Abstract
A 60-day experiment was conducted to evaluate the effect of dietary fish meal (FM) replacement with poultry by-product meal (PBM) on digestive enzymes, non-specific serum enzyme activity, and serum biochemical parameters of juvenile sobaity sea bream, Sparidentex hasta, weighing 29.27 ± 0.06 g. PBM replaced 0, 15, 25, 35, 45, and 55% of dietary FM in the isoproteic (50%) and isocaloric (21 kJ g−1) experimental diets. The final body weight, percentage weight gain, specific growth rate, and protein efficiency ratio were higher in PBM15, 25, and 35 than in control, PBM45, and 55. Digestive lipase and amylase activity did not differ significantly between the dietary treatments. Significantly lower trypsin and higher chymotrypsin activity were observed at PBM55 and 45, respectively. Digestive alkaline phosphatase (ALP) increased, whereas protein apparent digestibility coefficients (ADC) decreased significantly with increasing levels of PBM above 35%. Hematocrit, hemoglobin, mean corpuscular hemoglobin concentration, serum albumin, globulin, total protein, glucose, urea, uric acid, and aspartate aminotransferase did not show any significant differences between the treatments. Serum ALP, cholesterol, and calcium were higher in PBM diets than in the control diet, whereas an inverse trend was observed in triglyceride. Protein digestibility and trypsin activity and serum biochemical indices suggest that fish meal protein could be reduced up to 45% by the inclusion PBM in the formulated diets for S. hasta juveniles without any adverse effect on its overall performance.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the context of increasing importance of diversification in aquaculture, sobaity sea bream, Sparidentex hasta (Valenciennes 1830) has been propagated in Iran (Mozanzadeh et al. 2015). Sobaity sea bream is a commercially valuable carnivorous fish species distributed throughout the Western Indian Ocean. It spawns readily in captivity, demonstrates rapid growth under favorable conditions, and is amenable to a wide range of culture conditions (Pavlidis and Mylonas 2011). Dietary protein and lipid requirements (Hossian et al. 2014; Mozanzadeh et al. 2016) and effect of replacement of fish meal with plant proteins in the diet of sobaity sea bream (Yaghoubi et al. 2016) have been investigated.
Finding economically viable protein sources with homogeneous and well-balanced nutrient composition to replace fish meal in feeds is one of the main challenges for the sustainability of marine fish culture (Gatlin et al. 2007; Naylor et al. 2009). Fish meal is well recognized as the best dietary protein source for most marine carnivorous fish (NRC 2011) such as sobaity sea bream which require high dietary protein levels (Hossain et al. 2014). The need for optimizing the production of aqua feeds has taken several researchers to evaluate alternative protein sources (OlivaTeles et al. 2015; Silva et al. 2010; Xu et al. 2012). Poultry by-product meal (PBM) has been reported to be suitable for carnivorous fish species due to its palatability, high protein and energy contents, and high digestibility compared to other terrestrial animal proteins (Bureau et al. 1999). PBM has been used as a cost-effective and efficient protein source in diets for several marine species without any significant reduction in growth performance and feed utilization (Gunben et al. 2014; Hernandez et al. 2014; Shapawi et al. 2007; Wang et al. 2015; Zhou et al. 2011). Growth rate of fish depends on its digestive capacity, the metabolic capacity required to support tissue protein synthesis (Debnath et al. 2007). Studies on digestive secretions in fish can elucidate certain aspects of its nutritive physiology and help resolve nutritional problems, such as selection of feed ingredients and/or an artificial diet based on the digestive capabilities of fish (Xu et al. 2012). Changes in enzyme activity and blood parameters provide reliable information on metabolic disorders, nutrient deficiencies, and ability of fish to adjust to changes in nutritional conditions (Mozanzadeh et al. 2015; Peres et al. 2013).
The optimum level of FM substitution with alternative protein sources have been reported in many fish species based on productivity (Hernandez et al. 2014; Kureshy et al. 2000; Nengas et al. 1999; Shapawi et al. 2007), but studies on physiological response of fish to replacement of fish meal with terrestrial animal proteins are limited. The aim of this study was to examine the possibility of fish meal substitution with PBM in the diets of juvenile sobaity sea bream with an emphasis on digestive enzyme activities and serum biochemical parameters.
Material and methods
Ingredients and experimental diets
Six isoproteic (50%), isolipidic (18%), and isocaloric (21 kJ g−1) experimental diets were formulated in which 0 (PBM0 or the control diet, where kilka fish meal (FM) and soybean meal were the main protein sources), 15 (PBM15), 25 (PBM25), 35 (PBM35), 45 (PBM45), and 55% (PBM55) of protein from FM was replaced with protein from PBM. The proximate and amino acid compositions of the diets are given in Tables 1 and 2. Kilka oil, FM, PBM, and the other feed ingredients were provided by the Beyza Feed mill company (Table 1). Chromic oxide (Cr2O3) was included in the diets (0.5%) as an external marker for digestibility estimations. Starch and gelatin were cooked separately, added to the ingredients, and blended to produce a homogeneous mixture in a Hobart-type mixer. Kilka oil and water were added and thoroughly mixed; the wet pellets (3.0-mm diameter) were produced with a pellet machine (Houdtkhash, Tehran, Iran) and air-dried to about 10% moisture, sealed in plastic bags, and stored at − 20 °C prior to use in the feeding trial.
