Abstract
This study aimed to evaluate the effects of fish meal (FM) replacement by yeast hydrolysate (YH) on liver antioxidant capability, intestinal morphology, and inflammation-related genes of juvenile Jian carp (Cyprinus carpio var. Jian). A total of 600 fish (average initial weight 19.44 ± 0.06 g) were randomly selected and divided into five groups. Five isonitrogenous and isocaloric diets replacing FM by YH 0% (YH0), 1% (YH1), 3% (YH3), 5% (YH5), and 7% (YH7) were formulated. Each diet was tested in four replicates for 10 weeks. The results have shown that, compared to the control group (YH0), liver total superoxide dismutase (t-SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione (GSH) activities of fish fed YH1 and YH3 diets were significantly higher (P < 0.05). Liver malondialdehyde (MDA) concentration significantly increased as supplementation levels of YH increased from 1 to 7% (P < 0.05). Moreover, intestinal microvillus length of juvenile Jian carp fed YH diets were significantly higher than that of fish fed the control diet (P < 0.05). In proximal intestine, the relative expression levels of inflammation-related genes (ALP, IL-1β, and TNF-α) in YH7 were significantly higher than that in the control group (P < 0.05). However, in midintestine, the expression levels of these genes in YH3 were significantly lower compared to the control group (P < 0.05). The results of this study indicated that dietary replacement of FM by 3%YH could improve antioxidant capability and intestinal microvillus morphology, as well as enhance the non-specific immunity of juvenile Jian carp.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Jian carp (Cyprinus carpio var. Jian) is one of the most important cultivated species that inhabits in the lakes, rivers, and reservoirs. According to the data reported by FAO, the total global production of common carp was estimated at 4.33 million metric tonnes in 2015. However, the farmed fish have suffered from serious disease problems caused by viral infection and bacterial pathogens, which have caused significant economic losses (Sato and Okamoto 2010). Although antibiotic has been widely used to prevent or control pathogenic microorganisms, antibiotics usage is associated with problems such as bioaccumulation and development of antibiotic-resistant pathogens (Teuber 2001). Therefore, there has been a growing interest in the use of natural immunostimulants, probitotics, and prebiotics as an alternative to traditional disease-control treatments, such as yeast (Dimitroglou et al. 2011). However, the physiological basis for application of yeast in aquafeeds has still not been fully understood. In view of this, it is both economically and environmentally necessary to study the mechanism of yeast to promote growth and improve immunity.
Yeast, Saccharomyces cerevisiae, has been used as a probiotic in various aquatic species and the beneficial effects reported include growth promotion, enhancement of immunity, and protection against pathogen infection (Lara-Flores et al. 2003; Li and Dmiii 2003; Ortuno et al. 2002), which have often been attributed to the structural components of yeast cell wall, such as mannan oligosaccharides (MOS) and β-glucan. In fact, MOS have proved to be effective at enhancing growth performance of fish (Torrecillas et al. 2007; Dimitroglou et al. 2010), improving gut morphology (Salze et al. 2008), modulating the intestinal micro-biota (Dimitroglou et al. 2009), and elevating immunity and stress-resistant ability (Sang et al. 2009; Zhang et al. 2012). Besides, yeast could produce various enzymes, oligosaccharides, amino acids, peptides, organic acids, vitamins, and other metabolites (Ran et al. 2015; Gonzalez et al. 1997). It was previously reported that these metabolites of yeast could enhance health and growth performance of the fish (Mohsen et al. 2010; He et al. 2011). In addition, many studies demonstrated that yeast cultures have proved to be effective in anti-inflammatory effects of swine intestine (Zanello et al. 2011). Thus, further study is needed to ascertain the role played by yeast on overcoming the limitations and side effects of antibiotics and other drugs, leading to high production through enhanced growth, stimulated immune response, and increased resistance to pathogens of fish.
