bhogal et al. 1999; Owen et al. 2003; Ross et al. 2004; Fenn et al. 2005; Luxhøi et al. 2006; Fang et al. 2007). Rates of NNM often differed with ecotype, elevation, topographic conditions, and land use change, and the differences is attributed to variations in organic matter content, temperature, and water availability of soil (Gilliam et al. 2001; Jensen et al. 2005; Scott Bechtold and Naiman 2006). There are very few studies of NNM in the semi-arid temperate grassland in China (Wang et al. 2006; Xu et al. 2007; Zhou et al. 2009), despite the fact that it comprises nearly 12.5% of the global grassland areas. These preliminary results were extremely limited and the knowledge of NNM dynamics variation among grassland types along an environmental gradient in the Inner Mongolia grassland is still lacking.
The objective of the present study was to investigate simultaneously the seasonal variation of inorganic N pool and rates of NNM and nitrification in situ in surface soils from different grassland types along the Xilin River basin, Inner Mongolia, and to determine the main influencing factors of NNM and nitrification rates.
Materials and methods
Site description
The research site is located in the Xilin River basin, Inner Mongolia, China (43°26′–44°39′N, 115°32′–117°12′E). It is also in the Northeast China Transect (NECT) of the International Geosphere-Biosphere Program (IGBP) (Zhang et al. 1997). The elevation in the basin decreases progressively from ca. 1,500 m in southeast to ca. 1,000 m in northwest. The area is under a continental semi-arid monsoon climate in the temperate zone, with a frost-free period of 90–110 days. The mean annual temperature varies from −1 to 4°C, annual precipitation (PPT) in the basin also decreases progressively (from 450 to 150 mm), with a majority of it (70%) falling from June through September. Winter season ranges from November to late April and the growing season from May to September, being generally windy and warm. The PPT of this region from May to September in 2006 was 209.7 mm, but with comparably high rain (39.5 mm) in September (Fig. 1).
Meadow grassland and typical grassland are distributed along the Xilin River. Meadow grassland is distributed in the upper Xilin River, and belongs to more humid meadow grassland with representative formations of Stipa baicalensis grassland and Filifolium sibiricum grassland and soil type of chernozem. Typical grassland is mainly distributed in the low-lying middle and lower reaches of Xilin River, of which the representative formations of Aneurolepidium chinense grassland and Stipa grandis grassland are the main part in the middle reach with soil types of dark chestnut soil and typical chestnut soil. The representative formations in the light chestnut soil subzone at lower Xilin River are Stipa krylovii grassland and Artemisia frigida grassland, and it is a drier type of grassland in the typical grassland (Li et al. 1988; Liu and Liu 1988).
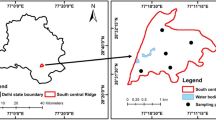
The present study selected Stipa baicalensis (SB), Aneulolepidum Chinense (AC), Stipa grandis (SG), and Stipa krylovii (SK) grassland along the elevation and precipitation gradient as our research sites which covered the meadow and typical grassland. The SB grassland is utilized as a mowing pasture at the frequency of mowing once a year in mid- or late August. The sampling plot of the AC grassland was situated in the permanent experiment site of the Inner Mongolian Grassland Ecosystem Research Station (IMGERS), Chinese Academy of Science, and it was fenced in 1979, from the time that grazing and fertilization were forbidden in this fenced field. About 5 km away from the AC grassland in the western direction is the SG grassland; the sampling plot was located in the permanent experimental site, which was also fenced in 1979. The sampling plot of the SK grassland was situated in the plain and the submontane diluvial fan area, 20 km northwest of Xilinhot City, which was grazed freely before it was fenced in June 2001. The four grasslands belong to several typical sub-ecozones of the semi-arid temperate grassland varying with terrain relief, flora composition, soil type and physiochemical properties, etc., so the research results possess extensive representative in this region. The distribution of grassland type and location of sampling plots in Xilin River basin were shown in Fig. 2. The features of the experimental sites and the soil physiochemical properties were listed in Tables 1 and 2.