Fish rearing facilities
This experiment was carried out in the Mariculture Research Station of the South Iranian Aquaculture Research Center, Sarbandar, Iran. Three-hundred-and-sixty healthy sobaity sea bream juveniles (mean body weight 29.27 ± 0.06 g, mean ± standard error), produced at the same hatchery, were randomly distributed into 18 300-L cylindrical polyethylene tanks and acclimated for 2 weeks before the onset of the feeding trial. During the acclimatization period, fish were fed twice daily to satiation with a commercial pelleted diet of 46% protein and 15% fat (Biomar, Scotland, UK). Tanks were supplied with filtered running seawater 1 (L min−1) in a flow-through system. Salinity ranged between 47 and 49‰ (48.0 ± 0.5‰) and temperature between 22.17 and 24.79 °C (23.48 ± 1.2) during the experimental period. Average values for dissolved oxygen and pH were 6.8 ± 0.4 mg L−1 and 7.37 ± 0.2, respectively. A 12-h photoperiod was used during the trial. Triplicate groups of fish were hand-fed one of the above mentioned diets to visual satiation thrice daily (08:00 h, 13:00 h, and 17:00 h) for 60 days.
Sample collection techniques
At the termination of the feeding trial, 24 h after the last feeding, fish in each tank were anesthetized using ethylene glycolmonophenylether (0.5 ml L−1) and individually weighed to the nearest 0.1 g. Blood samples of three fish from each tank were collected gently from the caudal vein for hematological tests. Two aliquots of blood were taken; one was used for estimation of hematocrit and hemoglobin and the other, after separating serum by centrifuging at 3000g for 10 min at 4 °C, was transferred to Eppendorf tubes, and stored at − 80 °C until analysis. The same fishes were scarified and their digestive tracts were removed, frozen in liquid nitrogen, and stored at − 80 °C until analysis. The remaining fish were returned to the respective tanks for digestibility analysis.
Digestibility study
At the end of the feeding trial and sampling, the remaining fish in each tank were fed to visual satiation twice daily for feces collection. Uneaten pellets were collected 30 min after each feeding period. Before beginning the experiment, preliminary trials were conducted to determine the length of time needed for optimal fecal production. The maximal fecal production occurred 2–5 h after feeding. Fresh feces were collected by siphoning the tank bottom 2 h after each feeding. The feces were rinsed with distilled water, dried over a filter paper for 24 h at 60 °C in an oven, and immediately frozen at − 20 °C (Shapawi et al. 2007). Feces collection was done daily from each tank until sufficient amount was obtained. Acid digestion method was used to determine chromic oxide concentration in the experimental feeds and feces (Furukawa and Tsukahara 1966). Apparent digestibility coefficients (ADCs) were calculated using standard formulae:
Biochemical analyses
Proximate analyses of ingredients, diets, whole-body composition, and feces were determined using standard methods (AOAC 2005). Moisture was determined using a moisture analyzer (AM B5 0, ADAM, UK). Protein was determined by measuring nitrogen using the Kjeldahl method (BÜCHI, Auto-KjeldahlK-370, Switzerland). To convert total nitrogen to total protein content, as a percentage of dry weight, the factor 6.25 (100/16) was used. Total lipid was extracted by petroleum benzene using the Soxhlet method (Barnstead/Electrothermal, UK). Fiber content was analyzed with a fiber analyzer (VELP® Scientifica, Italy) and the ash content was determined by incinerating the dry samples in a muffle furnace (Finetech, Shin Saeng Scientific, South Korea) at 600 °C for 8 h. Amino acids (except for tryptophan because of susceptibility of this AA to acid hydrolysis) were determined after hydrolysis of the diets. Freeze-dried samples (Freeze dryer, Operon, OPRFDU 7012, South Korea) were hydrolyzed in 6 N HCl for 24 h at 110 °C in glass vials filled with nitrogen. The o-phthaldialdehyde (OPA) was used as a pre-column derivatization reagent according to Lindroth and Mopper (1979). Total AA levels were determined by HPLC (Knauer, Germany) using C18 column (Knauer, Germany) at the flow rate of 1 mL min–1 with fluorescence detector (RF-530, Knauer, Germany).