Intestine, one of the main entry surfaces for infection and host-pathogen interactions, plays a key role in defending the host against attack from the external environment (Cerezuela et al. 2013). It was previously showed that the endogenous microbiota in intestine of fish provided a barrier against enteric infections from pathogens (Merrifield et al. 2010; Nayak 2010). In fact, intestine is considered to be the first line of defense against an increasingly toxic environment, which the intestinal surface area is constantly bombarded by antigens from external microorganisms (Cerezuela et al. 2013). Thus, maintaining intestine health and stability is essential for growth and defense of fish. In addition, oxidative stress of fish results from disruption of the pro-oxidant/antioxidant balance by reactive oxygen species (ROS) and other radicals or oxidants (Prior 2004). In fact, antioxidant defenses in organism have the capacity to scavenge ROS or prevent ROS-mediated cellular damage (Valavanidis et al. 2006). The defense system includes enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione (GSH), malondialdehyde (MDA), and other low molecular weight scavengers (López-López et al. 2011). These enzymes are found virtually in all tissues of vertebrates, but show in general, high activity in the liver (Lemaire et al. 1994). Therefore, the maintenance of healthy liver is vital to overall health (Treadway 1998). However, there are fewer reports on effects of fish meal replacement by yeast on intestine, liver antioxidant capability of fish. Bearing these in mind, the major goal of the present study is to elucidate the potential effects of dietary YH on antioxidant capability, intestinal morphology, and inflammation-related gene expression of juvenile Jian carp. The data obtained here may give some instructions for the application of YH in freshwater fish.
Materials and methods
Experimental diets
The components and nutrient contents of the basal diets were given in Table 1. Five isonitrogenous and isocaloric diets replacing FM by YH 0% (YH0), 1% (YH1), 3% (YH3), 5% (YH5), and 7% (YH7) were formulated. Yeast hydrolysate used in this experiment was provided by Guangdong Hinabiotech CO., Ltd. (Guangdong, China). YH was a complex product derived from enzymatic hydrolysis of the yeast, which contains 56.50% crude protein, 0.5% crude lipid, 4.4% moisture, and 9.6% ash. The increased fish oil supplemented the decreased lipid source induced by the increasing replacement levels with YH. The crude protein levels in diets were adjusted by wheat bran. Besides, fish meal, soybean meal, rapeseed meal, and cottonseed meal were used as protein sources. Wheat flour was regarded as carbohydrate source. The process of the feed-making has been previously described by Zhang et al. (2014) and Li et al. (2010), then the experiment diets stored in black plastic bags at − 20 °C until used.
Fish and feeding trial
The fish came from the fish hatchery of Freshwater Fisheries Research Institute of Jiangsu Province (Nanjing, China). A total of 600 fish (initial average weight 19.44 ± 0.06 g) were randomly selected and divided into five groups, each group was four replicates, 30 fish per replicate. All groups were farmed in the floating net cages (L:W:H, 2.0 × 1.0 × 1.7 m). Prior to experiment, the fish were reared in net cages for 2 weeks to acclimate the experiment condition. During the feeding trial, all fish of net cages were hand-fed three times daily at 7:30 a.m., 12:00 a.m., and 4:30 p.m., respectively, for 10 weeks under natural photoperiod. Fish were hand-fed to apparent satiation with utmost care to minimize feed waste. In addition, water temperature fluctuated from 24 to 28 °C, dissolved oxygen in the water was maintained approximately at 4.8 mg L−1, and pH varied between 6.6 and 7.4 during the trial period, total ammonia nitrogen less than 0.04 mg L−1.
Sample collection
At the end of the feeding trial, fish were starved for 24 h and then sampled. Four fish from each replicate were anesthetized using an overdose of MS222 (tricaine methanesulfonate, Sigma, USA), and weighed one by one. Liver of the selected fish was dissected over an ice for enzymatic analysis. Furthermore, the proximal intestine and midintestine of two fish from each replicate were processed for transmission electron microscope (TEM) analysis, another two proximal intestine and midintestine samples were used for real-time PCR (RT-PCR) analysis. All samples for RT PCR from fish were immediately snap-frozen and stored in liquid nitrogen in order to prevent RNA degradation.
