Abstract
The present research aimed to evaluate the effects of long-term fasting and refeeding on the growth and antioxidant defenses in the liver and serum in Yangtze sturgeon (Acipenser dabryanus). The results showed that body mass and hepatosomatic index significantly decreased with long-term fasting, but they could be recovered after 4 weeks refeeding. Compared with controls, the antioxidant defense parameters of starvation indicated that the malondialdehyde (MDA) levels increased significantly in both tissues; the activities of superoxide dismutase (SOD) and glutathione peroxidase (GPx) increased obviously in serum and liver, respectively (p < 0.05). However, the activities of catalase (CAT) always decreased in two tissues including liver and serum during the whole starvation, as was the SOD in the liver (p < 0.05). Interestingly, the T-AOC levels of Yangtze sturgeon presented higher at early stage of starvation and dropped down at the end of starvation (p < 0.05). However, all of the antioxidant index above returned to origin level after 4 weeks refeeding. In conclusion, the present study indicated that long-time fasting induced oxidative stress in Yangtze sturgeon and it may easily adjust their physiological status under situations characterized by a long-term starvation and refeeding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In general, many fish species are routinely affected in the wild by periods of starvation of a variable length. Starvation is one of the most important factors that affect the normal growth, development, breeding, and survival of fishes (Furné et al. 2009; Bayir et al. 2011). Compared to vertebrates other than fish that can withstand starvation only for brief periods, fishes can withstand prolonged periods of food deprivation in their natural environments during migrations, reproduction (Miller et al. 2009), and also in fishes farming (Furné et al. 2012). When fishes are deprived of food, they can utilize the stored lipids as a major source of metabolic energy through oxidative pathways (Morales et al. 2004; Bar 2014). However, it has been reported that most of the detrimental effects of food deprivation could be mainly attributed to the participation of ROS generated under food deprivation (Morales et al. 2004). And oxidative stress reaction caused by adverse factors involves the overproduction of reactive oxygen species (ROS) (Sies 1987).
It is well known that oxidative stress occurs when the rate of generation of ROS exceeds that of their removal (Livingstone 2001). During this oxidation process, production of reactive oxygen species (ROS) takes place as a result of univalent reduction of O2−. And these radicals are considered to be a harmful byproduct of oxidative metabolism, which can cause molecular damage in living cells (Bowden 2008), such as DNA, protein, and lipids (Martínez-Álvarez et al. 2005), finally leading to cell death (Regoli and Giuliani 2014); because free radicals are particularly unstable and lively, they have the unique oxidation characteristic of attacking adjacent molecules, which form chain reactions, and ultimately damage tissues (Martin et al. 2010)
Fish is the low-variable temperature vertebrate, and their abilities of specificity immune response are relatively low, but fish are equipped with a variety of enzymatic and non-enzymatic antioxidant scavenging systems to maintain endogenous ROS at relatively low levels and to attenuate the damage related to the high reactivity of ROS (Filho et al. 2001; Halliwell and Gutteridge 2004). The key antioxidant enzymes in this antioxidant defense system include superoxide dismutase (SOD), which detoxifies superoxide anions; catalase (CAT), which reduces H2O2; and glutathione peroxidase (GPx) which reduces both H2O2 and organic peroxides by a glutathione-dependent reaction, and so on (Martínez-Álvarez et al. 2005). It has also been widely reported that the intracellular levels of some non-enzymatic antioxidants, such as glutathione, influence the activity of the enzymatic antioxidants (Halliwell and Gutteridge 2004). These antioxidant defenses can be influenced by adverse factors such as starvation, hypoxia, heavy metals, and high or low temperature (Bayir et al. 2011; Davis and Gaylord 2011). Recently, attention has been paid to the pertinent literature to know the response to fasting of multiple hematological (Morshedi et al. 2011), biochemical (Luo et al. 2013), immunological parameters (Akbary and Jahanbakhshi 2016) and antioxidant defenses (Caruso et al. 2011; Zheng et al. 2016).