Soil sampling and incubation
The experiment was conducted in the growing season of 2006 using the resin-core technique (Bhogal et al. 1999; Hatch et al. 2000), which is a modification of procedure proposed by Raison et al. (1987) and Hübner et al. (1991). The method confined soil cores in situ in an open tube in the ground, with an anion exchange resin bag at the base to intercept any leached N. In every experimental site, six sampling plots of 2 × 2 m were randomly demarcated on the first sampling date of 11 May of 2006. The PVC tubes were sharpened in advance with a bevel to the outside at the base to avoid compression of soil. After the aboveground vegetation was clipped at the ground level and removed together with litter, one of a pair of PVC tubes (5 cm diameter and 12 cm long) was inserted 10 cm into the ground in every plot, then it was taken out for determining the initial inorganic N (NH4 +–N and NO3 −–N) concentration; the other tube was inserted 12 cm into the ground to confine a soil core, with soil structure undamaged, and then 2 cm soil layer was excavated from the base of the soil core with screwdriver. The pre-prepared filter paper, one resin bag, filter paper, and one cylindrical block of gypsum were put into this tube in order (the height of resin bag and plaster is about 2 cm), the resin bag was made by nylon and containing 3 g of anion exchange resin beads (717#, produced by the Huizhi resin Plant of Shanghai). Our past study showed that the NH4 +–N incepted by the resin bag was negligible in this semiarid region (Liu et al. 2007). The resin bag fit tightly within the PVC tube. The tube was then carefully placed back into its original soil hole, making sure that a solid contact was made between the bottom resin and the soil, and left for in situ incubation. This was repeated at 4 week interval until the experiment ended on 12 October (6 sampling tubes in total for the first time at each site, and 12 sampling tubes in total for the following sampling time at each site). Vegetation re-growth was controlled during incubation time.
In addition, in order to examine the effect of the length of incubation time on NNM, we selected two incubation time treatments. Some soil cores were incubated at 4 week interval during the entire growing season, and others were incubated at 2 week interval during the peak growth period from June to August (6 sampling tubes in total for the first time at each site, and 12 sampling tubes in total for the following sampling time at each site). All the initial and incubated soil samples and the collected resin bags were stored in refrigerator at 4°C temporarily, and extracted within 24 h.
Soil samples and resin bags extraction and chemical analysis
Soil samples (initial and incubated) were thoroughly mixed and sieved through a 2 mm screen after stones and coarse roots were manually removed. A 10 g subsample from each sample was extracted with 50 ml of 0.01 M CaCl2 solution. The soil suspension was filtered (Whatman No. 1 filter paper, 12.5 cm in diameter). The resin bags were washed with deionized water and dried at room temperature prior to extraction. The resin bags were extracted with 1 M NaCl solution (resin: NaCl 1: 5 w/v), and then they were washed with fresh NaCl solution. The filtrates of soil and the solution of extracted resin bags were kept frozen before they were analyzed for NH4 +–N and NO3 −–N concentration on an automated flow injection analysis (Braun & Lübbe, Norderstedt, Germany). Soil water content (SWC) was determined gravimetrically by oven-drying at 105°C for 24 h.
The soil samples were further air dried and then used to measure soil pH, total organic C (TOC), and total soil N (TN). Soil pH values were determined in water (water:soil = 2.5:1) suspension. TOC was analyzed using the H2SO4–K2Cr2O7 oxidation method with an Alpkem autoanalyzer (Kjektec System 1026 Distilling Unit, Sweden). TN was measured using the Kjeldahl acid-digestion method. Soil bulk density was measured using the core method. Soil temperature at 10 cm depth was measured with Model SN 2202 digital thermo detector (produced by the Sinan Instrument Plant of Beijing Normal University) at the time of sampling.
