Abstract
Worldwide, the frequencies and magnitudes of hypoxic events in estuarine waters have increased considerably over the past two decades. Fish populations are suitable indicators for the assessment of quality of aquatic ecosystems and often comprise a variety of adaptation systems by triggering oxidants, antioxidants and hypoxia-responsive signaling proteins. Signaling pathway may lead to cell survival or cell death which is fine-tuned by both positive and negative factors, which includes hypoxia-inducible factor-1α (HIF1α), heat-shock protein-70 (HSP70), phospho-c-Jun N-terminal kinase 1/2 (p-JNK1/2) and apoptosis signal-regulating kinase-1 (ASK1). In the present study, we attempt to determine stress-mediated signaling changes and molecular mechanism behind the cell survival by comparing adipocytes of fish from field hypoxic condition and laboratory-induced hypoxic condition (in vitro hypoxia). Comparison of field and laboratory studies in fish adipocytes showed differential expression of HIF1α, HSP70, p-JNK1/2 and ASK1 with altered oxidants and antioxidants. Further, the results also suggest that in vitro hypoxic conditions mimic field hypoxic conditions. Trends of hypoxia response were same in in vitro hypoxia of control adipocytes as in Ennore estuary, and hypoxia response was more pronounced in the test adipocytes under in vitro hypoxic condition. Results of the present work suggest that hypoxia is the major crusade of water pollutants affecting fish by differential regulation of pro- and antiapoptotic proteins probably through HSP70. This may play a vital role by providing cytoprotection in pollutant-induced stressed fish adipocytes substantiated by the in vitro hypoxic studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dissolved oxygen (DO) is a fundamental requirement for maintaining a diverse aquatic ecosystem. The environment is increasingly challenged by the consequences of human actions which results in hypoxic ecosystem (Volodymyr et al. 2001; Storey 1996). Hypoxia is a primary problem in estuaries, and it is a complex ecological phenomenon arising from the convergence of several environmental factors such as freshwater runoff, water column stratification, biological processes and weather conditions (Adams 2005). Previously, we have demonstrated that these environmental stressors initiate the complex stress responses which promote oxidative stress (OS) and activation of many relevant signaling molecules for the adaptation in fish hepatocytes and adipocytes (Padmini and Vijaya Geetha 2009; Padmini and Tharani 2014; Padmini and Parimala 2015). In particular, our previous study observed that hypoxia has pervasive effect on fish adipocytes, which is evidenced by low DO-induced HIF1α and its mediated signaling proteins expression, suggesting the key role of hypoxia due to environmental pollutants in Ennore estuary (Padmini and Parimala 2015); hence, it is considered as the site of field hypoxia. Since fish from Kovalam estuary do not experience hypoxia due to pollutants, it is taken as control site. In order to ascertain the effect of hypoxia, adipocytes from both estuaries were subjected to in vitro hypoxia and evaluated the oxidant–antioxidant status along with analysis of the expression of HIF1α and HSP70 and its associated effect on ASK1 and p-JNK1/2 in adipocytes of Mugil cephalus (M. cephalus, commonly known as grey mullets).
Adipocytes have been studied with increasing intensity as they serve as an integrator of various physiological pathways. In particular, its role in energy storage makes adipocytes well suited to the regulation of energy balance (Macia et al. 2006). Therefore, knowledge of adipocyte biology is crucial for understanding wide array of homeostatic processes. Although most multicellular organisms have adipocytes, anatomical location of fat tissues varies considerably between species (Rosen and Spiegelman 2006). Its principal role is storing excess energy as fat and releasing fatty acids (FAs) into the circulation for reviving fuel diminution (Pond 1998). These FAs are generated by breaking down triglycerides (TG), which contain more energy per unit mass. In addition to regulating fat mass and nutrient homeostasis, adipocytes are involved in the immune response, controlled blood pressure, bone mass, thyroid and reproductive function (Trayhurn 2005).
The synthesis of TG in the adipocyte uses both pre-made and de novo made FAs, whereas the glycerol backbone comes from glucose-derived glycerol-3-phosphate (Mariana et al. 2008). Lipogenic capacity of adipocyte was assessed by the specific activity of the enzyme glycerol-3-phosphate dehydrogenase (G3PDH), which catalyzes the formation of the glycerol backbone of triglycerides from dihydroxyacetone phosphate provided via glycolysis (Moustaid et al. 1996). This enzyme is considered rate limiting for triglyceride synthesis in adipose tissue (Mariana et al. 2008). G3PDH acts as a lipogenic marker in mature adipocytes which is supported by the upregulation of its mRNA and activity (Rumberger et al. 2003). However, hypoxia-mediated lipogenesis and lipolysis alterations are not elucidated earlier in fish.
Hypoxia-inducible factor-1 (HIF1), the key regulator of many cellular antihypoxic responses, is induced as an adaptive response to tissue hypoxia in human adipose tissue (Hosogai et al. 2007). In human adipocyte, HIF1α serves as a central regulator of adipocyte metabolism and energy expenditure (Krishnan et al. 2012). HIF1 is a heterodimer consisting of HIF1α and HIF1β (Greijer and van der Wall 2004). HIF1β is constitutively expressed, and its levels are not regulated by hypoxia; in contrast, HIF1α induction is functionally more rate limiting (Rocha 2007). Even though it is also constitutively expressed, HIF1α is highly prone to ubiquitination and is rapidly degraded in the presence of oxygen (Kallio et al. 1999). However, under hypoxic conditions, HIF1α is stabilized, and the accumulated protein quickly translocates into the nucleus, where it forms heterodimeric complexes with HIF1β (Bruick and McKnight 2001; Ivan et al. 2001). The heterodimers bind to genomic hypoxia response elements (HREs) and transactivate a wide variety of genes, including genes whose protein products are involved in cell survival, glycolysis, erythropoiesis and angiogenesis (Brahimi-Horn and Pouyssegur 2007; Sun et al. 2013).