As a typical endemic important species in China, the Yangtze sturgeon (Acipenser dabryanus) has been a critically endangered species, and its wild resources have a large decline and have been on the verge of extinction due to overfishing and water pollution caused by hydroelectric projects (Zhang et al. 2011; Yang et al. 2018). It is for granted that the sturgeon is protected by a great deal of proliferation activities. However, the sturgeon entering the wild environment for the first time will inevitably face the threat of starvation, as well as sturgeon during the migration process (Zhuang et al. 1997). And because of its preciousness and rarity, there are few reports about starvation in Yangtze sturgeon. Therefore, studying about starvation in Yangtze sturgeon is of great significance to know and protect it better. Thus, the objectives of the study were (1) to investigate the effect of starvation on the antioxidant defenses and (2) to evaluate physiological status of Yangtze sturgeon after refeeding.
Materials and methods
Ethics approval and consent to participate
All experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals in China. All experimental procedures and sample collection were approved by the Institutional Animal Care and Use Committee (IACUC) of the College of Animal Science and Technology of Sichuan Agricultural University, Sichuan, China, under permit no. DKY-S20176933.
Animals and maintaining
Healthy Yangtze sturgeon weighing 193.67 ± 30.75 g were obtained from Fish Hatchery Center, Fisheries Institute of Sichuan Academy of Agricultural Science, Sichuan province. They were kept in two big tanks for 2 weeks. The tank diameter was 3 m, and water depth was 0.80 m. The fish were fed at the rate 2% total body weight at 9:00 and 16:00 per day. The commercial diet (42% protein, 8% lipids, 5% fiber, 2.2% lysine,1.4%total phosphorus, 16% ash, and 12.5% water content) was produced by TONGWEI company, Sichuan, China. In the whole experiment, water was supplied to each tank at a rate of 2 L/min in recycling aquaculture system with continuous aeration and natural light. Water temperature, pH, ammonia nitrogen, nitrite, and dissolved oxygen was held at 20 ± 1 °C, 7.3 ± 0.3, < 0.1 mg/L, < 0.01 mg/L, and 6.6 ± 0.4 mg/L.
Experimental design
Before performing the experiment, 135 sturgeons were randomly distributed into 9 fiberglass tanks (1.5 m diameter and 0.80 m water depth) to accommodate the culture environment for 2 days. This experiment was divided into three treatment groups (3 treatment groups with 3 parallels per treatment; Table 1). Three treatment groups were group A, control 8 weeks (0 week starved, 8 weeks fed, S/F = 0:8); group B, 4 weeks starved and 4 re-fed (S/F = 4:4); and group C, 8 weeks starved (S/F = 8:0), respectively. During the experiment, the fish were fed by the means mentioned above in relevant treatment.
Sampling
Nine fish of each treatment (three fish of each tank; Table 1) were captured at weeks 0, 2, 4, and 8. In the per sample, fish were measured body mass after being euthanized with overdoes buffered MS-222 as quickly as possible. Prior to blood sampling, fish were immersed in the anesthetic solution until they reached a stage of complete immobility, and blood was subsequently sampled from the blood vessel just below the anal fin with a 2-mL sterile syringe. The blood was injected into separate centrifuge tubes and stored at 4 °C. After preliminary delamination, the blood was separated by centrifugation at 8000×g for 10 min until it was completely stratified. The clear serum on the upper layer of the solution was then transferred into a new centrifuge tube by pipette for measurement. Then, livers were excised and frozen immediately in liquid nitrogen, also the serum. All the samples were transferred and stored at − 80 °C. Hepatosomatic indexes (HSI) of fish were determined as liver mass/fish mass × 100.
Determination of enzyme activities and lipid peroxidation levels
The activities of SOD, GPx, CAT activities, and the levels of T-AOC and MDA in the serum and liver of fish were measured using reagent kits (Product A001-1, Code A005, A007-1, A015-2 and A003-1, respectively; Jiancheng Bioengineering Institute, Nanjing, China) via spectrophotometric analysis with a microplate reader.
The serum samples used for the determination of SOD, GPx, CAT activities, and the levels of T-AOC and MDA were divided into five parts. Each part was used to measure an enzyme activity or parameter, according to the kit instructions.
