Abstract
Fluorescent protein (FP) transgenesis is used in the ornamental aquarium trade to produce new colour morphs in tropical fish. Understanding whether such genetic modification could alter ability to survive temperate waters, or interactions with native fish, should such fish be released to natural systems is critical in developing policy on their commercial use. We examined the competitive foraging ability and cold tolerance of unrelated pet-trade sourced adult green FP transgenic tetra and non-transgenic white tetra (Gymnocorymbus ternetzi), as well as white non-transgenic and green FP transgenic juvenile progeny of these groups. FP transgenesis did not affect the foraging success or aggressive behaviour in either adult or juvenile fish, indicating FP transgenesis may not influence potential hazards through this pathway. During a cold temperature tolerance trial, adult green tetras had greatly diminished cold tolerance relative to unrelated adult white fish, while sibling juvenile offspring of these groups had intermediate cold tolerance between adult fish groups that were not affected by FP transgenesis. This data suggests background genetics, rearing history and/or life stage may play larger roles in cold tolerance than FP transgenesis in this species. Unexpectedly, both adult and juvenile white tetras were 3.8 times more likely to take refuge in shelters when temperature declined than green tetras. These data indicate FP transgenic fish may pose equal or lesser risk than non-transgenic fish, should they be released to natural environments. Results also demonstrate that unrelated pet-trade sourced fish may not always be appropriate models for examining effects of FP transgenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetically modified tropical fish with fluorescent protein (FP) transgenes are used to produce unique colours for the ornamental pet-trade. Such fish are available in various species and colours in some countries (e.g. Taiwan, USA, and Canada). In Canada, commercial use of genetically modified fish is regulated under the Canadian Environmental Protection Act (CEPA) New Substances Notification Regulations (Organisms) [NSNR(O)]. Environmental risk assessments conducted to guide regulatory decisions on commercial use of genetically modified tetras (Gymnocorymbus ternetzi Boulenger, 1895) under CEPA noted there was limited data on the effects of FP transgenesis on behaviour and/or physiology in tropical fish (DFO 2018, 2019). This resulted in uncertainty in conclusions on potential for the genetically modified tetras to pose hazards to the environment should the fish be released to natural water systems (DFO 2018, 2019).
When conducting risk assessments associated with use of transgenic fish, effects of both the intended phenotype (e.g. fluorescent colouration) and any off-target effects must be considered. For some genetic modifications, off-target effects can be strong and greatly influence potential for the genetically modified organism to pose risk to natural environments (e.g. growth hormone transgenic salmonids can have greatly altered appetite and competitive behaviour, with potential for negative consequences to wild populations, Devlin et al. 2004; Devlin et al. 2015). In contrast, FP transgenesis is considered a “neutral” marker, where the only phenotypic effect expected is targeted fluorescent colouration (see Stewart 2006). However, when examined, there have been some behavioural and physiological effects of FP transgenesis in fish (see below). There have been off-target reports in other models as well. For example, enhanced green FP (eGFP) transgenesis altered metabolic pathways in mice (Li et al. 2013), and there are some reports of eGFP altering gene expression in cell lines (Badrian and Bogoyevitch 2007; Baens et al. 2006; Coumans et al. 2014; Mak et al. 2007).
The effect of FP transgenesis on behaviour in fish has been primarily restricted to effects of a red FP (RFP) in zebrafish (Danio rerio), and reported effects are often conflicting. Howard et al. (2015) reported non-transgenic male zebrafish were aggressively superior to related RFP transgenic zebrafish and sired more young. In another study, related RFP and non-transgenic zebrafish females were reported to prefer to associate with RFP relative to non-transgenic males in mating but not shoaling contests, although this did not influence mating success in this study (Owen et al. 2012). In contrast, Snekser et al. (2006) reported non-transgenic zebrafish did not have a preference for associating with non-transgenic or unrelated RFP fish in a shoaling or mating context. In terms of trophic interactions, RFP zebrafish were reported to be more preyed upon (Hill et al. 2011), less preyed upon (Jha 2010), and equally preyed upon (Cortemeglia and Beitinger 2006b) than unrelated non-transgenic zebrafish. The different results among these experiments may be due to differences in rearing history or genetic backgrounds of the unrelated strains and/or experimental design (e.g. type of predator and environmental complexity). There have been no studies on effects of FP transgenesis on ability to compete for food in tropical fish. Fluorescent protein transgenesis could theoretically alter foraging if production of FP places increased metabolic demand in fish or if presence of FP, particularly in the sense organs (e.g. vision), interferes with foraging ability.