Calculation
NNM on a dry mass basis was calculated as the changes in inorganic N (NH4 +–N and NO3 −–N) in the initial and incubated samples. Rates of net ammonification (R a), net nitrification (R n), and net mineralization (R m) during the time interval (Δt) from t i to t i+1 were calculated using the following equations (Hübner et al. 1991; Valenzuela-Solano et al. 2005):
For a time interval Δt = t i+1 − t i
where
where t i and t i+1 are the initial and post incubation time, respectively; [NH4 +–N] i and [NH4 +–N]i+1 are the mean concentrations of ammonium N in the initial and incubated samples, respectively; [NO3 −–N] i and [NO3 −–N]i+1 are the mean concentrations of nitrate N in the initial and incubated samples; [NO3 −–N]resin is the mean concentration of nitrate N in the resin. A m, A n, and A a are the accumulation of total inorganic N (NH4 +–N and NO3 −–N), NO3 −–N, and NH4 +–N; R m, R n, and R a are the NNM rate, net nitrification rate, and net ammonification rate, respectively.
Statistical analysis
Two-way analysis of variance (ANOVA) was used to test the effects of sampling time on NH4 +–N, NO3 −–N, and inorganic N concentration within each grassland type, and it was also used to test the effects of incubation period on R m and R n. We contrast pairs of grassland types by using SE. Relationships between R m, R n and soil characteristics were tested using Pearson correlation analysis. All analyses were performed using the SPSS 11.0 statistical software package.
Results
Seasonal dynamics of inorganic N concentration
During the study period, the inorganic N (NH4 +–N and NO3 −–N) in surface soil layer (0–10 cm) ranged from 1.13 to 6.62 μg g−1 for the SB grassland, 1.15 to 5.85 μg g−1 for the AC grassland, 2.70 to 12.18 μg g−1 for the SG grassland, and 2.69 to 7.63 μg g−1 for the SK grassland, respectively (Fig. 3). Results from two-way ANOVA demonstrated that sampling time had a significant effect on the inorganic N concentration in each grassland type. Soil NH4 +–N concentration ranged from 0 to 2.63 μg g−1 and peaked in July to August for the four grasslands. The SB grassland had a significantly higher NH4 +–N concentration than the other three grasslands in most of the sampling period (P < 0.01), while soil NO3 −–N concentration peaked in late August to early September. The SG grassland had a significantly higher NO3 −–N concentration than others in most of the sampling period (P < 0.01). The ratio between NH4 +–N and NO3 −–N was approximately 1:4.2 during the growing season in the four grasslands, NO3 −–N was the dominant inorganic N. Soil inorganic N and NO3 −–N in the four grasslands showed similar seasonal variation patterns (Fig. 3).
Net N mineralization and nitrification
The seasonal patterns of daily R m under 2 weeks incubation were similar among the four grasslands. R m ranged from −0.10 to 1.03 μg g−1 d−1 and varied significantly during the growing season with two peaks in late June and early August (Fig. 4). The mean R m of the SB, AC, SG, and SK grassland were 0.36, 0.57, 0.40, and 0.28 μg g−1 d−1, respectively. The R m of the AC grassland was significantly higher than the other three grasslands (P < 0.01) during the incubation period except for 11–26 July, the R m of the SG grassland were also higher than the R m of the SB and SK grasslands. During 11–26 August, the R m of the SB and the SK grasslands decreased significantly and sharply because of drought. However, in the AC and SG grasslands, there were a lot of litter cover due to long period enclosure, soil evaporation was largely reduced, so the soil water were maintained, which likely caused higher R m in the two grasslands during the drought period.