Preparation of extracts and determination of digestive enzyme activities
Enzyme extraction was performed by adding homogeneous buffer solution (Tris-HCl 50 mM, KCl 50 mM, and CaCl 20 mM) into the digestive tracts at 9:1 (v/w) ratio at pH 7.5, before homogenization (IKA, Ultra-turrax®, USA). Homogenized samples were poured into 1.5-mL vials. The homogenates were centrifuged in a refrigerated centrifuge (K System Centurion Model) at 30,000g for 10 min at 4 °C. Then, the resulting supernatant was dispensed into 1.5 vials and used for enzyme assays. Protein content of homogenates was measured following Bradford (1976). Trypsin, chymotrypsin, lipase, α-amylase, and alkaline phosphatase (ALP) were determined in homogenates from intestinal. Trypsin activity (E.C.3.4.21.4) was assayed with a-r-toluenesulfonyl-L-arginine methyl ester hydrochloride (TAME). Crude extracts were incubated for 2 min (25 °C) in 2 mL of Tris/CaCl2 buffer, pH 8.1. Chymotrypsin activity (E.C.3.4.21.1) was assayed with benzoyl tyrosine ethylester (BTEE). Crude extracts were incubated for 2 min in 2 mL of Tris/CaCl2 buffer, pH 7.8. Both trypsin and chymotrypsin were assayed in duplicate and enzyme activities were recorded at 253 and 256 nm, respectively, according to protocol by Hummel (1959). One unit of enzyme was defined as the amount of enzyme needed to hydrolyze 1 mg of substrate (TAME or BTEE) per min per mg protein. Lipase, amylase, and ALP activity was measured using Pars Azmoon Biochemical Photometric Kit, Tehran, Iran, by Mindray BS-200, China. Auto-analyzer were recorded at 580, 405, and 405 nm (Tietz and Shuey 1993).
Hematological assay
Estimation of hematocrit and hemoglobin (Hb) levels were measured based on Sahli’s method. Hematocrit (Hct) was estimated by microhematocrit method. Mean corpuscular hemoglobin concentration (MCHC) was calculated from Hb and Hct according to the following formula (Campbell and Ellis 2007):
Serum biochemical parameters were analyzed using an auto-analyzer (Mindray BS-200, China), with commercial clinical investigation kits (Pars Azmoon Kit, Tehran, Iran). Biochemical measurements were carried out for glucose, total protein (TP), albumin, total cholesterol, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lipase, amylase, calcium (Ca), inorganic phosphorous (P), and magnesium (Mg). Globulin was calculated by subtracting the albumin values from the total plasma protein (McClatchey 2002).
Calculations
Standard formulae were used to assess growth performance: percentage weight gain (WG%), specific growth rate (SGR), survival rate (SR), and intestinal quotient (IQ); feed utilization: feed intake (FI), feed conversion ratio (FCR), and protein efficiency ratio (PER), were calculated as follows: WG% = 100 × (Wt − W0) / W0; SGR (% fish−1) = 100 × (LnWt – LnW0) / t; SR (%) = 100 × Nt / N0; IQ = Lg / Ls; FI (% day−1) = 100 × I / [(W0 + Wt) / 2 × t]; FCR = I / (Wt − W0); PER = (Wt − W0) / (I × Cpf), where I (g) is the total amount of the test diets fed on a dry matter basis, W0 (g) is the total initial body weight and Wt (g) is the total final body weight, Lg (cm) and Ls (cm) are the digestive tract length and body standard length, t (day) is the duration of the feeding trial, Nt is the number of fish at the end of the feeding trial and N0 at the start, and Cpf (%) is the crude protein contents of the test diets.
Statistics
The data are presented as mean ± standard error of the mean calculated from three replicates. The data for each parameter were tested for normality and homoscedasticity. A one-way analysis of variance was performed with diet as the independent variable. A Tukey’s HSD test was used for post hoc performed after a significant ANOVA (P < 0.05). Data were analyzed using SPSS version 19.0 (Chicago, IL, USA). The Pearson product moment correlation test was used to determine any correlation among parameters.
Results
Growth performance and feed utilization
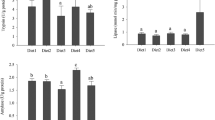
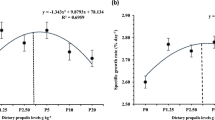
The survival rate of experimental fish ranged from 96 to 100% and was not affected by dietary treatments (P > 0.05; Table 3). There were no significant differences in FBW, WG%, SGR, and PER among fish fed PBM0, 35, 45, and 55 (P > 0.05). Significantly higher growth performance and PER values were found among fish fed PBM15 and 25 than PBM0, 45, and 55 (P < 0.05). Standard length (SL) and intestinal quotient (IQ) did not show significant difference between fish fed FM-based diet and fish fed diets including PBM (P > 0.05). Among the PBM treatments, significantly higher IQ value was observed in PBM45 as compared to PBM15 and 35 (P < 0.05). No significant differences were found in FI between the dietary treatments. PBM15 and 25 showed significantly lower FCR than PBM0 and 45 (P < 0.05). According to the quadratic regression, 24.06–25.87% substitution of FM by PBM could result in the highest WG% (Fig. 1), SGR, PER, and the lowest values of FCR (Table 3).