The most examined off-target effect of FP transgenesis in fish is its effect on cold tolerance as this is a critical factor in determining the potential for a tropical fish to establish and spread as an exotic in temperate climates. RFP transgenic zebrafish have been reported to be less tolerant of extreme temperatures than unrelated non-transgenic zebrafish (Cortemeglia and Beitinger 2005, 2006a), while only one of three research lines of eGFP zebrafish had diminished cold tolerance than the progenitor non-transgenic strain when all were reared in equal conditions (Leggatt et al. 2018a). Of the six commercially available colours of FP transgenic tetras, GloFish LLC (WI, USA) reported five of the six lines had significantly diminished cold tolerance relative to non-transgenic sibling fish (see DFO 2018, 2019). Available studies do indicate that diminished cold tolerance is a common, but not universal, off-target effect of FP transgenesis in tropical fish, although the effect of background genetics and rearing history is unknown in several studies.
The current study examines whether FP transgenesis affects competitive behaviour and cold tolerance in the tetra (G. ternetzi), in a line currently approved for commercial use in the USA and Canada due to its lack or limited potential to survive in temperate climates (see DFO 2018; USFWS 2017). The study also examines whether similar conclusions would be drawn if unrelated genetically modified and non-transgenic fish sourced from the pet-trade were used compared to offspring of these fish (i.e. would background genetics and/or rearing history effect the results of the experiment). This will help guide future studies and risk assessments of FP transgenic tropical fish as different species and lines enter the market.
Materials and methods
Fish
All experiments were conducted under approval of the Pacific Region Animal Care Committee (AUP18-014) following guidelines established by the Canadian Council on Animal Care (Ottawa, ON, Canada). Young adult GloFish® Electric Green® Tetras (GloFish LLC, hereafter called green tetras) and white tetras (both colour variants of the black tetra G. ternetzi, either genetically modified or from selective breeding of a naturally occurring phenotype, respectively) were obtained from a local aquarium distributor (Surrey, British Columbia, Canada). Fish were reared in 76 L static aquaria with waterfall-type filters maintained at approximately 27 °C and enriched with gravel, 4 inch PVC pipe shelters, and plastic plants. Fish were fed a mix of tropical fish flakes and frozen bloodworms two times per day. After 3 months acclimation, when fish were confirmed to have breeding activity and eggs were found in the gravel, fish were bred in small groups (one green female and two white males in 1 L tank) and in large groups (ten green and ten white fish of mixed sex in 37 L tank) in static breeding tanks with false floors to prevent adults eating fertilized eggs. Both methods were used as there was limited success with small groups. Embryos and early juveniles were reared in aerated static tanks containing E2 Embryo Media (http://zebrafish.org/documents/protocols.php) in a 27 °C incubator until large enough to be reared in filtered, heated aquaria as per adults above. Juveniles were fed a mix of newly hatched decapsulated brine shrimp, GEMMA Micro (Skretting, NB, Canada), and Nutrafin Max Baby (Hagen Inc., QC, Canada) and, when large enough, frozen calanus shrimp, frozen bloodworms, and crushed tropical fish flakes. Juvenile offspring of small crosses produced approximately 50 or 100% green offspring, indicating green adults were a mix of fish hemizygous or homozygous for the FP transgene.