Analysis of proximate composition in diets
Feeds were analyzed for proximate composition according to AOAC. (1995). Moisture was estimated by oven drying at 105 °C until constant weight; crude protein (nitrogen × 6.25) was measured by the micro-Kjeldahl method after acid digestion; crude lipid was analyzed by solvent extraction using a Soxtec System (Soxtec System HT6, Tecator, Höganäs, Sweden); ash was determined by combustion at 550 °C for 6 h; gross energy in diets was measured using a bomb calorimeter (Parr 1281; Parr Instrument Company, Moline, IL, USA).
Antioxidant enzyme assay
The liver was carefully weighed and homogenized in an ice bath with nine volumes (v/w) of chilled saline in a tissue homogenizer. The extract was later centrifuged at 3000 rpm/min at 4 °C for 10 min. The supernatant was then stored at − 20 °C for subsequent analysis. The total superoxide dismutase (t-SOD) was measured with the kit, according to the xanthine oxidase method (Marklund and Marklund 1974). Catalase (CAT) was measured with the colorimetric method (Sinha 1972). Glutathione peroxidase (GPX) activity was measured using the method described by Dabas et al. (2012). Glutathione (GSH) was determined with a commercial kit based on the recycling reaction of GSH with DNTB (5,5-dithios-2-205 nitrobenzoic acid). Malondialdehyde (MDA) was measured using the thiobarbituric acid test (Satho 1978). These kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
Intestinal microvillus morphology
The proximal intestine and midintestine from fish were conducted at the end of the trial using transmission electron microscopy (TEM), this was previously described by Merrifield et al. (2009). Firstly, samples were fixed in 2.5% glutaraldehyde for 24 h, post-fixed in 1% osmium tetroxide (OsO4) for 1 h, stored at 4 °C for 24 h, and then sections were embedded in epoxy resin Epon812 and cut by an RMC PowerTome XL microtome at 70 nm thickness. Finally, the sections were post-stained with 0.2% lead citrate and examined using Image J software version 1.45 (National Institutes of Health, USA). For each group, samples of eight replicate fish were processed and analyzed, with each fish giving one TEM image. TEM (4000×) images were analyzed to measure the microvillus length as detailed by Hu et al. (2007).
Real-time PCR analysis
Firstly, total RNA from these samples was isolated using RNAiso Plus Reagent (TaKaRa, Japan) following the manufacturer’s instruction, and then the concentration and integrity of the extracted RNA were measured by using ultra micro spectrophotometer (Thermo scientific, American) and electrophoresis in 1% agarose gel, the isolated RNA generally was purified when their OD260/OD280 ratio was between 1.8 and 2.0. Subsequently, the first-strand of cDNA was synthesized using reverse transcription kit (PrimeScripte RT Master Mix, Takara, Japan) according to the manufacturer’s protocols. Finally, the PCR primer sets were designed using primer premier 5.0 program based on sequences deposited in the Gene Bank (accession numbers of the primer sequences were listed in Table 2), all primers in this experiment were synthesized by Shanghai Generay Biotech Co., Ltd. (Shanghai, China). The PCR reaction was piloted under the following conditions: 40 cycles of 95 °C for 5 s, 60 °C for 30 s, followed by a melt curve analysis of 15 s from 95 to 60 °C, 1 m for 60 °C and then up to 95 °C for 15 s. The results of gene expression were determined using the 2−ΔΔct method, which was described by Reyes-Becerril et al. (2013) and Livak and Schmittgen (2001).
Statistical analysis
The experimental data were analyzed using version SPSS 19.0 (SPSS Inc., Michigan Avenue, Chicago, IL, USA). All results were presented as means ± S.E.M (standard error of the mean). The differences between mean values were compared using LSD. Differences between groups were seemed to be significant when the F value was significant at the level of probability 0.05.