Biochemical composition
Whole-body proximate composition was not affected by the dietary PBM inclusion (Table 4). Crude protein varied from 16.1 (PBM0) to 17.2 (PBM35) and crude lipid from 6.9 in PBM0 to 7.8 in PBM25. Dietary inclusion of PBM did not seem to affect the amino acid profile of fish (P > 0.05). TEAA of fish fed the experimental diets ranged from 44.7 to 46.1 g 100 g−1 protein in PBM45 and PBM0 and TNEAA from 45.2 to 46.6 in PBM25 and PBM35 (Table 4).
Apparent digestibility coefficient and digestive enzyme activities
Protein ADC was significantly lower in PBM35, 45, and 55 than other experimental diets (P < 0.05). Apparent digestibility of lipid and dry matter did not vary significantly between the control and PBM diets (P > 0.05; Table 5). The dry matter ADC in PBM15 was significantly higher than in PBM55 (P < 0.05). The highest and the lowest trypsin activities were found in PBM 15 and 55, respectively (P < 0.05; Table 5). Chymotrypsin activity was significantly higher in PBM35, 45, and 55 than in other diets (P < 0.05). Pearson correlation revealed a significant positive correlation of digestive lipase activity with lipid ADC (r = 0.70, P < 0.05), WG%, and SGR (r = 0.88, P < 0.05). There were no significant differences in lipase activity between the control and PBM35, 45, and 55 diets (P > 0.05). Amylase activity did not differ significantly among the diets except in PBM35. Fish fed PBM35 and 45 showed significantly higher ALP than fish fed other experimental diets (P < 0.05).
Hematological and serum biochemical parameters
Dietary inclusion of PBM did not seem to affect hematocrit, hemoglobin, MCHC, albumin, globulin, total protein, glucose, and uric acid of fish (P > 0.05; Table 6). The lowest and the highest cholesterol values were observed in PBM0 and PBM55, respectively. Serum cholesterol content showed a significant negative correlation with taurine and methionine contents of the experimental diets (r = − 0.0.773, r = − 0.723, respectively, P < 0.05). Significantly higher triglyceride value was recorded in fish fed the FM-based diet (P < 0.05; Table 6) and lower values were observed in PBM25 and 35 (P < 0.05). Serum urea did not vary significantly among the control and PBM diets (P > 0.05). The serum Ca content did not differ significantly between the PBM diets and was the lowest in the control diet. Serum Mg was significantly higher in PBM25 and 45 than in PBM0. The lowest serum P content was found in PBM 35 (P < 0.05) and showed a significant positive correlation with phosphorus content of the diets (r = 0.67, P < 0.05). Serum amylase and AST values did not show any significant variation between the diets (P < 0.05; Table 6). Serum ALT was significantly higher in PBM35 and 55 than in the control diet. Serum ALP activity increased significantly with increasing level of PBM in diets. Serum lipase activity showed a decreasing trend with increase in PBM levels above 35% replacement of FM (P < 0.05). Serum lipase activity showed a significant positive correlation with digestive lipase activity (r = 0.71, P < 0.05) and lipid ADC.
Discussion
No significant growth depression was observed in sobaity sea bream fed the diets including PBM. Capacity to utilize PBM protein for growth has been reported in many marine species such as juvenile Sciaenops ocellatus, Rachycentron canadum, and Sparus aurata up to 50% replacement (Kureshy et al. 2000; Nengas et al. 1999; Zhou et al. 2011). Gunben et al. (2014), Wang et al. (2015), and Yang et al. (2006) reported that PBM at low inclusion levels had positive impact on growth performance and feed utilization in fish. In our study replacement of FM with PBM up to 35% resulted in better growth and efficient feed utilization. Whole-body proximate composition and amino acid content of sobaity sea bream were not affected by the replacement of FM with PBM. Similar observations were made by Takagi et al. (2000) and Gunben et al. (2014) on red sea bream and grouper, respectively, when fed different levels of dietary PBM. Rawles et al. (2006) suggested that the decrease in whole-body protein and increase in energy depots as PBM replaced fish meal could indicate a nutrient imbalance in the diets. It appears that in sobaity sea bream juveniles low level of FM (280 g kg−1) in combination with PBM (390 g kg−1) in the diet could provide sufficient nutrients and energy for normal growth and body composition.
In present study increased protein digestibility was observed in treatments with higher growth performance. The higher nutrient digestibility probably allowed higher nutrient and amino acid bioavailability, thus resulting in the higher growth performance in comparison with other PBM diets. Merrifield et al. (2010) suggested that improved ADC of nutrients could be due to an optimum balance and better absorption of amino acids (AA). Positive correlation between dietary essential AA (EAA) content and protein ADC of the experimental diets were observed in sobaity sea bream (Hekmatpour et al. 2018). Hence, the reduced protein digestibility (at levels of PBM above 35) could be due to the lower levels of EAA (Hekmatpour et al. 2018; Hernandez et al. 2014; Nengas et al. 1999; Shapawi et al. 2007). The reduced protein ADC could be attributed to the imbalance in AA profile or to the presence of feathers, connective tissue, and skin in the PBM which are not easily digested by fish (Nengas et al. 1999). PBM used in our study had higher lipid content than FM, and hence, the percentage inclusion of fish oil decreased with increasing level of inclusion PBM. Probably decrease in intestinal lipase activity led to reduction of lipid ADC in diets over 35% substitutions.