The cellular response to hypoxic stress involves changes in many levels of gene regulation, including transcription, messenger RNA (mRNA) processing and translation (Biamonti and Caceres 2009). The heat-shock response is one of the best-characterized stress response pathways, during which heat-shock proteins (HSPs), a class of molecular chaperones, are regulated in response to widespread protein misfolding (Richter et al. 2010). The induction of HSPs in fish in response to stress serves to guard against the initial insult, augment recovery and produce a state of resistance to subsequent stress (Basu et al. 2002). In addition, HSP can function in cellular protection by modulating the engagement and/or progression of apoptosis induced by a variety of stress stimuli (Huang et al. 2001). The 70-kDa family of heat-shock protein-70 (HSP70), in particular, plays a vital role in cellular protection; it has been detected in various tissues subject to stress (Jang et al. 2008). Although the functions of HSP70 and its interaction with other signaling molecules have been studied in various tissues in detail, still the significance in fish adipocyte under pollution stress remains obscure. Hence, the present study analyzed the HSP70 in field and in vitro hypoxia in fish adipocytes.
Variety of stimuli such as hypoxia and inflammation promotes the activation of mitogen-activated protein kinases (MAPKs), which are ubiquitously expressed and highly conserved serine/threonine kinases. Apoptosis signal-regulating kinase-1 (ASK1) is a member of the MAPKKK family and activates both the MKK4/MKK7–JNK and MKK3/MKK6–p38 MAP kinase signaling cascades and constitutes a pivotal signaling pathway in various types of stress-induced apoptosis. ASK1 functions as an important molecular sensor of degree of internal and/or external environmental stresses to determine cell fate, such as survival, differentiation or apoptosis (Atsushi et al. 2002). JNK, also called the stress-activated MAPK (SAPK), is activated by cell responses to cytokines or stress. In addition to influencing the inflammatory response, metabolism and cell transformation through activation of various transcription factors, JNK plays an important role in apoptosis/cell survival by inhibiting the antiapoptotic proteins Bcl-2 and Bcl-xl (Davis 2000; Cuevas et al. 2007).
Hence, in the present study, in vitro hypoxia was employed to study the ability of the adipocytes to adapt the changing environment and the mechanisms of adaptation in fish during field hypoxia.
Materials and methods
Study site and study animal sampling
Grey mullets (n = 20) with an average length of 30–32 cm were collected from Kovalam (control) and Ennore (test) estuaries using baited minnow traps, which were situated on the east coast of India. Contamination of this estuary by heavy metals and the difference in physical, chemical and biological characteristics has already been confirmed by previous studies (Padmini and Vijaya Geetha 2007a, b; Padmini and Parimala 2014). Water quality was assessed by analyzing dissolved oxygen level of both Kovalam and Ennore estuaries. Dissolved oxygen level was estimated by CHEMLINE portable dissolved oxygen meter CL-930, and it is expressed as ppm. Simultaneously, fish were collected from both estuaries and placed immediately into insulated containers filled with aerated estuarine water at ambient temperature (25–30 °C) and salinity (24–29 ppt). Fish were maintained in the above-specified conditions for 4–5 h until the start of the experimental procedure for the isolation of adipocytes. The experiments were divided into three batches with minimum five samples at each time.
Adipocytes isolation
Adipose tissue was carefully removed from each fish (n = 20), then it was washed with distilled water separately, and the adipocytes were isolated by the method of Rodbell (1964) with some minor modifications (at temperature 18 °C). Briefly, adipose tissue was cut into small pieces and incubated in polypropylene tubes with isosmotic Krebs buffer (pH 7.4, 280 mM) containing collagenase type II (0.3 mg/ml) and 1 % BSA without glucose for 60 min in a water bath under gentle shaking at 18 °C. The cell suspension was filtered through a 100-µm filter to remove large undigested tissue particles and centrifuged at 700×g for 10 min. Then the pellet was washed by flotation. Finally, floating cells were carefully removed as it contains mature adipocytes. The isolated adipocytes from each fish (control and test fish) were separately used for following experiments.
Incubation of adipocytes under hypoxia
A known volume of isolated adipocytes (both control and test) was immediately suspended in Dulbecco’s modified Eagle’s medium (DMEM) containing 10 % FBS, 2 mM l-glutamine, 10 mM HEPES and 9 mM bicarbonate antibiotics. Condition of hypoxia was carried out by incubating adipocytes in 1 % O2, 94 % N2 and 5 % CO2 at different time intervals (1, 2 and 3 h) in the Forma water-jacketed CO2/O2 incubator (model: 3131, Thermo Fisher Scientific, USA) with the temperature maintenance of 18 °C. Following hypoxic incubation, cell viability was assessed by the following method to determine the effective incubation time which was mimicking the wild hypoxia; then it was utilized for the further comparative studies on biochemical analysis.
Cell viability assay
Viability of adipocytes (both field condition and incubated under hypoxia) was determined by trypan blue staining (Strober 2001). This dye exclusion test was used to determine the number of viable cells present in a cell suspension and was based on the principle that live cells possess intact cell membrane that exclude dyes such as trypan blue, whereas dead cells do not exclude dyes. In brief, suspension cells are harvested by centrifugation. An equal volume of 0.4 % (w/v) trypan blue is added to a cell suspension at a concentration of approximately 1 × 106 per mL. The cells were then incubated for 3 min and loaded into a hemacytometer. Nonviable, deep blue cells as well as viable, clear cells are counted in three separate fields using bright-field optics. The viability percentage was calculated by dividing the number of viable cells by the number of total cells and multiplying it by 100.
Estimation of protein
Adipocytes were harvested in cell suspension buffer (Krebs buffer pH 7.4), centrifuged (15 min at 2000 rpm) and resuspended in cell lysis buffer (20 mM Tris pH 7.5, 1 % Triton X-100, 1 mM ethylenediamine tetraacetic acid (EDTA), 1 mM ethylene glycol-bis (2-aminoethyl)-tetraacetic acid (EGTA), 1 mM phenylmethyl sulphonylfluoride (PMSF), 5 mM sodium pyrophosphate, 2 mM sodium orthovanadate and protease inhibitor). The cell suspension was incubated for 30 min at 4 °C, with occasional shaking, and centrifuged at 16,000×g for 10 min in a 4 °C to remove the cellular debris. The supernatant was the cell lysate, whose protein concentration was determined by the classical method of Bradford (1976) with Coomassie Brilliant Blue G-250, using bovine serum albumin as a standard. The protein concentration was expressed as mg protein/g of fresh weight of adipose tissue. Based on the requirement, sample was diluted to acquire 100 µg of protein by using Krebs buffer. Then, 100 µg of protein of each sample was used for the following analyzes, except for Western blotting (only 50 µg of protein required and used).