The hepatic samples used for the determination of SOD, GPx, CAT activities, and the levels of T-AOC and MDA were homogenized in a cold 0.9% sodium chloride buffer solution (sample/buffer solution (mg/ml), 1/9) on ice using an electrically driven tissue homogenizer. The homogenate was centrifuged at 3500×g for 10 min at 4 °C. The 1-mL supernatant was collected and immediately transferred into an Eppendorf tube for enzyme activity analysis. The protein concentration of the tissues was determined using BCA protein assay reagent, according to the method of Bradford (Bradford 1976). All assays were completed within 1 week when the supernatant was collected.
Statistical analysis
All data analysis was performed at SPSS 22.0. Data were presented as mean ± SEM (standard error of the mean) of the mean. Data were analyzed by one-way analysis of variance (ANOVA), and significant differences between feeding, starvation, and starvation-refeeding groups in different sample weeks were determined by Duncan’s multiple-range post-hoc test. Statistical significance was accepted at P < 0.05 (n = 9).
Results
Body mass and hepatosomatic index
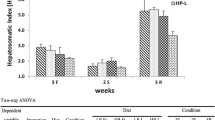
Changes in the body mass and HSI induced by long-term starvation and refeeding in Yangtze sturgeon are shown in Table 2. The result showed that starved fish lost their body mass during the entire experiment time. Similarly, starvation led a significant decreased trend in HSI values with increasing starvation time. However, starved fish had restored their HSI values to those of the control group after 4 weeks of refeeding (P < 0.05; Table 2).
Oxidative stress in Yangtze sturgeon
MDA contents, SOD, GPx, CAT activities, and T-AOC in liver
The effects of starvation and refeeding on the hepatic in Yangtze sturgeon are presented in Fig. 1. The MDA contents measured in liver increased significantly in the starved group and returned to normal level after refeeding (Fig. 1a). Nevertheless, the activities of SOD in liver just decreased significantly for starved group at the fourth week, and similar results were found on the activities of CAT in liver (Fig. 1b, d). On the contrary, the activities of GPx increased significantly in starvation at the fourth week (Fig. 1c). Interestingly, the level of T-AOC in liver underwent a process that first decreased, then increased, and finally decreased, and returned to normal level after refeeding (Fig. 1f).
MDA contents, SOD, GPx, CAT activities, and T-AOC in liver of Yangtze sturgeon during starvation and refeeding periods. Note: (a) for Malondialdehyde, (b) for Superoxide Dismutase, (c) for Glutathione Peroxidase, (d) for Catalase, (e) for Total Antioxidant Capacity. The fish in the experiment groups were treated differently (8F = control: 8 weeks feed; 4S4F: 4 weeks starve and 4 weeks refeed; 8S: 8 weeks starve). Data are means ± S.E. with n = 9. Asterisks indicate significant differences between values of control fish and the other experimental groups and at the different weeks of sampling; *P < 0.05 (n = 9)
MDA contents, SOD, GPx, CAT activities, and T-AOC in serum
The effects of starvation and refeeding in serum in Yangtze sturgeon are presented in Fig. 2. It was showed that the MDA contents in serum just increased significantly in starvation at the second week (Fig. 2a). No change was found in the serum GPx (Fig. 2c). Interestingly, the activities of SOD in serum increased significantly in the starved group, but the significant decrease of the activities of CAT in serum occurred at the fourth and eighth week starvation, and both of them returned to normal level after refeeding (Fig. 2b, d). The level of T-AOC in serum presented a trend in starvation group that it increased firstly and then decreased and returned to normal level after refeeding (Fig. 2f).