In present study the lowest trypsin activity was observed in treatment with the lowest protein apparent digestibility. The reduction in trypsin activity and protein digestibility affected feed utilization in fish (Roosta et al. 2011; Santigosa et al. 2008; Xu et al. 2012). Chymotrypsin activity did not show any correlation with growth in sobaity sea bream where as a positive correlation was observed in Atlantic cod (Lemieux et al. 1999). Animal protein substitution did not affect chymotrypsin activity in S. ocellatus (Applebaum and Holt 2003). In our study, trypsin and chymotrypsin were inversely proportional as reported by Santigosa et al. (2008) in S. aurata. They suggested that changes in the trypsin and chymotrypsin activity may imply a differential availability of oligopeptides and amino acids causing an amino acid imbalance in diets. In the present study lipase activity showed positive correlation with lipid ADC and serum lipase activity. Substitution of FM with PBM up to 35% increased digestive lipase activity, consequently resulting in increased serum enzyme activity for transport and circulation of lipid within the blood stream (De Silva and Anderson 1995). Similar to our results, activity of α-amylase was lower than alkaline protease and lipase in carnivorous species such as red sea bream, annular sea bream, and common Pandora (Fernandez et al. 2001). The experimental diets, except PBM35, did not induce or suppress amylase activity in sobaity. Jalili et al. (2012) reported that protein source did not significantly affect amylase activity in Oncorhynchus mykiss. Higher digestive ALP activity in PBM35 and 45 probably indicating an increased nutrient absorption in enterocytes (Gawlicka et al. 2000) and brush boarder cells (Nya and Austin 2011) in fish. Intestinal quotient (IQ) was the highest in PBM45, which was also associated with the highest ALP activity. Higher IQ in Rhamdia quelen (Lazzari et al. 2010) and better developed intestinal villi and microvilli in S. aurata (Silva et al. 2010) were reported when fed animal protein diet. This adaptive characteristic of the enzymes activity can be successfully used to take advantage of the nutrient content of diets (Moraes and Bidinotto 2000).
In the present study hemoglobin values were not affected by experimental diets and varied between 7.47 and 9.34 g dL−1. Hematocrit is a reliable index for checking the anemic condition as well as fish health relative to nutrition, disease, and stress status (Brill et al. 2008), which in our study was not affected by replacement of fish meal. The MCHC value in sobaity sea bream was comparable with values reported for other fish species (Fazio et al. 2013; Peres et al. 2014). The range of hematological parameters recorded in the present study suggested that substitution of FM up to 55% by PBM did not lead to any sign of anemia in sobaity sea bream. As TP, albumin, and globulin did not differ between treatments, it could be stated that the replacement of FM with PBM had no adverse effect on control of serum protein synthesis and albumin and globulin contents (Xu et al. 2012). PBM had no significantly effect on serum glucose values of sobaity sea bream. Serum cholesterol content fluctuated significantly in response to the inclusion of PBM in diets probably due to the characteristic of the protein source. Cholesterol showed a significant negative correlation with methionine and taurine content of the experimental diets. Higher serum cholesterol was observed in marine species fed low taurine (Gaylord et al. 2007) and methionine diets (Zhou et al. 2011). The lowest triglyceride level was observed in treatments which was associated with the higher growth and nutrient performance. Cheng et al. (2010) and Mozanzadeh et al. (2016) suggested that the reduction in serum triglyceride could be attributed to higher rate of lipid hydrolysis and consequently higher metabolism, in fish fed these diets in comparison to fish fed the control diet, suggesting an effect on energy metabolism. Similar to our results, comparable levels of serum urea and uric acid between treatments were observed by Perera et al. (1995). Decreased urea excretion has been shown in rainbow trout fed diets containing balanced EAA (Me ´dale et al. 1998). The serum Ca, P, and Mg levels were comparable with the values reported in sobaity sea bream (Mozanzadeh et al. 2015) and S. aurata (Peres et al. 2013). The Ca:P ratio (1.7:1) in S. hasta was comparable to the 1.4:1 ratio reported in S. aurata (Peres et al. 2013). Sobaity sea bream showed higher serum Ca and lower P in PBM diets which could be attributed to the composition of PBM.