Estimation of triglycerides
Triglyceride was estimated by using commercially available kit based on GPO (glycerol phosphate oxidase) method (Padmini and Lavanya 2014).
Estimation of glycerol
Glycerol level was quantified in adipocytes using ELISA kit (Cat. No: 10010755, Cayman Chemicals CO, India) according to the manufacturer’s instruction.
Estimation of FA
FA was estimated by the colorimetric method of Falholt et al. (1973). Fifty microliters of sample is added to l ml phosphate buffer (pH 6.4) and 6 ml chloroform/heptane/methanol solution in a test tube. This is shaken for 10 min and centrifuged at 4000 rpm for 10 min. The buffer is removed carefully by suction, and 5 ml of organic phase is shaken with 2 ml of a solution containing 0.05 mol/l Cu(NO3)2 and 0.1 mol/l triethanolamine, pH 8.1 for 5 min. After centrifugation at 4000 rpm for 5 min, 3 ml of the upper phase is transferred to a test tube containing 0.5 ml of a solution prepared from 10 ml of 4 g/l, 1,5-diphenylcarbazide in ethanol and 0.1 ml of 1 mol/l triethanolamine and mixed carefully. Palmitic acid was used as the standard. Colorimetric determination is made after 15 min at 550 nm.
Assay of GPDH
GPDH activity was measured by following Sottile and Seuwen (2001) with slight modifications. Cells were washed with PBS, and the assay mixture was added to the tube (0.1 M triethanolamine, 2.5 mM EDTA, 0.1 mM b-mercaptoethanol and 334 mM NADH, pH 7.7), and tubes were incubated for 10 min at 30 °C. The reaction was started by adding 4 mM dihydroxyacetone phosphate. GPDH activity was measured spectrophotometrically at 340 nm. GPDH served as the standard for the assay, and results were expressed as mU/mg protein (1 U = 1 mmol NADH/min).
Lipid peroxide (LPO) and nitrite (NO2 −)
The levels of LPO were determined by the method of Ohkawa et al. (1979) and expressed as nm MDA/mg protein. Estimation of NO2 − was based on the method of Yokoi et al. (1996) and expressed as μmol/mg protein.
Antioxidant status
The activity of super oxide dismutase (SOD) was estimated by the method of Misra and Fridovich (1972). The activity of catalase (CAT) was estimated by the method of Beer and Seizer (1952). The SOD and CAT activities were expressed as units/minutes/mg protein. Total antioxidant capacity (TAC) was evaluated by the method described by Prieto et al. (1999) and expressed as Trolox equivalent in mmol/L.
Quantification of HIF1α, HSP70 and ASK1 using ELISA kit
HIF1α, HSP70 and ASK1 in adipocytes were quantified using respective ELISA kits (CSB-E12112H, CUSA BIOTECh, China, 96 T; MBS706016, Mybiosource, USA; E91358Hu 96 T, Uscn Life Science Inc, USA) according to the manufacturer’s instruction.
Western blotting of JNK1/2
Aliquots of the sample (after cell lysis as mentioned in the estimation of protein) containing optimal protein concentration of 50 µg were run on 10 % SDS–polyacrylamide gel. Gel was run on an electrophoretic system and was then be blotted on to PVDF membrane (Biotrace, Germany) according to the method of Towbin et al. (1979) after which the blotted membrane was blocked specifically. The blot was then be washed thoroughly and cut into two equal halves. One part of the blotted membrane was incubated with antibody against phosphor JNK1/2 (rabbit polyclonal (KAP-SA011); 1:500 dilution), along with an anti-β-actin antibody (rabbit polyclonal (CSA-400); 1:1000 dilution) being used to assess equal loading for overnight at 4 °C for optimal time duration. The membrane was incubated with appropriately diluted alkaline phosphatase-labeled IgG secondary antibody prepared in TBS–Tween buffer. The 3-bromo-4-chloro-indolylphosphate-nitroblue tetrazolium (BCIP-NBT) substrate system was used to detect the alkaline phosphatase conjugate of all the blots separately. The band intensities were quantified using TOTAL-LAB software.
Statistical analysis
Data were analyzed using statistical software package version 7. One-way analysis of variance (ANOVA) was used to ascertain the significance of variations between control and test fish adipocytes with hypoxia and without hypoxia. Differences were considered significant at p < 0.05, p < 0.01 and p < 0.001.
Results
Dissolved oxygen level
Decreased level of DO by 29 % (p < 0.01) in Ennore estuary (3.67 ppm) water when compared to Kovalam estuary (5.19 ppm) water was observed.
Cell viability
Figure 1 depicts the cell viability. Decreased cell viability in test adipocytes (17 %, p < 0.05) was observed when compared to control adipocytes. Under in vitro hypoxic condition for 1 h, decrease in the cell viability (p < 0.05, 15 and 22 %, respectively) in control and test adipocytes was observed when compared to control and test adipocytes without hypoxic incubation. Control adipocytes on 1-h hypoxia closely mimic the test adipocytes from field condition, depicted by insignificant difference in cell viability between them (2 %, NS), whereas on hypoxic incubation for 2 and 3 h, cell viability further decreased in control by 24 and 34 % and in test adipocytes by 36 and 45 % when compared to adipocytes without hypoxic induction. Hence, adipocytes were incubated under hypoxia for 1 h and used for the further experiments.