Net N nitrification rates (R n) were comparable to R m in magnitude in the four grasslands, and R n was positively correlated with R m. Results from two-way ANOVA demonstrated that incubation period had a significant effect on R m and R n in each grassland type. The mean R n of the SB, AC, SG, and SK grasslands were 0.36, 0.55, 0.40, and 0.27 μg g−1 d−1, respectively. R n was significantly higher than the rates of ammonification (R a) in the four grasslands (P < 0.01; Fig. 4), and the higher the rate of nitrification, the lower the rate of ammonification in one incubation period, indicating that NH4 + was transformed into NO3 − or it was immobilized, and therefore the soil NNM in the temperate semi-arid grassland was dominated by the processes of soil net nitrification.
Incubation time had significant impacts on R m and R n in the four grasslands (P < 0.01). However, the seasonal variation patterns of R m, R n, and R a under 4 weeks incubations were similar to those under 2 weeks incubations (Figs. 4, 5), but the R m, R n, and R a were different from each other among the four grasslands significantly (P < 0.01). The daily mean R m of the SB, AC, SG, and SK grasslands under 4 weeks incubation were 0.20, 0.25, 0.17, and 0.14 μg g−1 d−1, respectively. The daily mean R n of the SB, AC, SG, and SK grasslands under 4 weeks incubation were 0.20, 0.27, 0.23, and 0.16 μg g−1 d−1, respectively. Compared to the treatment of incubation of 2 weeks, the R m and R n of incubation of 4 weeks reduced significantly during the same period (P < 0.01).
Correlations of NNM and nitrification with environmental factors
Some previous studies have demonstrated that R m and R n were affected by soil temperature, soil moisture, TOC, TN, and soil C:N, etc. (Gilliam et al. 2001; Cookson et al. 2002; Dalias et al. 2002; Dilly et al. 2003). We also studied the correlations of R m and R n with environmental factors (Table 3). The results showed that both R m and R n had positively significant correlations with SWC, TOC, and TN, but not significantly correlated with soil temperature. Both R m and R n had a decreasing trend with the available N. But there is an increasing trend in R m and R n with soil pH and the C:N ratio of soil organic matter (SOM).
Discussion
Effects of length of the incubation time on NNM
Regardless of grassland type, the length of incubation time had significant influence on R m (P < 0.01); and the longer the incubation time, the lower the R m (P < 0.01). Some studies also demonstrated that the accumulation of nitrate and inorganic N increased whereas net nitrification and mineralization rates decreased with prolonged incubation time (Wang et al. 2006; Amador et al. 2005; Cookson et al. 2002). The increasing accumulation of inorganic N with incubation time is clearly attributed to the lack of plant uptake. The higher inorganic N concentration of soil core restrained the organic N from further mineralization to a certain extent, and therefore the longer the incubation time, the lower the R m. Soil NNM had long and well been studied in grassland ecosystems both in the field and in the laboratory (Ledgard et al. 1998; Barretta and Burke 2000; Booth et al. 2003; Wang et al. 2006). However, the incubation time differs in different researches, for example, the incubation time as short as 1 day and as long as more than 1 year have been reported (Verchot et al. 2002; Dalias et al. 2002), so the inconsistency in incubation time make it difficult to compare the NNM across different ecosystem and region.
Rm and Rn were positive in nearly all incubation times. Ra was negative most of the incubation time (Figs. 4, 5). The negative values of Ra could be explained by N immobilization, or by gaseous losses at nitrification (Maag and Vinther 1996). The negative Ra also indicated that the available NH4+–N maybe oxidized into NO3−–N.