Results
Liver antioxidant capability
As can be seen from Fig. 1, the liver antioxidant capabilities of the juvenile Jian carp were significantly affected by dietary YH levels. Total superoxide dismutase (t-SOD), catalase (CAT), glutathione peroxidase (GPX), and glutathione (GSH) activities of fish fed YH1 and YH3 diets were significantly higher than that of fish fed the control diet (P < 0.05). In addition, malondialdehyde (MDA) concentration of fish fed YH diets were significantly higher than that of fish fed the control diet (P < 0.05).
Fish meal replacement by YH on antioxidant capacities (t-SOD (a), CAT (b), GPX (c), MDA (d), and GSH (e)) in liver of juvenile Jian carp. Each data represents the means ± SEM of four replicates. Bars assigned with different letters are significantly different (P < 0.05) when comparing to the control group
Intestinal morphology
As can be seen from Table 3 and Figs. 2 and 3, intestinal microvillus length was affected by dietary YH levels and the highest values of microvillus length were occurred in the YH3. In proximal and midintestine, microvillus length of juvenile Jian carp fed YH diets were significantly higher than that of fish fed the control diet (P < 0.05). Besides, intestinal microvillus density of fish fed YH3 diet was higher than that of fish fed the control diet (Figs. 2 and 3).
Expression level of inflammation-related genes
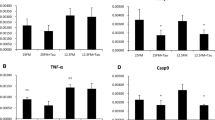
As can be seen from Fig. 4, the relative expression levels of intestinal inflammation-related genes in YH7 were significantly higher than that in the control group (P < 0.05). In proximal intestine, the expression levels of these genes in YH3 and YH5 were lower compared with the control group, but exhibited no significant difference (P > 0.05). However, in midintestine, the expression levels of these genes in YH3 and YH5 were significantly lower compared to the control group (P < 0.05).
Fish meal replacement by YH on the expression levels of inflammation-related genes (ALP in proximal intestine (a), IL-1β in proximal intestine (b), TNF-α in proximal intestine (c), ALP in midintestine (d), IL-1β in midintestine (e), and TNF-α in midintestine (f)) of juvenile Jian carp. Each data represents the means ± SEM of four replicates. Bars assigned with * are significantly different (P < 0.05) when comparing to the control group
Discussion
Generally, oxidative stress could render fish susceptible to diseases when balance between the generation and removal of reactive oxygen species (ROS) was broken (Sheikhzadeh et al. 2012). However, the fish body has several effective ways to counter oxidative stress, including enzymes (t-SOD, CAT and GPX) and functionalized molecules (GSH) (Wang et al. 2014). In the present study, the highest t-SOD and CAT activities of fish were observed in YH3. The results indicated that both SOD and CAT took part in oxygen reduction and avoided the damage from oxidative stress. This was further supported by the relatively higher GPX levels observed in fish fed YH3. These results can be attributed to the fact that SOD-CAT system constitutes the first defense against oxidative stress (Farombi et al. 2007). t-SOD can catalyze the dismutation of superoxide radicals (O2−) into O2 and hydrogen peroxide (H2O2) (Kiron 2012) and then CAT broken H2O2 into O2 and H2O, in order to control free radicals (Jovanović-Galović et al. 2004; Winston and Giulio 1991). Similarly, the results obtained in leopard grouper (Mycteroperca rosacea) (Reyes-Becerril et al. 2010). MDA is commonly regarded as an indicator to evaluate lipid peroxidation, it has a strong biotoxicity and will damage the cell structure and function (Parvez and Raisuddin 2005). In this study, fish fed with YH5 and YH7 diets had higher liver MDA content and lower SOD and CAT activities, indicating the production of ROS increased and therefore imbalance between the generation and removal of reactive oxygen species (ROS) existed in these groups. This suggests further that the fish in YH5 and YH7 had undergone oxidative damage. Therefore, the SOD, CAT, and MDA contents of liver can accurately reflect the antioxidant status in fish. Furthermore, in the present study, GSH activity of the fish firstly increased and then decreased with the replacement levels of YH increased, and the highest values were observed in YH3. A similar trend was also observed in liver GPX activities. This could be attributed to the fact that GPX decomposes peroxides using GSH as its co-substrate (Halliwell 2006). This again confirmed that appropriate replacement levels might reduce stress oxidative response of juvenile Jian carp.