Adipocytes viability of M. cephalus during field and in vitro (1, 2 and 3 h) hypoxic conditions. Values are expressed as mean ± SD (n = 20 fish per site). CA control adipocytes, TA test adipocytes, CH1 control adipocytes under hypoxia (1 h), TH1 test adipocytes under hypoxia (1 h), CH2 control adipocytes under hypoxia (2 h), TH2 test adipocytes under hypoxia (2 h), CH3 control adipocytes under hypoxia (3 h), TH3 test adipocytes under hypoxia (3 h). ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Level of protein
Level of protein is depicted in Fig. 2. Lower protein concentration (71 % (p < 0.001)) was observed in test adipocytes compared to control adipocytes. On hypoxic incubation, level of protein decreased significantly in control and test adipocytes by 39 and 26 % when compared to control and test adipocytes without hypoxic incubation. Significant difference (p < 0.001) in protein concentration was observed between control adipocytes on hypoxia and test adipocytes from field condition.
Level of protein in adipocytes of M. cephalus during field and in vitro hypoxic (for 1 h) conditions. Values are expressed as mean ± SD (n = 20 fish per site). C control adipocytes, T test adipocytes, CH control adipocytes under hypoxia, TH test adipocytes under hypoxia. ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Level of TG
Figure 3 indicates the level of TG. Lower TG concentration (53 %, p < 0.001) was observed in test adipocytes compared to control adipocytes. Significant decrease in the level of TG by 34 % (p < 0.001) and 28 % (p < 0.01) in control and test adipocytes on hypoxic incubation when compared to control and test adipocytes without hypoxic incubation was observed. Level of TG (p < 0.001) varied between control adipocytes on hypoxia and test adipocytes from field condition.
Level of TG in adipocytes of M. cephalus during field and in vitro hypoxic (for 1 h) conditions. Values are expressed as mean ± SD (n = 20 fish per site). C control adipocytes, T test adipocytes, CH control adipocytes under hypoxia, TH test adipocytes under hypoxia. ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Level of glycerol and FA
Figure 4 exhibits the level of glycerol and FA. Higher glycerol and FA level (41 and 61 %; (p < 0.001)) was observed in test adipocytes compared to control adipocytes. Significant raise in the level of glycerol and FA by 33 and 42 % (p < 0.001), and 26 and 23 % (p < 0.01) was observed in control and test adipocytes on hypoxic incubation when compared to control and test adipocytes without hypoxic incubation. No significant difference in the level of glycerol and FA was observed between control adipocytes on hypoxia and test adipocytes from field condition.
Level of FA and glycerol in adipocytes of M. cephalus during field and in vitro hypoxic (for 1 h) condition. Values are expressed as mean ± SD (n = 20 fish per site). C control adipocytes, T test adipocytes, CH control adipocytes under hypoxia, TH test adipocytes under hypoxia. ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Level of G3PDH
Figure 5 demonstrates the G3PDH activity. In test adipocytes, decrease in the activity of G3PDH (27 %) (p < 0.01) compared to control adipocytes was found. On hypoxic incubation, further decrease in G3PDH activity by 21 % (p < 0.01) and 24 % (p < 0.01) in control and test adipocytes was observed when compared to control and test adipocytes without hypoxic incubation. However, insignificant difference for G3PDH activity was observed between control adipocytes on hypoxia and test adipocytes from field condition.
Level of G3PDH in adipocytes of M. cephalus during field and in vitro hypoxic (for 1 h) conditions. Values are expressed as mean ± SD (n = 20 fish per site). C control adipocytes, T test adipocytes, CH control adipocytes under hypoxia, TH test adipocytes under hypoxia. ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Oxidant status
Figures 6 and 7 display the OS markers (LPO and NO2 −) and depict increase in the level of LPO and NO2 − (61 and 88 %) (p < 0.001) in test adipocytes compared to control adipocytes. Level of LPO and NO2 − increased significantly by 50 and 93 % (p < 0.001) in control on hypoxic incubation when compared to control adipocytes without hypoxic incubation. A significant increase in the level of LPO and NO2 − by 22 and 23 % (p < 0.01) in test adipocytes on hypoxic incubation was observed when compared to control and test adipocytes without hypoxic incubation. No significant difference in the oxidant status was observed between control adipocytes on hypoxia and test adipocytes from field condition.
Level of LPO in adipocytes of M. cephalus during field and in vitro hypoxic (for 1 h) conditions. Values are expressed as mean ± SD (n = 20 fish per site). C control adipocytes, T test adipocytes, CH control adipocytes under hypoxia, TH test adipocytes under hypoxia. ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Level of NO2 − in adipocytes of M. cephalus during field and in vitro hypoxic (for 1 h) conditions. Values are expressed as mean ± SD (n = 20 fish per site). C control adipocytes, T test adipocytes, CH control adipocytes under hypoxia, TH test adipocytes under hypoxia. ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Antioxidant status
Table 1 represents the antioxidant status (SOD, CAT and TAC). It depicts decrease in the level of SOD, CAT and TAC (61, 51 and 75 %; p < 0.001) in test adipocytes compared to control adipocytes. After hypoxic incubation of control adipocytes, level of SOD, CAT and TAC decreased significantly by 56, 55 and 72 % (p < 0.001) when compared to control adipocytes without hypoxic incubation. When compared to test adipocytes without hypoxic incubation, level of SOD, CAT and TAC decreased significantly by 28, 26 and 27 % (p < 0.01) in test adipocytes on hypoxic incubation. Less significant difference (p < 0.05) in SOD and TAC and no significant (NS) difference in CAT were observed in control adipocytes on 1-h hypoxia when compared to test adipocytes from field hypoxic condition.
Expression of HIF1α
HIF1α is expressed in Fig. 8. It depicts the increased expression of HIF1α (38 %; p < 0.001) in test adipocytes compared to control adipocytes. On hypoxic incubation, HIF1α increased significantly by 22 % (p < 0.01) in control when compared to control adipocytes without hypoxic incubation. When compared to test adipocytes without hypoxic incubation, expression of HIF1α increased significantly by 22 % (p < 0.01) in test adipocytes on hypoxic incubation. Less significant (p < 0.05) difference was observed between control adipocytes on in vitro hypoxia and test adipocytes from field condition.