Effects of soil temperature and moisture on NNM
The seasonal variation patterns of soil inorganic N pools, R m and R n in the four grasslands were examined in this study. As expected, the daily R m and R n were higher from June to August, which are consistent with previous results (Ross et al. 2004; Wang et al. 2006). R m and R n was positively correlated with SWC (P < 0.05) and not significantly correlated with soil temperature (P > 0.05) during the 2006 growing season, but it also indicated that an increase in soil temperature was also likely to stimulate soil mineralization. The likely reason is that the experimental region is located in the semi-arid climatic zone where SWC was usually low for a long period of time, the average SWC at the 0–10 cm soil layer during the growing season was only 8.2% in 2006. The mean air temperature, however, was higher than 10°C in most of the growing season in the region. Wang et al. (2006) found that soil moisture content limited NNM when the moisture was less than 15% in Inner Mongolia grassland, and R m and R n increased with soil temperature between 5 and 35°C. Throughout the incubation period, SWC was less than 15% (Fig. 6) in most of the incubation period while daily mean air temperature was between 5 and 22°C (Fig. 1), which suggested that NNM and nitrification was likely subjected to soil water content during the growing season in the semi-arid grassland.
Other previous studies also indicated the highest rates of N mineralization usually occurred in summer and coincided with the higher temperature and soil moisture conditions for microbial activities, and then rapidly decline in fall and winter, which appeared to follow seasonal patterns of temperature and rainfall (Wang et al. 2006, 2008). In this study, R m and R n also peaked from June to August, and then obviously declined in September.
Nitrogen mineralization and nitrification are microbe-governed processes, and soil temperature has been consistently reported to control soil microbial processes (Dalias et al. 2002). Some studies indicated that R m was positively correlated with seasonal temperature and less sensitive to soil moisture (Hatch et al. 1991; Sierra 1997). Other researchers reported that soil N transformation was usually sensitive to temperature and oxygen supply in wet ecosystems (Paul et al. 2003), and sensitive to soil moisture in arid and semi-arid ecosystems (Vangestel et al. 1993; Xu et al. 2007). SWC regulates microbial processes by changing water availability and controlling oxygen diffusion within the soil, and therefore affects N mineralization (Amador et al. 2005).
Variations of NNM among the four grasslands
Land use types can profoundly impact soil N cycling through the alteration of abiotic and biotic characteristic of soils (Templer et al. 2005; Zhang et al. 2008). It is expected that the R m and R n at the AC and SB grasslands would be higher, because they were freed from grazing since 1979 and had higher amount of SOM or higher carbon and nitrogen concentrations, which supplied plenty of substrate for the microbial activity and thereby accelerated N mineralization (Hacin et al. 2001; Wang et al. 2008). The lower R m and R n at the SB grassland were likely to be associated with its long-term land use, which was utilized as a mowing pasture. Large amount of soil nutrients was lost and therefore the soil TOC and TN decreased. However, the nutrients returned to the soil through residue or leaf litter is an important source of C for microbes.
We also observed R m and R n had significantly positive correlations with TOC and TN (Table 3), suggesting that an increase in TOC and TN was likely to stimulate soil NNM. This is consistent with other studies (Hacin et al. 2001; Wang et al. 2008). Both TOC and TN are highest for the AC grassland, followed by the SB, SG, and SK grasslands, respectively. But the inter-seasonal variation, however, are usually small.
Rm and Rn were negatively correlated with the soil inorganic N content, and NO3−–N was the main form of inorganic N in the four grasslands. This was consistent with previously result (Robertson 1982). Since soil ammonium supply was the main substrate for nitrification, net rates of N nitrification related to soil ammonium supply.
Effects of species diversity and soil C:N ratio on NNM
The NNM and nitrification showed similar seasonal variation trends among the four grasslands, which demonstrated the long-term climate was the major controlling factor for NNM at a regional scale. However, the effect of species diversity on NNM at the local scale was supported by observations from other regions. For example, species diversity was also found to be related to N mineralization dynamics in the South American temperate forest (Pérez et al. 1998), and in the subtropical forest of southwestern China (Wang et al. 2008). We also found the similar relationships between species diversity and N mineralization in the four grassland ecosystems. The order of species richness (defined by number of species per square meter) in the four grasslands was AC > SB > SG > SK (Table 2), which accord with the rates of NNM and nitrification. Correlation between number of plant species and net rates should be investigated in grassland in future experiments.