Competition trials
For competition trials, pairs of approximately size-matched fish (one green one white) were placed in static aerated 3 L (adults) or 2 L (juvenile) Plexiglas tanks containing a heater, gravel, and plastic plant for refuge. Pairs of fish were placed in the tanks and allowed to acclimate for approximately 32 h with feeding. To initiate the trial, fish were fed a single bloodworm, and which fish ate the bloodworm was recorded. Individual bloodworms were added continuously in this manner until either four had been added in a row with no fish eating them or 20 worms were added, which ever came first. Behaviour of juveniles was video recorded before, during, and after feeding, and number of chases 5 min pre-feed, during feed, and for 5 min post-feed counted and averaged. Fish were then lightly anaesthetized with buffered 50 mg/L tricaine methanesulfonate (Syndel Canada, BC, Canada), and mass and fork length were recorded. Adult competition trials took place after 5 months rearing in equal conditions, and juvenile competition trials took place 7 months post-fertilization. In total, 23 pairs of adults and 18 pairs of juveniles were used in the competition trials.
Cold tolerance trial
For the cold tolerance trial, two 76 L tanks were set up as described for adults above, and 10 white adult, 10 green adult, 10 white juvenile, and 10 green juvenile tetras were added to each tank. Remaining adult and juvenile fish were reared together in a 37 L tank for controls. Fish were reared at approximately 20 °C for 2 months prior to start of the trial. Cold tolerance trials were conducted as per Leggatt (2019). In brief, the two experimental tanks were connected to in-line chillers, and temperature was dropped rapidly by 1 °C at approximately 0820 each day and maintained at this temperature (± 0.5 °C) for 24 h. Fish were monitored two times a day for activity level, feeding behaviour, and ability to maintain equilibrium until fish stopped eating and then monitored four times per day. Once fish started losing equilibrium, fish were monitored a minimum of every 15 min during the day. When temperature was dropped to 11 °C, it was noted that a number of fish were taking refuge in the 4 inch pipes. The proportion of each group of fish (adult/juvenile, white/green) was recorded approximately every 30 min between 11 °C and 10 °C until large numbers of fish losing equilibrium prevented further measurements. When a fish lost equilibrium, it was removed and time and temperature were recorded. Adult fish were euthanized by 200 mg/L buffered tricaine methanesulfonate at current tank temperature, and fish mass and fork length were recorded. Fork length of juvenile fish was recorded while fish were in a net in the experimental tank, then juvenile fish were moved to a recovery tank held a few degrees above the temperature they lost equilibrium at. Temperature in these tanks was slowly increased over the day, and then fish were moved to a 20 °C holding tank. The cold tolerance trial took place after adult groups had been reared in equal conditions for 9 months and 5 months post fertilization for juvenile groups.
Statistical analyses
All statistical analyses were performed using R (R Core Team 2018) or SigmaPlot (Systat Software Inc., IL, USA). Survival (loss of equilibrium) data were analysed via Log Rank analysis, and all other data were analysed by Anova and/or linear regression. Proportional data were logit transformed prior to analysis, and if normality failed, data were analysed via Kruskal-Wallis Anova on Ranks. In the cold tolerance trial, temperature at which 50% of individuals lost equilibrium (LD50) for each group was calculated using the dose.p function in the MASS package (Venables and Ripley 2002) in R. All data are given as mean ± standard error of the mean, and differences were considered significant if p < 0.05.
Results
Mass, length, and condition factor (CF = mass ÷ length3 × 100) of fish used in the competition and cold tolerance trials are shown in Tables 1 and 2, respectively. There were no significant differences in size or CF among colour genotypes within age group for either trial.