In our study, the serum ALP showed clear separation from other variations according to principal component analysis. According to Mozanzadeh et al. (2017), ALP could be used as a reliable hepatic biomarker for assessing the nutritional and health conditions in sobaity sea bream. The serum ALP increased with the increasing level of inclusion PBM. This increase could be attributed to the processing of energy substrates by the liver leading to increased trans-membrane transport of ions and water, elevation of hepatic ALP activities, and increased leakage of these enzymes into the blood (Congleton and Wagner 2006). On the contrary, serum AST and ALT did not show any relation/trend with increasing level of PBM in diets suggesting that inclusion of PBM did not have any adverse effect on liver (Harikrishnan et al. 2011). The digestive lipase, serum lipase, and lipid ADC reduced as PBM substitution increased above 35%. Serum lipase activity is related to lipid mobilization in liver and adipose tissues, supplying fatty acids to peripheral tissues, also is a health clinical index of pancreas and increase in its leakage in serum indicated pancreatic disorders (Navarro and Gutierrez 1995). This overall decline in lipase could be attributed to a decrease of enzyme synthesis and turnover rates in fish fed these diets (Evans and Watterson 2009). Serum amylase activity did not vary between the treatments probably suggesting that the replacement of FM by PBM did not affect the normal functioning of the pancreas.
Our findings suggest that fish meal protein could be reduced by poultry by-product meal in the formulated diets for S. hasta juveniles up to 45% (280 g kg−1 FM + 340 g kg−1 PBM) without any adverse effect on its overall performance.
References
AOAC (2005) Official methods of analysis. Association of Official Analytical Chemists. Arlington, Virginia
Applebaum SL, Holt GJ (2003) The digestive protease, chymotrypsin, as an indicator of nutritional condition in larval red drum (Sciaenops ocellatus). Mar Biol 142:1159–1167
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brill RW, Bushnell P, Schroff R, Seifert R, Galvin M (2008) Effects of anaerobic exercise accompanying catch-and-release fishing on blood-oxygen affinity of the sandbar shark (Carcharhinus plumbeus, Nardo). J Exp Mar Biol Ecol 354:132–143
Bureau DP, Harris AM, Cho CY (1999) Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhyncus mykiss). Aquac 180:345–358
Campbell TW, Ellis CK (2007) Avian and exotic animal hematology and cytology. Blackwell, Ames
Cheng Z, Ai Q, Mai K, Xu W, Ma H, Li Y, Zhang J (2010) Effects of dietary canola meal on growth performance, digestion and metabolism of Japanese seabass, Lateolabrax japonicus. Aquac 305:102–108
Congleton JL, Wagner T (2006) Blood -chemistry indicators of nutritional status in juvenile salmonids. Fish Biol 69:473–490
Debnath D, Pal AK, Sahu NP, Yengkokpam S, Baruah K, Choudhury D, Venkateshwarlu G (2007) Digestive enzymes and metabolic profile of Labeo rohita fingerlings fed diets with different crude protein levels. Comp Biochem Physiol, Part B 146:107–114
De Silva SS, Anderson TA (1995) Fish nutrition in aquaculture. Chapman and Hall, London
Evans GO, Watterson CL (2009) General enzymology. In: Animal clinical chemistry, a practical guide for toxicologists and biochemical researchers, 2nd edn. CRC Press, New York
Fazio F, Marafioti S, Filiciotto F, Buscaino G, Panzera M, Faggio C (2013) Blood Hemogram profiles of farmed onshore and offshore Gilthead Sea bream (Sparus aurata) from Sicily, Italy. Turk J Fish Aquat Sci 13:415–422
Fernandez I, Moyano F, Dıaz M, Martınez T (2001) Characterization of α-amylase activity in five species of Mediterranean sparid fishes (Sparidae, Teleostei). J Exp Mar Biol Ecol 262:1–12
Furukawa A, Tsukahara H (1966) On the acid digestion method for the determination of chromic oxide as an index substance in the study of digestibility of fish feed. Bull Jpn Soc Sci Fish 32:502–506
Gatlin DM, Barrows FT, Brown P, Dabrowski K, Gaylord TG, Hardy RW, Herman E, Hu G, Krogdahl Å, Nelson R, Overturf K, Rust M, Sealey W, Skonberg D, Souza EJ, Stone D, Wilson R, Wurtele E (2007) Expanding the utilization of sustainable plant products in aquafeeds: a review. Aquac Res 38:551–579
Gawlicka A, Parent B, Horn MH, Ross N, Opstad I, Torrissen OJ (2000) Activity of digestive enzymes in yolk-sac larvae of Atlantic halibut (Hippoglossus hippoglossus): indication of readiness for first feeding. Aquac 184:303–314