There were no significant variations in the C:N ratio of SOM and soil pH among the four grasslands and with seasonality. The C:N ratios of SOM were about 10 for the four grasslands, which was much lower than other ecosystems, such as young Hawaii tropical forests (C:N = 110), which might suffer N limitation (Herbert and Fownes 1999; Carmosini et al. 2003; Luxhøi et al. 2006). Our results suggested the C:N ratio of SOM had a very weak correlation with N mineralization and nitrification.
The research way need to cut off the aboveground plant during the installation of mineral soil incubations, which may have resulted in reduced N-uptake by the plant, we may underestimate the rate of NNM. However, we have no way of quantifying this disturbance effect on our N-flux estimates. Of the in situ methods, the RCT maintained soil conditions that were most representative of actual soil condition.
Conclusions
We documented the seasonal variation patterns in soil inorganic N pools, NNM and nitrification rates across the grassland types that differed in elevation, species composition, and soil characteristics in the semi-arid temperate grassland. The similarity in the seasonal variation of NNM and nitrification rates among the SB, AC, SG, and SK grasslands suggested that the climate condition was the most dominant controlling factor, and it regulated the soil N mineralization by controlling soil temperature and SWC. There were obvious differences in NNM and nitrification rates among the four grassland types, and the difference was mainly caused by land use type.
Abbreviations
- AC:
-
Aneulolepidum Chinense
- NNM:
-
Net N mineralization
- SB:
-
Stipa baicalensis
- SG:
-
Stipa grandis
- SK:
-
Stipa krylovii
- SOM:
-
Soil organic matter
- SWC:
-
Soil water content
- TN:
-
Total soil N
- TOC:
-
Total soil organic C
References
Amador JA, Görres JH, Savin MC (2005) Role of soil water content in the carbon and nitrogen dynamics of Lumbricus terrestris L. burrow soil. Appl Soil Ecol 28:15–22
Barretta JE, Burke IC (2000) Potential nitrogen immobilization in grassland soils across a soil organic matter gradient. Soil Biol Biochem 32:1707–1716
Bhogal A, Hatch DJ, Shepherd MA, Jarvis SC (1999) Comparison of methodologies for field measurement of net nitrogen mineralization in arable soils. Plant Soil 207:15–28
Booth MS, Stark JM, Caldwell MM (2003) Inorganic N turnover and availability in annual- and perennial-dominated soils in a northern Utah shrub-steppe ecosystem. Biogeochemistry 66:311–330
Carmosini N, Devito KJ, Prepas EE (2003) Net nitrogen mineralization and nitrification in trembling aspen forest soils on the Boreal Plain. Can J For Res 33:2262–2268
Cobo JG, Barrios E, Kass DCL, Thomas R (2002) Nitrogen mineralization and crop uptake from surface-applied leaves of green manure species on a tropical volcanic-ash soil. Biol Fertil Soils 36:87–92
Cookson WR, Cornforth IS, Rowarth JS (2002) Winter soil temperature (2–5°C) effects on N transformations in clover green manure amended or unamended soils: a laboratory and field study. Soil Biol Biochem 34:1401–1415
Dalias P, Anderson JM, Bottner P, Couteaux MM (2002) Temperature responses of net nitrogen mineralization in conifer forest soil incubated under standard laboratory conditions. Soil Biol Biochem 34:691–701
Davidson EA, Hart SC, Firestone MK (1992) Internal cycling of nitrate in soils of a mature coniferous forest. Ecology 73:1148–1156
Davidson EA, Matson PA, Vitousek PM, Riley R, Dunkin K, Garciamendez G, Maass JM (1993) Processes regulating soil emissions of NO and N2O in a seasonally dry tropical forest. Ecology 74:130–139