In the current study, intestinal microvillus length of fish fed YH diets was significantly higher than that of fish fed the control diet. This indicated that dietary YH can improve the intestinal microvillus length. It has also reported that yeast was able to increase microvillus length in different fish species (Zhu et al. 2012). Similar results have also been observed in rainbow trout (Oncorhynchus mykiss) (Pouramini et al. 2008). In fact, microvillus length is usually thought to be directly related to the absorptive surface area of the intestine (Cerezuela et al. 2012). Therefore, juvenile Jian carp fed the diet supplemented with YH diets presented a higher microvillus length which translated to increased enterocyte absorptive area and subsequently resulted in the improved growth performance when compared with fish fed the control diet. Controversially, Dimitroglou et al. (2010) found the microvilli density and length improvements did not translate into improved nutrient utilization or growth performance in gilthead sea bream (Sparus aurata). Therefore, further research is needed to obtain sufficient information on the endocytic activity. In addition, in this study, microvillus density of fish fed YH3 diet was higher than that of fish fed the control diet, indicating that appropriate YH level could produce more microvilli structures which should provide the potential possibility of improving nutrient capture. The result could be attributed to yeast structural components like MOS, β-glucan, and nucleic acids (Ran et al. 2015). Similarly, improvements of gut morphology were previously reported in gilthead sea bream (Sparus aurata) (Dimitroglou et al. 2010).
Alkaline phosphatase (ALP) is responsible for the hydrolysis of phosphate groups of many types of molecules, including bacterial lipopolysaccharides (Péres et al. 1998). In the present study, down-regulation of the ALP gene in midintestine of fish fed YH3 diet. This indicated that appropriate level of dietary YH decreased the expression levels of the ALP. Similar result was observed in Nile tilapia (Oreochromis niloticus). It found that lower gut alkaline phosphatase activity in the yeast supplement groups reflected significantly protection of the host against pathogen (Ran et al. 2015). In aquatic animals, the inflammatory response plays an important role in the intestinal immunity response (Niklasson et al. 2011), which is primarily mediated by cytokines (Secombes et al. 2001). Therefore, the cytokine gene expression in intestine of juvenile Jian carp was assayed. In the present study, the pro-inflammatory cytokines interleukin-1ß (IL-1ß) and tumor necrosis factor-a (TNF-α) in mid intestine of fish fed with YH3 and YH5 diets significantly decrased compared to the control group. These results indicated that appropriate levels of fish meal replacement by YH attenuated the intestinal inflammatory response. The explanation may be that the YH enhanced the innate immunity response (Yuan et al. 2017). One reasonable explanation was that MOS derived from yeast cell wall can reproduce probiotic bacteria and restrain the adherence and colonization of pathogen in gut (Kaneko et al. 1995), thus resulting in the enhanced immunity of fish. Therefore, in our study, it again confirmed that dietary YH was very helpful in enhancing non-specific immunity.
Conclusions
In conclusion, the current study reveals that dietary replacement of FM by 3%YH could improve antioxidant capability and lower inflammatory response of juvenile Jian carp. In addition, dietary supplementation of YH resulted in a significant improvement in intestinal microvillus length and density.