Gaylord TG, Barrows FT, Teague AM, Johansen KA (2007) Supplementation of taurine and methionine to all- plant protein diets for rainbow trout (Oncorhynchus mykiss). Aquac 269:514–524
Gunben EM, Senoo S, Yong A, Shapawi R (2014) High potential of poultry by-product meal as a Main protein source in the formulated feeds for a commonly cultured grouper in Malaysia (Epinephelus fuscoguttatus). Sains Malaysiana 43:399–405
Harikrishnan R, Kim MC, Kim JS, Balasundaram C, Heo MS (2011) Probiotics and herbal mixtures enhance the growth, blood constituents, and nonspecific immune response in Paralichthys olivaceus against Streptococcus parauberis. Fish Shellfish Immunol 31:310–317
Hekmatpour F, Preeta Kochanian P, Marammazi JG, Zakeri M, Mohammad Mousavi SM (2018) Inclusion of poultry by-product meal in the diet of Sparidentex hasta: effects on production performance, digestibility and nutrient retention. Anim Feed Sci Technol 241:173–183
Hernandez C, Sanchez-Gutierrez Y, Hardy RW, Benitez- Hernandez A, Dominguez- Jimenez P, Gonzalez-Rodriguez B, Bosuna-Osuna L, Tortoledo O (2014) The potential of pet-grade poultry by-product meal to replace fish meal in the diet of the juvenile spoted rose snapper Lutjanus guttatus (Steindachner, 1869). Aquac Nutr 20:623–631
Hossain M, Al-Abdul-Elah K, El-Dakour S (2014) Evaluation of different commercial feeds for culture of juvenile sobaity (Sparidentex hasta, Valenciennes) in Kuwait. APCBEE Proc 8:310–316
Hummel BCW (1959) A modified spectrophotometric determination of chymotrypsin, trypsin and thrombin. Can J Biochem Physiol 37:1393–1399
Jalili R, Noori F, Agh N (2012) Effects of dietary protein source on growth performance, feed utilization and digestive enzyme activity in rainbow trout (Oncorhynchus Mykiss). J Appl Biol Sci 6:61–68
Kureshy ND, Davis A, Arnold CR (2000) Partial replacement of fish meal with meat and bone meal, flash- dried poultry by- product meal, and enzyme- digested poultry by-product meal in practical diets for juvenile red drum. N Am J Aquac 62:266–272
Lazzari R, Neto JR, de Araújo Pedron F, Loro VL, Pretto A, Gioda CR (2010) Protein sources and digestive enzyme activities in jundiá (Rhamdia quelen). Sci Agric 67:259–266
Lemieux H, Blier P, Dutil JD (1999) Do digestive enzymes set a physiological limit on growth rate and food conversion efficiency in the Atlantic cod (Gadus morhua)? Fish Physiol Biochem 20:293–303
Lindroth P, Mopper K (1979) High performance liquid chromatographic determination of subpicomole amounts of amino acids by precolumn fluorescence derivatization with o-phthaldialdehyde. Anal Chem 51:1667–1674
McClatchey KD (2002) Clinical laboratory medicine. Williams and Wilkins, Philadelphia
Me’ dale F, Boujard T, Valle ´e F, Blanc D, Mambrini M, Roem A, Kaushik SJ (1998) Voluntary feed intake, nitrogen and phosphorus losses in rainbow trout (Oncorhyncus mykiss) fed increasing dietary levels of soy protein concentrate. Aquat Living Resour 11:239–246
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bøgwald J, Castex M, Ringø E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquac 302:1–1
Moraes G, Bidinotto PM (2000) Induced changes in the amylohydrolytic profile of the gut of Piaractus mesopotamicus (Holmberg, 1885) fed different levels of soluble carbohydrate: its correlation with metabolic aspects. Rev Ictiol 8:47–51
Mozanzadeh MT, Yaghoibi M, Yavari V, Agh N, Marammazi JG, Topic-Popovic N (2015) Reference intervals for haematological and plasma biochemical parameters in sobaity seabream juveniles (Sparidentex hasta, Valenciennes 1830). Comp Clin Pathol 595
Mozanzadeh MT, Agh N, Yavari V, Marammazi JG, Mohammadian T, Gisbert E (2016) Partial or total replacement of dietary fish oil with alternative lipid sources in silvery-black porgy (Sparidentex hasta). Aquaculture 451:232–240
Mozanzadeh M, Marammazi J, Yaghoubi M, Yavari V, Agh N, Gisbert E (2017) Somatic and physiological responses to cyclic fasting and re-feeding periods in sobaity sea bream (Sparidentex hasta, Valenciennes 1830). Aquac Nutr 23:181–191
Navarro I, Gutierrez J (1995) Fasting and starvation. Elsevier, Amsterdam
Naylor RL, Hardy RW, Bureau DP, Chiu A, Elliott M, Farrell AP, Forster I, Gatlin DM, Goldburg RJ, Hua K (2009) Feeding aquaculture in an era of finite resources. PNAS 106:15103–15110
Nengas I, Alexis MN, Davies SJ (1999) High inclusion levels of poultry meals and related by products in diets for gilthead seabream Sparus aurata L. Aquac 179:13–23
NRC (2011) Nutrient requirements of fish. National Academy Press, Washington, DC