Level of HIF1α in adipocytes of M. cephalus during field and in vitro hypoxic (for 1 h) conditions. Values are expressed as mean ± SD (n = 20 fish per site). CA control adipocytes, TA test adipocytes, CH control adipocytes under hypoxia, TH test adipocytes under hypoxia. ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Expression of HSP70
Figure 9 presents expression of HSP70. Increased expression of HSP70 (35 %) (p < 0.001) was observed in test adipocytes compared to control adipocytes. Expression of HSP70 increased significantly by 22 % (p < 0.01) in control adipocytes on hypoxic incubation when compared to control adipocytes without hypoxic incubation. However, expression of HSP70 decreased by 14 % (p < 0.05) in test adipocytes on hypoxic incubation when compared to test adipocytes without hypoxic incubation. No significant (NS) difference was observed between control adipocytes on in vitro hypoxia and test adipocytes from field condition.
Level of HSP70 in adipocytes of M. cephalus during field and in vitro hypoxic (for 1 h) conditions. Values are expressed as mean ± SD (n = 20 fish per site). C control adipocytes, T test adipocytes, CH control adipocytes under hypoxia, TH test adipocytes under hypoxia. ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Expression of ASK1
ASK1 expression is given in Fig. 10. It depicts the increased expression of ASK1 (20 %; p < 0.05) in test adipocytes compared to control adipocytes. On hypoxic incubation, ASK1 increased significantly by 13 % (p < 0.05) in control when compared to control adipocytes without hypoxic incubation. Further increase in expression of ASK1 (27 %; p < 0.01) in test adipocytes on hypoxic incubation was observed when compared to test adipocytes without hypoxic incubation. No significant (NS) difference in expression of ASK1 was observed between control adipocytes on in vitro hypoxia and test adipocytes from field condition.
Level of ASK1 in adipocytes of M. cephalus during field and in vitro hypoxic (for 1 h) conditions. Values are expressed as mean ± SD (n = 20 fish per site). CA control adipocytes, TA test adipocytes, CH control adipocytes under hypoxia, TH test adipocytes under hypoxia. ‘Ȼ’ represents comparison with control; ‘ʧ’ represents comparison with test. NS not significant; *p < 0.05; **p < 0.01; ***p < 0.001
Expression of p-JNK1/2
Blotting analysis showed a significant increase in expression of p-JNK1/2 in test adipocytes compared to control adipocytes. When compared to control adipocytes without hypoxic incubation, expression of p-JNK1/2 increased moderately in control on hypoxic incubation, whereas significant increase in the expression of p-JNK1/2 was observed in test adipocytes on hypoxic incubation when compared to test adipocytes without hypoxic incubation. The representative blots for proteins are given in Fig. 11.
Discussion
Oxygen availability is one of the most important environmental factors that influence fish biodiversity, particularly in coastal waters and estuaries. Hence, understanding the impact of oxygen depletion provides insights into the hypoxic consequences of fish populations and whole ecosystems. It is executed in the present work by analyzing DO level, oxidants, antioxidants and signaling molecules responses in the grey mullet adipocytes.
DO was monitored in both Kovalam and Ennore estuary waters, which is an important indicator in determining the water quality criteria of an aquatic ecosystem. The DO, an indicator of trophic status of the water body, is the key regulator that governs the metabolisms of the biological community as a whole. In the present study, DO in the Ennore estuary was decreased when compared to the Kovalam estuary which could be due to the turbidity, inflows from runoffs and decomposition of organic matter (Braide et al. 2004), implying that it is more polluted and hypoxic than Kovalam estuary. Our previous study also demonstrates the hypoxic nature of Ennore estuary and its mediated adaptive response in fish adipocytes by HIF1α elevation during pollution stress (Padmini and Parimala 2015).
Viability of mature adipocytes was analyzed to emphasize the effect of field hypoxia on adipocyte maturation. Low level of oxygen leads to various biochemical responses in the cells that ultimately can result in cell death (Strese et al. 2013). It was substantiated by the presence of decreased viable mature adipocytes in both field and in vitro ypoxic conditions (at 1, 2 and 3 h). However, hypoxia for 2 and 3 h caused extreme detrimental effects on cell viability of both Kovalam and Ennore estuarine fish adipocytes. Hence, hypoxia for 1 h was utilized for the further experimental studies. It is suggesting that hypoxia for 1 h with 1 % oxygen mimics the field hypoxia at Ennore estuary which is proved by comparing cell viability. It reflects that cell viability in control adipocytes on hypoxia was exerting similar status when compared to adipocytes of Ennore estuarine fish. However, in test adipocytes, hypoxia for 1 h caused a detrimental damage to cells which is observed by further decrease in cell viability.
Hypoxia has predominantly detrimental effects on adipocyte metabolism. In the present study, it was well confirmed by exposure of fish adipocytes to hypoxia, which led to a potent reduction in lipogenic mechanism which was observed through diminished TG and increase in the lipolytic pathways with increased glycerol and FA production in control adipocytes on in vitro ypoxic condition, which was similar to adipocytes from field hypoxic condition. It ultimately proves the adverse effect of hypoxia on adipocyte maturation as it involves the TG synthesis. This enhanced lipolysis and impaired lipogenic mechanism may be responsible for decreased mature adipocytes as observed in trypan blue staining. Present study with decreased TG, elevated glycerol and FA proves the pivotal role of oxygen in regulating metabolic functions like lipid metabolism (Famulla et al. 2012; Strosznajder and Domanska-Janik 1980; Wheaton and Chandel 2011). The activity of the lipogenic marker enzyme G3PDH was assessed in the present study which is a key enzyme for triglyceride biosynthesis. It has been used as differentiation marker that reflects the FA synthesis rate and the incorporation of glucose to the cellular lipids in adipocytes (He et al. 2009). Decreased activity of G3PDH was noted, demonstrating its crucial role in TG synthesis. Further decrease in G3PDH along with increase in glycerol and FA was observed in test adipocytes on hypoxia, suggesting that prolonged stress markedly alters the lipid metabolism.