MDA contents, SOD, GPx, CAT activities, and T-AOC in serum of Yangtze sturgeon during starvation and refeeding periods. Note: (a) for Malondialdehyde, (b) for Superoxide Dismutase, (c) for Glutathione Peroxidase, (d) for Catalase, (e) for Total Antioxidant Capacity. The fish in the experiment groups were treated differently (8F = control: 8 weeks feed; 4S4F: 4 weeks starve and 4 weeks refeed; 8S: 8 weeks starve). Data are means ± S.E. with n = 9. Asterisks indicate significant differences between values of control fish and the other experimental groups and at the different weeks of sampling: *P < 0.05 (n = 9)
Discussion
Body mass and hepatosomatic index
Starvation can decrease tissue metabolic capacities but, on the other hand, the food deprivation causes degradation of endogenous sources of energy (lipids, glycogen, and proteins) in order to maintain the fish physiological homeostasis, leading to weight loss (Zheng et al. 2016). Similarly, low HSI values in fish were usually correlated to nutritional problems, because the relative size of the liver is correlated with the nutritional status of the fish (EchevarríA et al. 1997). Body mass and HSI values evaluated in this study decreased in starved fish, which was in agreement to that reported by other authors (Pedro et al. 2003; Mohapatra et al. 2015). Thereby, one of the most important reasons for the reduction of HSI might be the consumption of a large amount of energy substances in the liver, such as liver glycogen, when lacking food (Pérez-Jiménez et al. 2007).
Oxidative stress in Yangtze sturgeon
Based on MDA levels, a metabolite derived from lipid peroxidation (Stephensen et al. 2002; Domenicali et al. 2001), the results clearly showed that prolonged starvation resulted in oxidative stress and that starved fish expressed a significant increase in liver and serum MDA compared with control fish, which was consistent with other studies (Robinson et al. 1997; Gomi and Matsuo 1998; Pandey et al. 2003). Although there was no significant increase in MDA in late post-starvation serum, these enzymatic activities showed a downward trend as the starvation time prolonged, except the increase in liver GPx and serum SOD. These results indicated that long-time fasting could induce oxidative stress in Yangtze sturgeon.
According to this study, Yangtze sturgeon may resist oxidative stress caused by starvation mainly through increasing liver GPx and serum SOD. Because only the liver GPX and serum SOD were significantly elevated in the enzyme system, when oxidative stress occurred due to starvation. On the contrary, other enzyme activities did not increase or even decreased (Figs. 1 and 2). These results were different from that all the antioxidant enzyme activities increased significantly in other fish that underwent starvation, such as Mugil cephalus (Akbary and Jahanbakhshi 2016). However, that the CAT activity decreased in fasting fish was consistent with Sparus aurata (Pascual et al. 2003). The diversity of this result may be due to different varieties. On the other hand, the antioxidant system of animals contains not only the enzyme system but also the non-enzymatic system, and it is established that the components of these non-enzymatic systems typically involve antioxidant compounds (e.g., NADH/NADPH, glutathione) and dietary micronutrients (e.g., vitamins E and C, carotenoids) (Lee et al. 2016). Yangtze sturgeon may be against oxidative stress due to starvation through a variety of defense systems synergy according to the results of T-AOC in this study, in which T-AOC increased early in starvation time.
Interestingly, the T-AOC in both tissues decreased significantly during late starvation. The decrease agreed to the findings by other researchers (Feng et al. 2011). One possible explanation for these results was as follows: as the starvation time was extended, the amount of free radicals in Yangtze sturgeon increased, and some effective substances were subsequently reduced, which might be due to the fact that catabolism inhibited the oxidation of exogenous electrophilic groups and avoided lipid peroxidation, and ultimately led to the reduction of T-AOC measurements (Feng et al. 2011). Another reason might be the long duration of starvation, which declined the body’s overall functioning of Yangtze sturgeon, as did its antioxidant capacity (Llesuy et al. 2001). Simultaneously, when the antioxidant system is not able to eliminate or neutralize the excess of ROS, there is an increased risk of oxidative damage because of lipid peroxidation accumulation, which may, in turn, decrease enzyme activities or even degrade the enzymes (Zhang et al. 2008). Additionally, starvation has been reported to decrease the expression of genes encoding a number of secreted immune-related proteins, including serum amyloid A, complement factor B, and serotransferrin in the Atlantic salmon (Martin et al. 2010). Therefore, we could not rule out a possibility that antioxidant enzyme activity would be downregulated by starvation with time, as demonstrated by Choi et al. 2012.