References
AOAC (Association of Official Analytical Chemists) (1995) Official methods of analysis. AOAC, Arlington, p 1298
Cerezuela R, Fumanal M, Tapia-Paniagua ST, Meseguer J, Moriñigo MA, Esteban MA (2012) Histological alterations and microbial ecology of the intestine in gilthead seabream (Sparus aurata L.) fed dietary probiotics and microalgae. Cell Tissue Res 350:477–489
Cerezuela R, Meseguer J, Esteban MÁ (2013) Effects of dietary inulin, bacillus subtilis and microalgae on intestinal gene expression in gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol 34:843–848
Dabas A, Nagpure N, Kumar R, Kushwaha B, Kumar P, Lakra W (2012) Assessment of tissue-specific effect of cadmium on antioxidant defense system and lipid peroxidation in freshwater murrel, Channa punctatus. Fish Physiol Biochem 38:469–482
Dimitroglou A, Merrifield DL, Moate R, Davies SJ, Spring P, Sweetman J, Bradley G (2009) Dietary mannan oligosaccharide supplementation modulates intestinal microbial ecology and improves gut morphology of rainbow trout, Oncorhynchus mykiss (Walbaum). J Anim Sci 87:3226–3234
Dimitroglou A, Merrifield DL, Spring P, Sweetman J, Moate R, Davies SJ (2010) Effects of mannan oligosaccharide (MOS) supplementation on growth performance, feed utilisation, intestinal histology and gut microbiota of gilthead sea bream (Sparus aurata). Aquaculture 300:182–188
Dimitroglou A, Merrifield DL, Carnevali O, Picchietti S, Avella M, Daniels C, Güroy D, Davies SJ (2011) Microbial manipulations to improve fish health and production a Mediterranean perspective. Fish Shellfish Immunol 30:1–16
FAO (Food and Agricultural Organization of the United Nations) (2015) Fisheries and aquaculture department, Rome, Italy. http://www.fao.org/fishery/statistics/global-commodities-production/en
Farombi E, Adelowo O, Ajimoko Y (2007) Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in African cat fish (Clarias gariepinus) from Nigeria Ogun River. Int J Environ Res Public Health 4:158–165
Gonzalez B, François J, Renaud M (1997) A rapid and reliable method for metabolite extraction in yeast using boiling buffered ethanol. Yeast 13:1347–1355
Halliwell B (2006) Reactive species and antioxidants redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
He S, Zhou Z, Meng K, Zhao H, Yao B, Ringø E, Yoon I (2011) Effects of dietary antibiotic growth promoter and Saccharomyces cerevisiae fermentation product on production, intestinal bacterial community, and nonspecific immunity of hybrid tilapia (Oreochromis niloticus female×Oreochromis aureus male). J Anim Sci 89:84–92
Hu C, Xu Y, Xia M, Xiong L, Xu Z (2007) Effects of Cu2+-exchanged montmorillonite on growth performance, microbial ecology and intestinal morphology of Nile tilapia (Oreochromis niloticus). Aquaculture 70:200–206
Huttenhuis HBT, Taverne-Thiele AJ, Grou CPO, Bergsma J, Saeij JPJ, Nakayasu C, Rombout JHWM (2006) Ontogeny of the common carp (Cyprinus carpio L.) innate immune system. Dev Comp Immunol 30:557–574
Jovanović-Galović A, Blagojević DP, Grubor-Lajšić G, Worland R, Spasić MB (2004) Role of antioxidant defense during different stages of preadult life cycle in European corn borer (Ostrinia nubilalis, Hubn.): diapause and metamorphosis. Arch Insect Biochem Physiol 55:79–89
Kaneko T, Yokoyama A, Suzuki M (1995) Digestibility characteristics of isomaltooligosaccharides in comparison with several saccharides using the rat jejunum loop method. Biosci Biotechnol Biochem 59:1190–1194
Kiron V (2012) Fish immune system and its nutritional modulation for preventive health care. Anim Feed Sci Technol 173:111–133
Lara-Flores M, Olvera-Novoa MA, Guzman-Méndez BE, López-Madrid W (2003) Use of the bacteria Streptococcus faeciumand and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquaculture 216:193–201