Competition trials
Of the 23 pairs of adults used in the competition trial, nine pairs did not eat and were excluded from the analysis. Of the remaining 14 pairs, green adult fish appeared to be slightly more successful than white fish in capturing individual worms in competition (see Fig. 1A), but overall green and white adults did not significantly differ in the percent of offered worms eaten under competition (Table 1). Neither mass nor length was a significant covariate in proportion of worms eaten in the adult trial (p = 0.214 F24,25 = 1.63, and p = 0.179 F24,25 = 1.92, respectively).
Number of worms eaten by green (grey squares) or white (white circles) unrelated adult (a, n = 14) and sibling juvenile (b, n = 17) tetras (Gymnocorymbus ternetzi) in paired competition trials. Paired green and white fish were offered individual blood worms one at a time until either four worms were added consecutively without being eaten or 20 worms were offered, whichever came first
Of the 18 pairs of juveniles used in the competition trial, one pair did not eat and was excluded from the analysis. Of the remaining 17 juvenile pairs, there was no clear pattern in genotype success in capturing individual worms in competition (see Fig. 1B) and no significant difference in proportion of worms eaten by white versus green fish (Table 1). Fish mass was not a significant covariate (p = 0.426, F30,31 = 0.651) but length was (p = 0.024, F30,31 = 5.68), although the two groups did not significantly differ in length overall (Table 1). Linear regression analysis showed a significant positive correlation between proportion of worms eaten and length for white fish (logit(proportion eaten) = 3.568 × length − 11.08, p = 0.002, F1,15 = 12.46; R2 = 0.454), but a negative correlation for green fish that was not statistically significantly different from zero (logit(proportion eaten) = − 0.494 × length + 1.03, p = 0.593, F1,15 = 0.130; R2 = 0.009). There was no difference in the proportion of chases performed by either white or green fish (Table 1). Neither mass (p = 0.721, F30,31 = 01301) nor length (p = 0.350, F30,31 = 0.901) was a significant covariate in proportion of chases performed. When the proportion of worms was plotted against the proportion of chases performed, there was a weak but significant positive relationship for green juveniles (proportion eaten = 0.504 × proportion chase + 0.150, p = 0.017, F1,15 = 7.14, R2 = 0.284), but not for white fish (p = 0.388, F1,15 = 0.790, R2 = 0.275).
Cold tolerance trial
There were no significant differences between experimental tank replicates for the cold trial in any variable measured, and patterns of survival among fish groups were similar between tank replicates (see Supplemental Fig. 1 for temperature and survival curves for two experimental tanks). Consequently data from the two experimental tanks was combined.
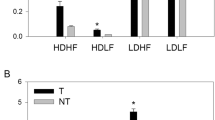
There was a significant difference among fish groups for loss of equilibrium rate and LD50 (p < 0.001 for both measures, see Fig. 2, Table 2), where fish lost equilibrium with decreasing temperature in order of adult green > juvenile green = juvenile white > adult white. When temperature at which each fish group lost equilibrium was plotted as a function of fish size, the only significant but weak negative correlations were for length in juvenile green fish (temperature = − 1.378 × length + 12.264, p = 0.023, F1,18 = 6.705, R2 = 0.256) and CF in adult green fish (temperature = − 2.147 × CF + 14.554, p = 0.032, F1,18 = 5.43, R2 = 0.232). No differences were observed in feeding and activity level between colour genotypes, but adult fish decreased and stopped eating at higher temperatures than juvenile fish (see Fig. 2). The proportion of white fish that sought refuge in 4 inch PVC tubes from 11 to 10 °C was on average 3.8-fold greater than proportion of green fish that sought refuge (p < 0.001, F1,56 = 80.8, see Fig. 3), and proportion of fish in tubes was not affected by age (p = 0.761, F1,56 = 0.093).