Refeeding
The present research also aims to evaluate the oxidative stress whether or not disappeared after refeeding, which was induced by starvation. The results obtained in refeeding Yangtze sturgeon indicated that HSI, peroxidation levels, and all the enzymatic activities of both antioxidant defenses and intermediary metabolism returned to origin values. Therefore, these results suggested that Yangtze sturgeon probably eliminated oxidative stress induced by starvation after 4 weeks refeeding, and these might reflect a sort of compensatory growth response. Compensatory growth is the phase of rapid growth, greater than normal or control growth, which occurs upon adequate refeeding following a period of malnutrition (Laizcarrión et al. 2012). In this study, although the body weight was not fully compensated after 4 weeks of refeeding, the weight gain rate reached normal levels (Table 2). These results were in consistent with those obtained in Gadus morhua by Jobling et al. (1994), on which the weight was not compensated after 3 weeks of refeeding. However, unlike other marine bony fishes such as Oreochromis mossambicus (Fox et al. 2010), full compensation was observed in fish subjected to starvation and 2 weeks of refeeding. This might be due to the differences resulting in different fish species.
In addition, the inhomogeneous fish size might affect the results in the study due to its rarity and preciousness, as well as the difficulty of reproduction to obtain adequate uniform size (Zheng et al. 2016). Furthermore, the effect of experimental conditions, fish age, and sex probably masked the food deprivation effecting per se. Therefore, according to the present findings, it is suggested that to avoid misinterpretation of experimental results, any assay involving sturgeon in aquarium conditions must take into account the acclimation period to this “artificial new environment.” Climatic and environmental conditions during animal sampling should also be considered due to the fact that they might affect the physiological status of Yangtze sturgeon.
Conclusion
The rationale for the present research was to investigate whether Yangtze sturgeon may face a long fasting followed by refeeding without any significant damage for health in order to optimize in proliferation protection and its production in aquaculture. In the present study, it was observed that Yangtze sturgeon could alter the level of their antioxidant defense to cope with oxidative stress under starvation and refeeding. Knowledge of the duration of these alterations and their reversibility in response to refeeding may provide useful insight for a better understanding of the physiology of Yangtze sturgeon in proliferation and release and intensive rearing.
References
Akbary P, Jahanbakhshi A (2016) Effect of starvation on growth, biochemical, hematological and non-specific immune parameters in two different size groups of grey mullet, Mugil cephalus, (linnaeus, 1758). Acta Ecol Sin 36(3):205–211
Bar N (2014) Physiological and hormonal changes during prolonged starvation in fish. Can J Fish Aquat Sci 71(10):1447–1458
Bayir A, Sirkecioglu AN, Bayir M, Haliloglu HI, Kocaman EM, Aras NM (2011) Metabolic responses to prolonged starvation, food restriction, and refeeding in the brown trout, Salmo trutta: oxidative stress and antioxidant defenses. Comp Biochem Physiol B 159(4):191–196
Bowden TJ (2008) Modulation of the immune system of fish by their environment. Fish Shellfish Immunol 25(4):373–383
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Caruso G, Denaro MG, Caruso R, Mancari F, Genovese L, Maricchiolo G (2011) Response to short term starvation of growth, haematological, biochemical and non-specific immune parameters in European sea bass (Dicentrarchus labrax) and blackspot sea bream (Pagellus bogaraveo). Mar Environ Res 72(1):46–52
Choi CY, Shin HS, Choi YJ, Kim NN, Lee J, Kil GS (2012) Effect of led light spectra on starvation-induced oxidative stress in the cinnamon clownfish Amphiprion melanopus. Comp Biochem Physiol A 163(3–4):357–363