Under low oxygen conditions, an increased formation of ROS may occur which may cause the damage of macromolecules and disruption of cell homeostasis (Murphy 2009; Sies 1996). Similarly, in the present work increased concentration of LPO and NO2 − was observed in both field and in vitro hypoxic adipocytes. Enhancement of FA oxidation in adipocytes has also been proposed to reduce adiposity in mice (Elheiga et al. 2001) which is concomitant to the present work where elevated OS marker with decreased TG was observed, ultimately depicting the lipolytic mechanism. However, the cellular homeostasis is protected by a defence system that consists of antioxidant compounds as well as antioxidant enzymes. In our research work, the levels of the antioxidant enzymes (Table 1) such as SOD, CAT and TAC were monitored and they were found to be essentially affected by hypoxia, depicting the extreme utilization of antioxidants as adaptive mechanism (Merino et al. 2014). Drastic elevation in the level of LPO and NO2 − along with decrease in SOD, CAT and TAC was observed in test adipocytes on in vitro ypoxia, depicting the loss of cellular homeostasis.
HIF1α activity is influenced in adipose tissue by hypoxia (He et al. 2011). The reprogramming of cellular metabolism by HIF1α involves a switch toward altered glycolysis and TG synthesis (Krishnan et al. 2009; Wellen and Thompson 2010), implicating a central role for HIF1α in the change of metabolic strategy following reduction in oxygen concentration. As a major hypoxia-responsive gene, the protein concentration of HIF1α was elevated in both field and in vitro ypoxic adipocytes. The results coincide with the observation of Zhang et al. (2010) which postulated that hypoxia in mice instigates adipocytes damage through activation of HIF1α. Increase in the expression of HIF1α was observed in test adipocytes under in vitro ypoxic condition, suggesting the promising role of HIF1α as an oxygen sensor.
Welker et al. (2013) emphasized that under hypoxic conditions, aquatic animals adapt different strategies to cope with ROS. Upregulation of HSPs is known to occur as part of the cellular defense system against environmental stresses (Subjeck and Shyy 1986; Gehrmann et al. 2002). Huang et al. (2009) showed that HSP70 expression levels increased during hypoxia, and HIF1α upregulation preceded HSP70 induction. Similarly, in the present study, elevated HSP70 in both field and in vitro hypoxic control adipocytes was monitored, substantiating the provision of cytoprotection during polluted environment by HSP70. Thereby it confirms the cytoprotective role of HSP70 in fish by interfering with the apoptotic program by inhibiting the JNK signaling cascade and also well established as a negative regulator of apoptosis signal-regulating kinase-1 (Gabai et al. 1998; Lee et al. 2005; Padmini and Vijaya Geetha 2009; Padmini and Tharani 2014), which is concomitant to the present study. However, in in vitro hypoxic test adipocytes, HSP70 level was decreased. It may be due to the occurrence of severe stress which ultimately ends signals that inhibit HSP70 and favors cell death (Gorman et al. 1999). Lee et al. (2005) demonstrated that HSP70 deficiency leads to the activation of JNK and caspase-3 in the apoptotic pathway. Similar results were achieved in the present study where increased expression of ASK1 and p-JNK1/2 was observed with decreased HSP70. Hence, the present study suggests that HSP70 expression has a key role in regulating apoptosis, and it plays a vital part in cell survival under various apoptotic stress conditions.
Apoptosis signal-regulating kinase-1 (ASK1) has been reported to regulate cell fate in many injurious conditions and disease models. ASK1 is a MAP3K preferentially activated by oxidative stress and inflammation which activates the p38 MAPK and the c-Jun N-terminal-activating kinase pathways and eventually triggers cell death (Song et al. 2013). HIF1α plays an important role in the survival of cells in a low-oxygen environment by suppressing a hypoxia-induced ASK1/MKK4/JNK signaling cascade that promotes an apoptotic response (Zhou et al. 2004). Coherently we observed elevated HIF1α, ASK1 and p-JNK1/2 in both field and in vitro ypoxic adipocytes. However, ASK1 and p-JNK1/2 levels were significantly increased in in vitro hypoxic adipocytes when compared to those of field hypoxia. Hence, the present study depicts that during field hypoxia, HIF1α promotes protective role by suppressing the sustained activation of ASK1 and p-JNK1/2, whereas during prolonged hypoxia in test adipocytes, expression of ASK1 was overwhelmed, which ultimately leads to cell death (Fig. 1).
Results of the present work (Fig. 12) suggest that in vitro hypoxia for 1 h closely mimics the field hypoxic consequences. It is depicted by results achieved in control subjected to hypoxia which is close to field hypoxia. We hypothesized that HSP70 upregulation in response to hypoxia may facilitate adaptation to cellular stress imposed by field hypoxia, which is predicted by control adipocytes on 1-h hypoxia. However, in test adipocytes on in vitro hypoxia, level of HSP70 was decreased, suggesting the existence of toxicity which ultimately leads to apoptosis. Here we outline that cellular decision to undergo apoptosis/survival is determined by the integration of multiple signals. It is depicted by coordinated expression of HIF1α and HSP70 in rendering cytoprotective mechanism by suppressing ASK1 and p-JNK1/2 during hypoxia-induced stress conditions. In conclusion, our study proclaims that expression of HSP70 is not precisely regulated beyond the threshold level and its expression may be one of the key factors to maintain cellular homeostasis during hypoxia.