Survival (percent without loss of equilibrium) during gradual cold temperature exposure for green or white tetra (Gymnocorymbus ternetzi) in two different age groups: unrelated adult fish and sibling juvenile fish. Temperature was decreased rapidly by 1 °C daily. Letters (x,y,z) indicated statistically significant differences in survival curves among groups (p < 0.05). Arrows indicate at what temperature altered behaviour was observed in fish groups. n = 20 per colour/age group of fish
Proportion of unrelated adult and sibling juvenile white and green tetra fish groups (Gymnocorymbus ternetzi) that took refuge in 4 inch PVC pipe shelters from 11 °C to 10 °C during a cold temperature tolerance trial. Data are given as mean ± standard error of the mean. Significant differences among fish groups are indicated by letters (x, y, p < 0.001), n = 20 fish per age/colour
Adult and juvenile fish in the control tank had normal activity and feeding level throughout the trial, did not experience loss of equilibrium, and did not take refuge inside pipe shelters.
Discussion
This is the first known study to examine the effect of FP transgenesis on competitive foraging success and behaviour. There was no significant effect of FP transgenesis on competitive success nor on recorded aggressive behaviour in green and white tetras (G. ternetzi). In white juvenile tetras only, there was a significant correlation between fish length and competitive success, while in green juvenile tetras only, aggressive behaviour was positively correlated with competitive success; suggesting factors influencing success may differ between genotypes and/or age but without an overall effect on competitive success. This study suggests that under the given context, FP transgenic tetras do not differ in their potential to pose hazards through foraging competition relative to non-transgenic white tetras. Equal conclusions were drawn if unrelated adult tetras with unknown rearing history were compared or if sibling juvenile fish with equal rearing history were compared.
In contrast, the cold tolerance trial revealed very different results if unrelated adult fish (large effect of FP transgenesis) or sibling juvenile fish (no effect of FP transgenesis) were compared. For adult fish, green tetras lost equilibrium on average almost 2 °C warmer than white tetras, demonstrating diminished cold tolerance for FP fish previously reported in sibling fish for this FP transgenic line (see Leggatt et al. 2018b), four of five other FP transgenic tetra lines (see DFO 2019), RFP zebrafish compared to unrelated non-transgenic fish (Cortemeglia and Beitinger 2005, 2006a), and one of three eGFP research lines of zebrafish relative to non-transgenic progenitor strain (Leggatt et al. 2018a). The current study of adult fish represents the largest difference in cold tolerance reported between FP transgenic and non-transgenic fish, with other studies reporting differences between 0.17 and 1 °C between groups, including a difference of only 0.17 °C difference reported previously in this line of FP transgenic tetra (see Leggatt et al. 2018b). In contrast, green and white juvenile tetras did not significantly differ in cold tolerance, and difference in LD50 between the two juvenile groups was less than 0.1 °C.
There are numerous potential causes for the different results obtained from the two groups of fish. In other models, life stage or body size (Charo-Karisa et al. 2005; Truebano et al. 2018), rearing history (Schaefer and Ryan 2006; Travis et al. 1999), adaptation (Barrett et al. 2011), and genetic or population differences (Saillant et al. 2008; Tuckett et al. 2016) have all been reported to influence variation in a species thermotolerance. The origin of the two groups of adult white and green tetras relative to one another is not known; consequently the early rearing history and age of the two groups is not known. Whether age or early rearing influenced the relative cold tolerance of the fish cannot be determined. However, the two groups of fish were reared together for 9 months prior to the cold tolerance trial, which would in theory minimize effects of environmental history on cold tolerance. Juvenile offspring of the adult green and white tetras had intermediate cold tolerance relative to their parent strains, regardless of genotype, suggesting additive heritable cold tolerance traits were present in the two adult populations, although the potential for interacting effects of developmental stage and rearing history to account for differences cannot be excluded. The intermediate cold tolerance of juvenile offspring, and lack of effect of the FP transgene in this group, suggests background genetic effects or early rearing conditions may have had a greater influence on cold tolerance than the presence of the green FP transgene. Poor cold tolerance of the pet-trade sourced green transgenic tetra is consistent with Howard et al. (2015), who reported that fitness traits in pet-trade sourced RFP transgenic zebrafish were low relative to non-transgenic pet-trade sourced zebrafish, and the authors postulated this may be due to inbreeding depression in the former. The lack of FP transgenic effect on cold tolerance in sibling juvenile fish in the current study differed from diminished cold tolerance in FP fish from this line previously reported by GloFish LLC (see Leggatt et al. 2018b), potentially due to differences in experimental design (speed of temperature decline, sample size, rearing history, e.g. Schaefer and Ryan 2006) and/or differences in genetic background from different populations of white tetras use as well as genetic drift in the green tetra line (e.g. Howard et al. 2015; Tuckett et al. 2016).