Davis KB, Gaylord TG (2011) Effect of fasting on body composition and responses to stress in sunshine bass. Comp Biochem Physiol A 158(1):30–36
Domenicali M, Caraceni P, Vendemiale G, Grattagliano I, Nardo B, DallTAgata M, Santoni B, Trevisani F, Cavallari A, Altomare E, Bernardi M (2001) Food deprivation exacerbates mitochondrial oxidative stress in rat liver exposed to ischemia-reperfusion injury. J Nutr 131(1):105–110
EchevarríA G, MartíNez-Bebiá M, Zamora S (1997) Evolution of biometric indices and plasma metabolites during prolonged starvation in European sea bass (Dicentrarchus labrax, l.). Comp Biochem Physiol A 118(1):111–123
Feng GP, Shi X, Huang X, Zhuang P (2011) Oxidative stress and antioxidant defenses after long-term fasting in blood of Chinese sturgeon (Acipenser sinensis). Procedia Environ Sci 8(1):469–475
Filho DW, Tribess T, Gáspari C, Claudio FD, Torres MA, Magalhães ARM (2001) Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture 203(1–2):149–158
Fox BK, Breves JP, Davis LK, Pierce AL, Hirano T, Grau EG (2010) Tissue-specific regulation of the growth hormone/insulin-like growth factor axis during fasting and re-feeding: importance of muscle expression of IGF-I and IGF-II mRNA in the tilapia. Gen Comp Endocrinol 166(3):573–580
Furné M, Sanz A, García-Gallego M, Hidalgo MC, Domezain A, Domezain J, Morales AE (2009) Metabolic organization of the sturgeon acipenser naccarii: a comparative study with rainbow trout Oncorhynchus mykiss. Aquaculture 289(1–2):161–166
Furné M, Morales AE, Trenzado CE, Garcã-A-Gallego M, Carmen HM, Domezain A et al (2012) The metabolic effects of prolonged starvation and refeeding in sturgeon and rainbow trout. J Comp Physiol B 182(1):63–76
Gomi F, Matsuo M (1998) Effects of starving and food restriction on the antioxidant enzymes activity of rat livers. J Gerontol A Biol Sci Med Sci 53(3):1361–1367
Halliwell B, Gutteridge JMC (2004) Free radicals in biology and medicine. J Free Radic Biol Med 1(4):331–332
Jobling M, Meløy OH, Santos JD, Christiansen B (1994) The compensatory growth response of the Atlantic cod: effects of nutritional history. Aquac Int 2(2):75–90
Laizcarrión R, Viana IR, Cejas JR, Ruizjarabo I, Jerez S, Martos JA et al (2012) Influence of food deprivation and high stocking density on energetic metabolism and stress response in red porgy, Pagrus pagrus L. Aquac Int 20(3):585–599
Lee J, Choi J, Scafidi S, Wolfgang MJ (2016) Hepatic fatty acid oxidation restrains systemic catabolism during starvation. Cell Rep 16(1):201–212
Livingstone DR (2001) Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull 42(8):656–666
Llesuy S, Evelson P, Campos AM, Lissi E (2001) Methodologies for evaluation of total antioxidant activities in complex mixtures. A critical review. Biol Res 34(2):51–73
Luo G, Liu G, Tan HX (2013) Effects of stocking density and food deprivation-related stress on the physiology and growth in adult Scortum barcoo (Mcculloch & Waite). Aquac Res 44(6):885–894
Martin SA, Douglas A, Houlihan DF, Secombes CJ (2010) Starvation alters the liver transcriptome of the innate immune response in Atlantic salmon (Salmo salar). BMC Genomics 11(1):418
Martínez-Álvarez RM, Morales AE, Sanz A (2005) Antioxidant defenses in fish: biotic and abiotic factors. Rev Fish Biol Fish 15(1–2):75–88
Miller KM, Schulze AD, Ginther N, Li S, Patterson DA, Farrell AP, Hinch SG (2009) Salmon spawning migration: metabolic shifts and environmental triggers. Comp Biochem Physiol D Genomics Proteomics 4(2):75–89
Mohapatra S, Chakraborty T, Shimizu S, Urasaki S, Matsubara T, Nagahama Y, Ohta K (2015) Starvation beneficially influences the liver physiology and nutrient metabolism in Edwardsiella tarda infected red sea bream (Pagrus major). Comp Biochem Physiol A 189:1–10
Morales AE, Pérez-Jiménez A, Hidalgo MC, Abellán E, Cardenete G (2004) Oxidative stress and antioxidant defenses after prolonged starvation in Dentex dentex liver. Comp Biochem Physiol C 139(3):153–161