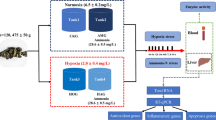
Schematic representation of expression of signaling molecules in response to field hypoxia and in vitro hypoxia. Schematic representation depicting the status of adipocytes during normal, field hypoxia and in vitro hypoxia. In normal condition, adipocytes store fat as TG and exhibited balanced oxidants and antioxidants, which ultimately results in cell homeostasis. Adipocytes of field hypoxic condition showed elevated lipolysis and superoxide generation due to membrane damage. Formation of FA in turn promotes the lifting of ROS/RNS and increased antioxidant utilization, which evokes the oxidant to be upgraded and antioxidants to be dropped off. Oxidant and antioxidant disequilibrium drives the cells to further damage by increasing the apoptotic proteins ASK1 and p-JNK1/2. However, adipocytes are surviving due to enhanced HIF1α, which induces HSP70 and thereby renders cytoprotection. Similar mechanism was observed in the control fish adipocytes incubated in hypoxic condition. When polluted fish adipocytes were incubated in in vitro hypoxic condition, oxidants were increased exacerbatingly with terrible loss of antioxidants. It further limits the cytoprotective mechanism by inhibiting HSP70 expression and activating apoptotic proteins ASK1 and p-JNK1/2. Thus, it explains the cytoprotective function of HSP70
References
Adams SM (2005) Assessing cause and effect of multiple stressors on marine systems. Mar Pollut Bull 51:649–657
Atsushi M, Hideki N, Kei T, Kohsuke T, Hidenori I (2002) Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid Redox Signal 4(3):415–425
Basu N, Todgham AE, Ackerman PA, Bibeau MR, Nakano K, Schulte PM, Iwama GK (2002) Heat shock protein genes and their functional significance in fish. Gene 295:173–183
Beer RF, Seizer TW (1952) Spectrophotometric method for measuring breakdown of hydrogen peroxide by catalase. J Biol Chem 115:130–140
Biamonti G, Caceres JF (2009) Cellular stress and RNA splicing. Trends Biochem Sci 34:146–153
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brahimi-Horn MC, Pouyssegur J (2007) Oxygen, a source of life and stress. FEBS Lett 581:3582–3591
Braide SA, Izonfuo WAL, Adiukwu PU, Chindah AC, Obunwo CC (2004) Water quality of Miniweja stream, swamp forest stream receiving non-point source waste discharges in Eastern Niger Delta. Niger Sci Afr 3:1–8
Bruick RK, McKnight SL (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294:1337–1340
Cuevas BD, Abell AN, Johnson GL (2007) Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene 26(22):3159–3171
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103(2):239–252
Elheiga AL, Matzuk MM, Abo-Hashema KA, Wakil SJ (2001) Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase. Science 291:2613–2616
Falholt K, Lund B, Falholt W (1973) An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clin Chim Acta 46:105–111
Famulla S, Schlich R, Sell H, Eckel J (2012) Differentiation of human adipocytes at physiological oxygen levels results in increased adiponectin secretion and isoproterenol-stimulated lipolysis. Adipocyte 1(3):132–181
Gabai VL, Meriin AB, Yaglom JA, Volloch VJ, Sherman MYE (1998) Role of Hsp70 in regulation of stress-kinase JNK: implications in apoptosis and aging. FEBS Lett 438(1–2):1–4
Gehrmann M, Pfister K, Hutzler P, Gastpar R, Margulis B, Multhoff G (2002) Effects of antineoplastic agents on cytoplasmic and membrane-bound heat shock protein 70 (Hsp70) levels. Biol Chem 383:1715–1725
Gorman AM, Heavey B, Creagh E, Cotter TG, Samali A (1999) Antioxidant-mediated inhibition of the heat shock response leads to apoptosis. FEBS Lett 445(1):98–102
Greijer AE, van der Wall E (2004) The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol 57:1009–1014
He ML, Yang WZ, Youv JS, Chaves AV, Mir PS, Benchaar C, McAllister TA (2009) Effect of garlic oil on fatty acid accumulation and glycerol-3-phosphate dehydrogenase activity in differentiating adipocytes. Asian-Aust J Anim Sci 22(12):1686–1692
He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J (2011) Regulation of HIF-1α activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. Am J Physiol Endocrinol Metab 300(5):877–885
Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56:901–911
Huang L, Mivechi NF, Moskophidis D (2001) Insights into regulation and function of the major stress-induced hsp70 molecular chaperone in vivo: analysis of mice with targeted gene disruption of the hsp70.1 or hsp70.3 Gene. Mol Cell Biol 21(24):8575–8591
Huang WJ, Xia LM, Zhu F, Huang B, Zhou C, Zhu HF, Wang B, Chen B, Lei P, Shen GX, Tian D (2009) Transcriptional upregulation of HSP70-2 by HIF-1 in cancer cells in response to hypoxia. Int J Cancer 124:298–305
Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 92:464–468
Jang HJ, Kwak JH, Cho EY, We YM, Lee YH, Kim SC, Han DJ (2008) Glutamine induces heat-shock protein-70 and glutathione expression and attenuates ischemic damage in rat islets. Transpl Proc 40:2581–2584
Kallio PJ, Wilson WJ, O’Brien S, Makino Y, Poellinger L (1999) Regulation of the hypoxia-inducible transcription factor 1alpha by the ubiquitin- proteasome pathway. J Biol Chem 274:6519–6525
Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Schlattner MT, Aasum E, Bogdanova A, Perriard E (2009) Activation of a HIF1a-PPARg axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell Metab 9:512–524
Krishnan J, Danzer C, Simka T, Ukropec J, Walter MK, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T, Krek W (2012) Dietary obesity-associated Hif1a activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD + system. Genes Dev 26:259–270
Lee JS, Lee JJ, Seo JJS (2005) Mechanisms of signal transduction: HSP70 deficiency results in activation of c-Jun N-terminal kinase, extracellular signal-regulated kinase, and caspase-3 in hyperosmolarity-induced apoptosis. Biol Chem 280:6634–6641
Macia L, Viltart O, Verwaerde C, Delacre M, Delanoye A, Grangette C, Wolowczuk I (2006) Genes involved in obesity; adipocytes, brain and microflora. Genes Nutr 1:189–212