Lemaire P, Matthews A, Forlin L, Livingstone DR (1994) Stimulation of oxyradical production of hepatic microsomes of flounder (Platichthys flesus) and perch (Perca fluvialis) by model and pollutant xenobiotics. Arch Environ Contam Toxicol 26:191–200
Li P, Dmiii G (2003) Evaluation of brewer’s yeast (Saccharomyces cerevisiae) as a feed supplement for hybrid striped bass (Morone chrysops×M. saxatilis). Aquaculture 219:681–692
Li XF, Liu WB, Jiang YY, Zhu H, Ge XP (2010) Effects of dietary protein and lipid levels in practical diets on growth performance and body composition of blunt snout bream (Megalobrama amblycephala) fingerlings. Aquaculture 303:65–70
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C (T)) method. Methods 25:402–408
López-López E, Sedeño-Díaz JE, Soto C, Favari L (2011) Responses of antioxidant enzymes, lipid peroxidation, and Na+/K+-ATPase in liver of the fish Goodea atripinnis exposed to Lake Yuriria water. Fish Physiol Biochem 37:511–522
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 47:469–474
Merrifield DL, Dimitroglou A, Bradley G, Baker R, Davies S (2009) Soybean meal alters autochthonous microbial populations, microvilli morphology and compromises intestinal enterocyte integrity of rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 32:755–766
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bøgwald J, Castex M, Ringø E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1–18
Mohsen A, Mamdouhaa M, Maalya M (2010) Use of live baker's yeast, Saccharomyces cerevisiae, in practical diet to enhance the growth performance of galilee tilapia, Sarotherodon galilaeus (L.), and its resistance to environmental copper toxicity. J World Aquacult Soc 41:214–223
Nayak SK (2010) Role of gastrointestinal microbiota in fish. Aquac Res 41:1553–1573
Niklasson L, Sundh H, Fridell F, Taranger G, Sundell K (2011) Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol 31:1072–1080
Ortuno J, Cuesta A, Rodríguez A, Esteban MA, Meseguer J (2002) Oral administration of yeast, Saccharomyces cerevisiae, enhances the cellular innate immune response of gilthead seabream (Sparus aurata L.). Vet Immunol Immunopathol 85:41–50
Parvez S, Raisuddin S (2005) Protein carbonyls: novel biomarkers of exposure to oxidative stress-inducing pesticides in freshwater fish Channa punctata (Bloch). Environ Toxicol Pharmacol 20:112–117
Péres A, Infante JLZ, Cahu C (1998) Dietary regulation of activities and mRNA levels of trypsin and amylase in sea bass (Dicentrarchus labrax) larvae. Fish Physiol Biochem 19:145–152
Pouramini M, Alghasem KA, Almajid HMA, Rasoul G, Morteza A (2008) Effect of feeding by yeast (Saccharomyces cerevisiae) as a probiotic, in contrast with salinity stress and on intestinal histology in rainbow trout (Oncorhynchus mykiss) fry. J Fisheries 2:33–40
Prior RL (2004) Biochemical measures of antioxidant status. Top Clin Nutr 19:226–238
Ran C, Huang L, Liu Z, Xu L, Yang YL, Philippe T, Eric A, Zhou ZG (2015) A comparison of the beneficial effects of live and heat-inactivated baker’s yeast on Nile Tilapia: suggestions on the role and function of the secretory metabolites released from the yeast. PLoS One 10:e0145448
Reyes-Becerril M, Tovar-Ramírez D, Ascencio-Valle F, Civera-Cerecedo R, Gracia-López V, Barbosa-Solomieu V, Esteban MÁ (2010) Effects of dietary supplementation with probiotic live yeast Debaryomyces hansenii on the immune and antioxidant systems of leopard grouper Mycteroperca rosacea infected with Aeromonas hydrophila. Aquac Res 42:1676–1686
Reyes-Becerril M, Guardiola F, Rojas M, Ascenciovalle F, Esteban MÁ (2013) Dietary administration of microalgae Navicula sp. affects immune status and gene expression of gilthead seabream (Sparus aurata). Fish Shellfish Immunol 35:883–891
Salze G, Mclean E, Schwarz MH, Craig SR (2008) Dietary mannan oligosaccharide enhances salinity tolerance and gut development of larval cobia. Aquaculture 274:148–152