Morshedi V, Ashouri G, Kochanian P, Yavari V, Bahmani M, Pourdehghani M et al (2011) Effects of short-term starvation on hematological parameters in cultured juvenile beluga. J Vet Res 66(4):363–368 381
Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river yamuna fish Wallago attu (bl. & schn.). Sci Total Environ 309(1–3):105–115
Pascual P, Pedrajas JR, Toribio F, López-Barea J, Peinado J (2003) Effect of food deprivation on oxidative stress biomarkers in fish (sparus aurata). Chem Biol Interact 145(2):191–199
Pedro ND, Delgado MJ, Gancedo B, Alonso-Bedate M (2003) Changes in glucose, glycogen, thyroid activity and hypothalamic catecholamines in tench by starvation and refeeding. J Comp Physiol B Biochem Syst Environ Physiol 173(6):475–481
Pérez-Jiménez A, Guedes MJ, Morales AE, Oliva-Teles A (2007) Metabolic responses to short starvation and refeeding in dicentrarchus labrax. Effect of dietary composition. Aquaculture 265(1):325–335
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93(1):106–117
Robinson MK, Rustum RR, Chambers EA, Rounds JD, Wilmore DW, Jacobs DO (1997) Starvation enhances hepatic free radical release following endotoxemia. J Surg Res 69(2):325–330
Sies H (1987) Biochemistry of oxidative stress. Eur J Cancer Clin Oncol 23(11):1798–1798
Stephensen E, Sturve J, Förlin L (2002) Effects of redox cycling compounds on glutathione content and activity of glutathione-related enzymes in rainbow trout liver. Comp Biochem Physiol C 133(3):435
Yang S, Wu H, Zhao LL, Xiao Q, Fu HM, Yang SY et al (2018) Morphology and histochemical analysis of glycoproteins in the digestive tract of Dabry’s sturgeon. J Appl Ichthyol 28(1):1–9
Zhang XD, Cai LS, Wu TX (2008) Effects of fasting on the meat quality and antioxidant defenses of market-size farmed large yellow croaker (Pseudosciaena crocea). Aquaculture 280(1–4):136–139
Zhang H, Wei QW, Du H, Li LX (2011) Present status and risk for extinction of the Dabry’s sturgeon (acipenser dabryanus) in the Yangtze river watershed: a concern for intensified rehabilitation needs. J Appl Ichthyol 27(2):181–185
Zheng JL, Zhu QL, Shen B, Zeng L, Zhu AY, Wu CW (2016) Effects of starvation on lipid accumulation and antioxidant response in the right and left lobes of liver in large yellow croaker Pseudosciaena crocea. Ecol Indic 66:269–274
Zhuang P, Ke F, Wei Q, He X, Cen Y (1997) Biology and life history of Dabry's sturgeon, acipenser dabryanus, in the Yangtze river. Environ Biol Fish 48(1–4):257–264
Acknowledgements
We thank Jie Du of Sichuan Agricultural University for assistance with rearing the fish, and thanks for Huadong Li of Fisheries Institute of Sichuan, Academy of Agricultural Science for rearing the fish and sampling tissues. More thanks would be given to the Fish Hatchery Center, Fisheries Institute of Sichuan Academy of Agricultural Science for supplying test fish. We also appreciated the assistance from Peter Shep (School of Freshwater Sciences, University of Wisconsin-Milwaukee) for assistance with manuscript revision.
Funding
The research was supported by China Agriculture Research System (CARS-46), which is also supported by the “Double Support Project” fund of Sichuan Agricultural University (SICAU; No. 03573018 and No. 03573013).
Author information
Authors and Affiliations
Author notes
S. Yang and K. He are co-first authors
Corresponding authors
Ethics declarations
All experiments were performed according to the Guidelines for the Care and Use of Laboratory Animals in China. All experimental procedures and sample collection were approved by the Institutional Animal Care and Use Committee (IACUC) of the College of Animal Science and Technology of Sichuan Agricultural University, Sichuan, China, under permit no. DKY-S20176933.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, S., He, K., Yan, T. et al. Effect of starvation and refeeding on oxidative stress and antioxidant defenses in Yangtze sturgeon (Acipenser dabryanus). Fish Physiol Biochem 45, 987–995 (2019). https://doi.org/10.1007/s10695-019-0609-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-0609-2