Surprisingly, the high LT50 of the green adult fish (9.88 °C) was much more similar to the LT50 formally reported for white tetra (9.81 °C, Leggatt et al. 2018a) than the LT50 for the white tetra in the current study (7.95 °C), despite similar experimental design between the two studies (i.e. decreasing temperature by 1 °C per day, starting at 20 °C). One potential cause for this could be differences in how temperature was decreased daily between the two temperatures (i.e. slow decrease in Leggatt et al. 2018a, rapid decrease in the current study). Tuckett et al. (2016) also reported strong differences thermotolerance in different populations of pet-trade sourced swordtails (Xiphophorus hellerii), and the current study supports the suggestion by Tuckett et al. that use of a single population would be inadequate to characterize cold tolerance of a species, particularly in terms of its potential to become established.
One unexpected observation was that white tetras were much more likely than green tetras to take refuge in pipe shelters during low temperature, in both adult and juvenile groups. These shelters may have had lower water current than in the main area of the aquaria and may have provided metabolic rest areas for the fish by minimizing swimming effort. While use of shelters did not result in improved cold tolerance in the juvenile fish, it could result in decreased potential for white fish to be preyed upon relative to green fish in natural temperate water bodies.
Conclusions
This current study indicates FP transgenic fish may pose equal or lesser risk than non-transgenic fish, should they be released to natural environments. While behaviour comparisons in both the competition and cold tolerance trials were similar if unrelated adult or sibling juvenile fish were used, very different conclusions were made in terms of effects of FP transgenesis on cold tolerance when adult or juvenile fish were compared. This demonstrates that unrelated pet-trade sourced fish may not always be appropriate models for examining effects of FP transgenesis. The results from the cold tolerance study indicate that diminished cold tolerance is not necessarily a universal off-target effect of FP transgenesis in tropical fish and population-level differences may have more effect on cold tolerance variation than FP transgenesis. This confirms the need to use multiple relevant populations to gain an accurate estimate of cold tolerance in different species for use in risk assessment (Tuckett et al. 2016), which would be particularly important in species whose cold tolerance may be close to the minimum temperatures of natural systems with potential for exposure to the organisms. Similar competitive ability of FP tetras and decreased potential to take shelter during extreme temperature exposure relative to non-transgenic tetras, combined with previously confirmed lack of overwintering potential in Canada and most of the USA for this species (Leggatt et al. 2018a; USFWS 2017), strengthen evidence that FP transgenesis will not result in greater risks to temperate environments than the non-transgenic species, should they be released to natural systems.
References
Badrian B, Bogoyevitch MA (2007) Changes in the transcriptional profile of cardiac myocytes following green fluorescent protein expression. DNA Cell Biol 26:727–736. https://doi.org/10.1089/dna.2007.0604
Baens J et al (2006) The dark side of EGFP: Defective polyubiquitination. PLoS ONE 1:e54. https://doi.org/10.1371/journal.pone.0000054
Barrett RDH, Paccard A, Healy TM, Bergek S, Schulte PM, Schluter D, Rogers SM (2011) Rapid evolution of cold tolerance in stickleback. Proc R Soc Lond B Biol Sci 278:233–238. https://doi.org/10.1098/rspb.2010.0923
Charo-Karisa H, Rezk MA, Bovenhuis H, Komen H (2005) Heritability of cold tolerance in Nile tilapia, Oreochromis niloticus, juveniles. Aquaculture 249:115–123. https://doi.org/10.1016/j.aquaculture.2005.04.029
Core Team R (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria Available from https://www.r-project.org/.