Nya EJ, Austin B (2011) Dietary modulation of digestive enzymes by the administration of feed additives to rainbow trout, Oncorhynchus mykiss Walbaum. Aquac Nutr 17:459–466
OlivaTeles A, Enes P, Peres H (2015) Replacing fishmeal and fish oil in industrial aquafeeds for carnivorous fish. In: Davis AD (ed) Feed and Feeding Practices in Aquaculture. Elsevier, Cambridge, pp 203–233
Pavlidis MA, Mylonas CC (2011) Sparidae biology and aquaculture of gilthead seabream and other species. Blackwell Publishing Ltd., UK
Perera WMK, Carter CG, Houlihan DF (1995) Feed consumption, growth and growth efficiency of rainbow trout, Oncorhynchus mykiss Walbaum fed diets containing bacterial single cell protein. Br J Nutr 73:591–603
Peres H, Santos S, Oliva-Teles A (2013) Selected plasma biochemistry parameters in gilthead seabream (Sparus aurata) juveniles. J Appl Ichthyol 29:630–636
Peres H, Santos S, Oliva-Teles A (2014) Blood chemistry profile as indicator of nutritional status in European seabass (Dicentrarchus labrax). Fish Physiol Biochem 40:1339–1347
Rawles SD, Riche M, Gaylord TG, Webb J, Freeman DW, Davis M (2006) Evaluation of poultry by-product meal in commercial diets for hybrid striped bass (Morone chrysops ♀×M. saxatilis ♂) in recirculated tank production. Aquac 259:377–389
Roosta H, Javadi T, Nazari F (2011) Isolation and characterization of trypsin inhibitors (Kunitz soybean trypsin inhibitor, Bowman-birk inhibitor) in soybean. Adv Environ Biol 145–153
Santigosa E, Sánchez J, Médale F, Kaushik S, Pérez-Sánchez J, Gallardo MA (2008) Modifications of digestive enzymes in trout (Oncorhynchus mykiss) and sea bream (Sparus aurata) in response to dietary fishmeal replacement by plant protein sources. Aquac 282:68–74
Shapawi R, Ng W-K, Mustafa S (2007) Replacement of fish meal with poultry by-product meal in diets formulated for the humpback grouper, Cromileptes altivelis. Aquaculture 273:118–126
Silva FCP, Nicoli JR, Zambonino-Infante JL, Le Gall MM, Kaushik S, Gatesoupe FJ (2010) Influence of partial substitution of dietary fish meal on the activity of digestive enzymes in the intestinal brush border membrane of gilthead sea bream, Sparus aurata and goldfish, Carassius auratus. Aquac 306(1–4):233–237
Takagi S, Hosokawa H, Shimeno S, Ukawa M (2000) Utilization of poultry by-product meal in a diet for red sea bream Pagrus major. Nippon Suisan Gakkaishi 66:428–438
Tietz NW, Shuey DF (1993) Lipase in serum- the elusive enzyme: an overview. Clin Chem 39:746–756
Wang Y, Wang F, Ji WX, Han H, Li P (2015) Optimizing dietary protein sources for Japanese sea bass (Lateolabrax japonicus) with an emphasis on using poultry by-product meal to substitute fish meal. Aquac Res 46:874–883
Xu QY, Wang CA, Zhao ZG, Luo L (2012) Effects of replacement of fish meal by soy protein isolate on the growth, digestive enzyme activity and serum biochemical parameters for juvenile Amur sturgeon (Acipenser schrenckii). Asian Australas J Anim Sci 25:1588–1594
Yaghoubi M, T. Mozanzadeh M, Gh. Marammazi J, Safari. O, Gisber E (2016) Dietary replacement of fish meal by soy products (soybean meal and isolated soy protein) in silvery-black porgy juveniles (Sparidentex hasta). Aquac 464:50–59
Yang Y, Xie SQ, Cui YB, Zhu XM, Lei W, Yang YX (2006) Partial and total replacement of fish meal with poultry by-product meal in diets for gibel carp, Carassius auratus gibelio Bloch. Aquac Res 37:40–48
Zhou QC, Zhao J, Li P, Wang HL, Wang LG (2011) Evaluation of poultry by-product meal in commercial diets for juvenile cobia (Rachycentron canadum). Aquaculture 322-323:122–127
Acknowledgments
Authors are thankful to Dr. Morteza Yaghoubi and Mr. Esmail Paghe for their technical help throughout the experiment. We are grateful to the Director and staff of the Mariculture Research Station, Sarbandar, Iran, for providing the necessary facilities for the experiment.
Funding
This study was financed by Khorramshahr University of Marine Science and Technology, Iran and South Iranian Aquaculture Research Center, Ahwaz, Iran.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hekmatpour, F., Kochanian, P., Marammazi, J.G. et al. Changes in serum biochemical parameters and digestive enzyme activity of juvenile sobaity sea bream (Sparidentex hasta) in response to partial replacement of dietary fish meal with poultry by-product meal. Fish Physiol Biochem 45, 599–611 (2019). https://doi.org/10.1007/s10695-019-00619-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00619-4