Sang HM, Ky LT, Fotedar R (2009) Dietary supplementation of mannan oligosaccharide improves the immune responses and survival of marron, Cheraxtenuimanus (Smith, 1912) when challenged with different stressors. Fish Shellfish Immunol 27:341–348
Satho K (1978) Serum lipid peroxidation in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta 190:37–43
Sato T, Okamoto N (2010) Induction of virus-specific cell-mediated cytotoxic responses of isogeneic ginbuna crucian carp, after oral immunization with inactivated virus. Fish Shellfish Immunol 29:414–421
Secombes CJ, Wang T, Hong S, Peddie S, Crampe M, Laing KJ, Cunningham C, Zou J (2001) Cytokines and innate immunity of fish. Dev Comp Immunol 25:713–723
Sheikhzadeh N, Tayefi-Nasrabadi H, Oushani AK, Enferadi MH (2012) Effects of Haematococcus pluvialis supplementation on antioxidant system and metabolism in rainbow trout (Oncorhynchus mykiss). Fish Physiol Biochem 38:413–419
Sinha AK (1972) Colorimetric assay of catalase. Anal Biochem 47:389–394
Teuber M (2001) Veterinary use and antibiotic resistance. Curr Opin Microbiol 4:493–499
Torrecillas S, Makol A, Caballero MJ, Montero D, Robaina L (2007) Immune stimulation and improved infection resistance in European seabass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish Immunol 23:969–981
Treadway S (1998) An Ayurvedic herbal approach to a healthy liver. Clin Nutr Insights 6:1–3
Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M (2006) Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicol Environ Saf 64:178–179
Wang LN, Liu WB, Lu KL, Xu WN, Cai DS, Zhang CN, Qian Y (2014) Effects of dietary carbohydrate/lipid ratios on non-specific immune responses, oxidative status and liver histology of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 426–427:41–48
Winston GW, Giulio RTD (1991) Prooxidant and antioxidant mechanisms in aquatic organisms. Aquat Toxicol 19:137–161
Yuan XY, Liu WB, Liang C, Sun CX, Xue YF, Wan ZD, Jiang GZ (2017) Effects of partial replacement of fish meal by yeast hydrolysate on complement system and stress resistance in juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol 67:312–321
Zanello G, Meurens F, Berri M, Chevaleyre C, Melo S, Auclair E, Salmon H (2011) Saccharomyces cerevisiae decreases inflammatory responses induced by F4+ enterotoxigenic Escherichia coli in porcine intestinal epithelial cells. Vet Immunol Immunopathol 141:133–138
Zhang J, Liu YJ, Tian LX, Yang HJ, Liang GY, Xu DH (2012) Effects of dietary mannan oligosaccharide on growth performance, gut morphology and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei. Fish Shellfish Immunol 33:1027–1032
Zhang YY, Liu B, Ge XP, Liu WB, Xie J, Ren MC, Cui YT, Xia SL, Chen RL, Zhou QL, Pan LK (2014) The influence of various feeding patterns of emodin on growth, non-specific immune responses, and disease resistance to Aeromonas hydrophila in juvenile Wuchang bream (Megalobrama amblycephala). Fish Shellfish Immunol 36:187–193
Zhu H, Liu H, Yan J, Wang R, Liu L (2012) Effect of yeast polysaccharide on some hematologic parameter and gut morphology in channel catfish (Ictalurus punctatus). Fish Physiol Biochem 38:1441–1447
Funding
This research was funded by the earmarked fund for China Agriculture Research System (CARS-45-14), the National Natural Science Foundation of China (31502178) and the Fundamental Research Funds for the Central Universities (KJQN201612).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuan, XY., Jiang, GZ., Wang, CC. et al. Effects of partial replacement of fish meal by yeast hydrolysate on antioxidant capability, intestinal morphology, and inflammation-related gene expression of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Physiol Biochem 45, 187–197 (2019). https://doi.org/10.1007/s10695-018-0552-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-018-0552-7