Cortemeglia C, Beitinger TL (2005) Temperature tolerances of wild-type and red transgenic zebra danios. Trans Am Fish Soc 134:1431–1437. https://doi.org/10.1577/T04-197.1
Cortemeglia C, Beitinger TL (2006a) Projected US distributions of transgenic and wildtype zebra danios, Danio rerio, based on temperature tolerance data. J Therm Biol 31:422–428. https://doi.org/10.1016/j.jtherbio.2006.01.011
Cortemeglia C, Beitinger TL (2006b) Susceptibility of transgenic and wildtype zebra danios, Danio rerio, to predation. Environ Biol Fishes 76:93–100. https://doi.org/10.1007/s10641-006-9011-x
Coumans JVF, Gau D, Polijak A, Wasinger V, Roy P, Moens PR (2014) Green fluorescent protein expression triggers proteome changes in breast cancer cells. Exp Cell Res 320:33–45. https://doi.org/10.1016/j.yexcr.2013.07.019
Devlin RH, D’Adrade M, Uh M, Biagi CA (2004) Population effects of growth hormone transgenic coho salmon depend on food availability and genotype by environment interactions. Proc Natl Acad Sci U S A 101:9303–9308. https://doi.org/10.1073/pnas.0400023101
Devlin RH, Sundstrom LF, Leggatt RA (2015) Assessing ecological and evolutionary consequences of growth-accelerated genetically engineered fishes. Bioscience 65:685–700. https://doi.org/10.1093/biosci/biv068
DFO (2018) Environmental and indirect human health risk assessment of the Glofish® Electric Green® Tetra and the Glofish® Long-Fin Electric Green® Tetra (Gymnocorymbus ternetzi): a transgenic ornamental fish. DFO Can Sci Advis Sec Sci Advis Rep 2018/027 23 pp. https://waves-vagues.dfo-mpo.gc.ca/Library/40712928.pdf
DFO (2019) Environmental and indirect human health risk assessment of the GloFish® Tetras (Gymnocorymbus ternetzi): five lines of transgenic ornamental fish. DFO Can Sci Advis Sec Sci Advis Rep 2019/002 26 pp. www.dfo-mpo.gc.ca/csas-sccs/Publications/SAR-AS/2019/2019_002-eng.pdf
Hill JE, Kapuscinski AR, Pavlowich T (2011) Fluorescent transgenic zebra danio more vulnerable to predators than wild-type fish. Trans Am Fish Soc 140:1001–1005. https://doi.org/10.1080/00028487.2011.603980
Howard RD, Rohrer K, Liu YY, Muir WM (2015) Mate competition and evolutionary outcomes in genetically modified zebrafish (Danio rerio). Evolution 69:1143–1157. https://doi.org/10.1111/evo.12662
Jha P (2010) Comparative study of aggressive behaviour in transgenic and wildtype zebrafish Danio rerio (Hamilton) and the flying barb Esomus danricus (Hamilton), and their susceptibility to predation by the snakehead Channa striatus (Bloch). Ital J Zool 77:102–109. https://doi.org/10.1080/11250000802629463
Leggatt RA (2019) Cold temperature tolerance of albino rainbow shark (Epalzeorhynchos frenatum), a tropical fish with transgenic application in the ornamental aquarium trade. Can J Zool 97:1–3. https://doi.org/10.1139/cjz-2018-0208
Leggatt RA, Dhillion RS, Mimeault C, Johnson N, Richards JG, Devlin RH (2018a) Low-temperature tolerances of tropical fish with potential transgenic applications in relation to winter water temperatures in Canada. Can J Zool 96:253–260. https://doi.org/10.1139/cjz-2017-0043