Dilly O, Blume HP, Sehy U, Jimenez M, Munch JC (2003) Variation of stabilized, microbial and biologically active carbon and nitrogen in soil under contrasting land use and agricultural management practices. Chemosphere 52:557–569
Fang SZ, Xie BD, Zhang HC (2007) Nitrogen dynamics and mineralization in degraded agricultural soil mulched with fresh grass. Plant Soil 300:269–280
Fenn ME, Poth MA, Terry JD, Blubaugh TJ (2005) Nitrogen mineralization and nitrification in a mixed-conifer forest in southern California: controlling factors, fluxes, and nitrogen fertilization response at a high and low nitrogen deposition site. Can J For Res 35:1464–1486
Gilliam FS, Yurish BM, Adams MB (2001) Temporal and spatial variation of nitrogen transformations in nitrogen-saturated soils of a central Appalachian hardwood forest. Can J For Res 31:1768–1785
Hacin J, Cop J, Mahne I (2001) Nitrogen mineralization in marsh meadows in relation to soil organic matter content and watertable level. J Plant Nutr Soil Sci 164:503–509
Hatch DJ, Jarvis SC, Reynolds SE (1991) An assessment of the contribution of net mineralization to N cycling in grass swards using a field incubation method. Plant Soil 138:23–32
Hatch DJ, Jarvis SC, Parkinson RJ (1998) Concurrent measurements of net mineralization, nitrification, denitrification and leaching from field incubated soil cores. Biol Fertil Soils 26:323–330
Hatch DJ, Jarvis SC, Parkinson RJ, Lovell RD (2000) Combining field incubation with nitrogen-15 labeling to examine nitrogen transformations in low to high intensity grassland management systems. Biol Fertil Soils 30:492–499
Herbert DA, Fownes JH (1999) Forest productivity and efficiency of resource use across a chronosequence of tropical montane soils. Ecosystems 2:242–254
Hooper DU, Johnson L (1999) Nitrogen limitation in dryland ecosystems: responses to geographical and temporal variation in precipitation. Biogeochemistry 46:247–293
Hübner C, Redl G, Wurst F (1991) In situ methodology for studying N-mineralization in soils using anion exchange resins. Soil Biol Biochem 23:701–702
Jensen LS, Salo T, Palmason F, Breland TA, Henriksen TM, Stenberg B, Pedersen A, Lundström C, Esala M (2005) Influence of biochemical quality on C and N mineralization from a broad variety of plant materials in soil. Plant Soil 273:307–326
Ledgard SF, Jarvis SC, Hatch DJ (1998) Short-term nitrogen fluxes in grassland soils under different long-term nitrogen management regimes. Soil Biol Biochem 30(10–11):1233–1241
Li B, Yong SP, Li ZH (1988) The vegetation of the Xilin River basin and its utilization. In: Inner Mongolia Grassland Ecosystem Research Station, CAS (eds) Research on grassland ecosystem no. 3, Science Press, Beijing, pp 84–183 (in Chinese)
Liu S, Liu ZL (1988) Outline of flora of the Xilin River basin, Inner Mongolia. In: Inner Mongolia Grassland Ecosystem Research Station, CAS (eds) Research on grassland ecosystem no. 3, Science Press, Beijing, pp 227–268 (in Chinese)
Liu XR, Dong YS, Qi YC, Domroes M (2007) Soil net mineralization in the typical temperate grassland. Environ Sci 28(3):633–640 (in Chinese)
Luxhøi J, Bruun S, Stenberg B, Breland TA, Jensen LS (2006) Prediction of gross and net nitrogen mineralization-immobilization-turnover from respiration. Soil Sci Soc Am J 70:1121–1128
Maag M, Vinther FP (1996) Nitrous oxide emission by nitrification and denitrification in different soil types and at different soil moisture contents and temperatures. Appl Soil Ecol 4:5–14
Owen JS, Wang MK, Wang CH, King HB, Sun HL (2003) Net N mineralization and nitrification rates in a forested ecosystem in northeastern Taiwan. For Ecol Manag 176:519–530