Mariana C, Cecilia A, Cecilia VR (2008) Differences in lipogenesis and lipolysis in obese and non-obese adult human adipocytes. Biol Res 41:197–204
Merino JJ, Roncero C, Gasque MJO, Naddaf Ahmad, González MP (2014) Antioxidant and protective mechanisms against hypoxia and hypoglycaemia in cortical neurons in vitro. Int J Mol Sci 15(2):2475–2493
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biochem 247:3170–3175
Moustaid N, Jones BH, Taylor JW (1996) Insulin increases lipogenic enzyme activity in human adipocytes in primary culture. J Nutr 126:865–870
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Ohkawa H, Ohishi M, Yagi K (1979) Assay for lipid peroxides in animal tissue with thiobarbituric acid reaction. Ann Biochem 95:351–354
Padmini E, Lavanya D (2014) Differential expression of heat shock proteins and inflammatory changes in preeclamptic placental explants. Int J Sci Res 3(4):858–864
Padmini E, Parimala P (2014) Cytoprotective role of hemeoxygenase-1 in polluted estuarine Mugil cephalus. IAJLB 2(1):32–46
Padmini E, Parimala P (2015) Role of HSP70 and the associated signaling molecules in fish adipocytes during pollutants induced hypoxia. IOSR J Environ Sci Toxicol Food Technol 9(7):16–26
Padmini E, Tharani J (2014) Heat- shock protein 70 modulates apoptosis signal-regulating kinase 1 in stressed hepatocytes of Mugil cephalus. Fish Physiol Biochem 40(5):1573–1585
Padmini E, Vijaya Geetha B (2007a) A comparative seasonal pollution assessment study on estuary with respect to metal accumulation in Mugil cephalus. Oceanol Hydrobiol Stud 35:1–13
Padmini E, Vijaya Geetha B (2007b) Seasonal influences on water quality parameters and pollution status of the Ennore estuary, Tamilnadu. Ind J Environ Hydrol 15:1–9
Padmini E, Vijaya Geetha B (2009) Modulation of ASK1 expression during overexpression of Trx and HSP70 in stressed fish liver mitochondria. Cell Stress Chaperon 14(5):459–467
Pond CM (1998) The fats of life. Cambridge Univ. Press, Cambridge
Prieto P, Pineda M, Aguilar M (1999) Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenum complex, specific application to the determination of vitamin E. Ann Biochem 26(9):337–341
Richter K, Haslbeck M, Buchner J (2010) The heat shock response: life on the verge of death. Mol Cell 40:253–266
Rocha S (2007) Gene regulation under low oxygen: holding your breath for transcription. Trends Biochem Sci 32:389–397
Rodbell M (1964) Metabolism of isolated adipocytes. I. Effects of hormones on glucose metabolism and lipolysis. J Biol Chem 239:375–380
Rosen ED, Spiegelman BM (2006) Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444(7121):847–853
Rumberger JM, Wu T, Hering MA, Marshall S (2003) Role of hexosamine biosynthesis in glucose-mediated up-regulation of lipogenic enzyme mRNA levels: effects of glucose, glutamine, and glucosamine on glycerophosphate dehydrogenase, fatty acid synthase, and acetyl-CoA carboxylase mRNA levels. J Biol Chem 278:28547–28552
Sies H (1996) Oxidative stress: oxidants and antioxidants. J Physiol 491:S2
Song J, Cho KJ, Cheon SY, Kim SH, Park KA, Lee WT, Lee JE (2013) Apoptosis signal-regulating kinase 1 (ASK1) is linked to neural stem cell differentiation after ischemic brain injury. Exp Mol Med 45:69
Sottile V, Seuwen K (2001) A high-capacity screen for adipogenic differentiation. Anal Biochem 293:124–128
Storey KB (1996) Oxidative stress: animal adaptations in nature. Braz J Med Biol Res 29:1715–1733
Strese S, Fryknas M, Larsson R, Gullbo J (2013) Effects of hypoxia on human cancer cell line chemosensitivity. BMC Cancer 13:331
Strober W (2001) Trypan blue exclusion test of cell viability. Curr Protoc Immunol. May, Appendix 3, Appendix 3B 18432654 (P, S, E, B, D)
Strosznajder J, Domanska-Janik K (1980) Effect of anoxia and hypoxia on brain lipid metabolism. Neurochem Res 5:583–589
Subjeck JR, Shyy TT (1986) Stress protein systems of mammalian cells. Am J Physiol 250:1–17
Sun K, Halberg N, Khan M, Magalang UJ, Scherera PE (2013) Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol Cell Biol 33(5):904–917
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci 76(9):4350–4354
Trayhurn P (2005) Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand 184:285–293
Volodymyr I, Ludmyla P, Alice AM, Lima MH (2001) Oxidative stress and antioxidant defenses in goldfish Carassius auratus during anoxia and reoxygenation. Am J Physiol Regul Integr Comp Physiol 280:100–107
Welker AF, Moreira DC, Campos EG, Hermes-Lima M (2013) Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp Biochem Physiol A: Mol Integr Physiol 165:384–404
Wellen KE, Thompson CB (2010) Cellular metabolic stress: considering how cells respond to nutrient excess. Mol Cell 40:323–332
Wheaton WW, Chandel NS (2011) Hypoxia- hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol 300:385–393
Yokoi I, Habu H, Kabuto H, Mori A (1996) Analysis of nitrite, nitrate, and nitric oxide synthase activity in brain tissue by automated flow injection technique methods. Enzymol 268:152–159
Zhang X, Lam KSL, Ye H, Chung SK, Zhou M, Wang Y, Xu A (2010) Adipose tissue-specific inhibition of hypoxia-inducible factor 1 α induces obesity and glucose intolerance by impeding energy expenditure in mice. J Biol Chem 285:32869–32877
Zhou G, Golden T, Aragon V, Honkanen RE (2004) Ser/Thr protein phosphatase 5 inactivates hypoxia-induced activation of an apoptosis signal-regulating kinase 1/MKK-4/JNK signaling cascade. J Biol Chem 279(45):46595–46605
Acknowledgments
The project funded by Department of Science and Technology, New Delhi, India, is acknowledged, Project Referral Number—DST: SB/SO/AS-046/2013.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Rights and permissions
About this article
Cite this article
Ekambaram, P., Parasuraman, P. & Jayachandran, T. Differential regulation of pro- and antiapoptotic proteins in fish adipocytes during hypoxic conditions. Fish Physiol Biochem 42, 919–934 (2016). https://doi.org/10.1007/s10695-015-0185-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0185-z