Leggatt RA, Johnson N, McGowan C (2018b) Environmental risk assessment of the Glofish® Electric Green® Tetra and the Glofish® Long-Fin Electric Green® Tetra: transgenic ornamental fish, imported to Canada, for sale in the pet trade. DFO Can Sci Advis Sec Res Doc 2018/049 xii + 54 pp. https://waves-vagues.dfo-mpo.gc.ca/Library/40722995.pdf
Li H, Wei H, Wang Y, Tang H, Wang Y (2013) Enhanced green fluorescent protein transgenic expression in vivo is not biologically inert. J Proteome Res 12:3801–3808. https://doi.org/10.1021/pr400567g
Mak GW-Y, Wong C-H, Tsui SK-W (2007) Green fluorescent protein induces the secretion of inflammatory cytokine interleukin-6 in muscle cells. Anal Biochem 362:296–298. https://doi.org/10.1016/j.ab.2006.12.017
Owen MA, Rohrer K, Howard RD (2012) Mate choice for a novel male phenotype in zebrafish, Danio rerio. Anim Behav 83:811–820. https://doi.org/10.1016/j.anbehav.2011.12.029
Saillant E, Wang XX, Ma L, Gatlin DM, Vega RR, Gold JR (2008) Genetic effects on tolerance to acute cold stress in red drum, Sciaenops ocellatus L. Aquac Res 39:1393–1398. https://doi.org/10.1111/j.1365-2109.2008.02008.x
Schaefer J, Ryan A (2006) Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J Fish Biol 69:772–734. https://doi.org/10.1111/j.1095-8649.2006.01145.x
Snekser JL, McRobert SP, Murphy CE, Clotfelter ED (2006) Aggregation behavior in wildtype and transgenic zebrafish. Ethology 112:181–187. https://doi.org/10.1111/j.1439-0310.2006.01139.x
Stewart CN (2006) Go with the glow: fluorescent proteins to light transgenic organisms. Trends Biotechnol 24:155–162. https://doi.org/10.1016/j.tibtech.2006.02.002
Travis J, McManus MG, Baer CF (1999) Sources of variation in physiological phenotypes and their evolutionary significance. Am Zool 39:422–433. https://doi.org/10.1093/icb/39.2.422
Truebano M, Fenner P, Tills O, Rundle SD, Rezende EL (2018) Thermal strategies vary with life history stage. J Exp Biol 221:1–5. https://doi.org/10.1242/jeb.171629
Tuckett QM, Ritch JL, Lawson KM, Lawson LL, Hill JE (2016) Variation in cold tolerance in escaped and farmed non-native green swordtails (Xiphophorus hellerii) revealed by laboratory trials and field introductions. Biol Invasions 18:45–56. https://doi.org/10.1007/s10530-015-0988-y
USFWS (US Fish & Wildlife Service) (2017) Black tetra (Gymnocorymbus ternetzi): ecological risk screening summary. Available at https://www.fws.gov/fisheries/ANS/erss/uncertainrisk/ERSS-Gymnocorymbus-ternetzi-FINAL-November2017.pdf. Accessed August 2019.
Venables WN, Ripley BD (2002) Modern applied statistics with S. Springer, New York
Acknowledgments
This project was funded by the Canadian Regulatory System for Biotechnology. Thanks go to A. Csuzdi, H. Tadey, and B. Yates for assistance in fish care and monitoring.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted (Pacific Region Animal Care Committee, AUP18-014).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 1045 kb)
Rights and permissions
About this article
Cite this article
Leggatt, R.A., Devlin, R.H. Fluorescent protein transgenesis has varied effects on behaviour and cold tolerance in a tropical fish (Gymnocorymbus ternetzi): implications for risk assessment. Fish Physiol Biochem 46, 395–403 (2020). https://doi.org/10.1007/s10695-019-00725-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00725-3