Paul KI, Ploglase PJ, Oconell AM, Carlyle JC, Smethcrst PJ, Khanna PK (2003) Defining the relation between soil water content and net nitrogen mineralization. Eur J Soil Sci 54:39–47
Pérez CA, Hedin LO, Armesto JJ (1998) Nitrogen mineralization in two unpolluted old-growth forests of contrasting biodiversity and dynamics. Ecosystems 1(4):361–373
Raison RJ, Connel MJ, Khanna PK (1987) Methodology for studying fluxes of soil mineral nitrogen in situ. Soil Biol Biochem 19:521–530
Robertson GP (1982) Factors regulating nitrification in primary and secondary succession. Ecology 63:1561–1573
Ross DS, Lawrence GB, Fredriksen G (2004) Mineralization and nitrification patterns at eight northeastern USA forested research sites. For Ecol Manag 188:317–335
Scott Bechtold J, Naiman RJ (2006) Soil texture and nitrogen mineralization potential across a riparian toposequence in a semi-arid savanna. Soil Biol Biochem 38:1325–1333
Sierra J (1997) Temperature and soil moisture dependence of N mineralization in intact soil cores. Soil Biol Biochem 29:1557–1563
Templer PH, Groffman PM, Flecker AS, Power AG (2005) Land use change and soil nutrient transformations in the Los Haitises region of the Dominican Republic. Soil Biol Biochem 37:215–225
Valenzuela-Solano C, Crohn DM, Downer JA (2005) Nitrogen mineralization from eucalyptus yardwaste mulch applied to young avocado trees. Biol Fertil Soils 41:38–45
Vangestel M, Merckx R, Vlassak K (1993) Microbial biomass responses to soil drying and rewetting: the fate of fast- and slow-growing microorganisms in soils from different climates. Soil Biol Biochem 25:109–123
Verchot LV, Groffman PM, Frank DA (2002) Landscape versus ungulate control of gross mineralization and gross nitrification in semi-arid grasslands of Yellowstone National Park. Soil Biol Biochem 34:1691–1699
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115
Wang CH, Wan SQ, Xing XR, Zhang L, Han XG (2006) Temperature and soil moisture interactively affected soil net N mineralization in temperate grassland in Northern China. Soil Biol Biochem 38:1101–1110
Wang LX, Wang J, Huang JH (2008) Net nitrogen mineralization and nitrification in three subtropical forests of southwestern China. Dyn Soil Dyn Plant 2(1):33–40
Xu YQ, Li LH, Wang QB, Chen QS, Cheng WX (2007) The pattern between nitrogen mineralization and grazing intensities in an Inner Mongolian typical steppe. Plant Soil 300:289–300
Zhang XS, Gao Q, Yang DA, Zhou GS, Ni J, Wang Q (1997) A gradient analysis and prediction on the northeast China Transect (NECT) for global change study. Acta Bot Sin 9:785–799
Zhang XL, Wang QB, Li LH, Han XG (2008) Seasonal variations in nitrogen mineralization under three land use types in a grassland landscape. Acta Oecol 34:322–330
Zhou LS, Huang JH, Lǚ FM, Han XG (2009) Effects of prescribed burning and seasonal and interannual climate variation on nitrogen mineralization in a typical steppe in Inner Mongolia. Soil Biol Biochem 41:796–803
Acknowledgments
This research was jointly funded by the “Hundred Talents” Program, Knowledge Innovation Project (KZCX2-YW-432) of the Chinese Academy of Sciences, the National Natural Science Foundation of China (Nos: 40501072 and 40673067), and the Ministry of Science and Technology of China (No. 2007BAC03A11).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, XR., Dong, YS., Ren, JQ. et al. Drivers of soil net nitrogen mineralization in the temperate grasslands in Inner Mongolia, China. Nutr Cycl Agroecosyst 87, 59–69 (2010). https://doi.org/10.1007/s10705-009-9312-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-009-9312-5