Abstract
Dipeptidyl peptidase-4 (DPP4) is a serine protease of great interest because it has been shown to modulate the activity of several peptidergic factors including peptide YY (PYY) and glucagon-like peptide-1/2. While PYY(1–36) is orexigenic in mammals, PYY(3–36) recently garnered interest as a potent anorexigen. In silico phylogenetic analysis found that the DPP4 cleavage sites are absent in fish PYY sequences. However, no studies were conducted to show that indeed PYY(3–36) is not produced by DPP4 in fish. If DPP4 does not cleave PYY(1–36), is PYY(3–36) an anorexigen in fish? The objectives of this research were to (1) test whether DPP4 cleaves goldfish PYY(1–36) and (2) determine whether PYY(3–36) is an anorexigen in goldfish. First, we identified the highly conserved catalytic region of DPP4 in goldfish. Abundant expression of DPP4 mRNA was found within the gastrointestinal tract. We also report the first MALDI-MS cleavage analysis of DPP4 effects on PYY(1–36) in a non-mammalian vertebrate. Our novel results indicate that DPP4 is unable to cleave goldfish PYY(1–36) to PYY(3–36) in vitro. It also confirms a previously held hypothesis that DPP4 is unable to cleave fish PYY(1–36) that contains N-terminal proline–proline residues. PYY(3–36) had no effects on food intake of goldfish. The appetite inhibitory effects of intraperitoneal and intracerebroventricular injections of 10 ng/g body weight gfPYY(1–36) were abolished by coinjections of BIBP3226, a Y1 receptor antagonist. These results are significant because it shows the lack of generation of endogenous PYY(3–36) and its anorectic effects in goldfish.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In vertebrates, food intake triggers the secretion of many peptidergic endocrine factors that facilitate tissue-specific responses required to induce satiety and for the efficient replenishment of energy stores. In addition, hypothalamic feeding centers are sensitive to peripheral hormonal and chemical changes that allow for immediate and precise homeostatic regulation. In recent years, a number of peptides homologous to mammalian appetite regulatory peptides have been identified and sequenced in fish (Volkoff et al. 2005). Peptide YY (PYY), a 36 amino acid peptide, is a meal-responsive hormone primarily produced from the gastrointestinal tract of mammals (Batterham et al. 2002) and fishes (Gonzalez and Unniappan 2010). Interestingly, the anorexigenic effects of PYY in mammals are not attributed to the full-length peptide but from its cleaved form, PYY(3–36), which is released postprandially from the gastrointestinal tract (Adrian et al. 1985; Batterham et al. 2002; Eberlein et al. 1989). In mammals, the serine protease, dipeptidyl peptidase-4 (DPP4), preferentially cleaves peptides with a proline and alanine residue within the second N-terminal position, including PYY(1–36), glucagon-like peptide-1 (GLP-1), glucagon-like peptide-2 (GLP-2), gastric inhibitory polypeptide (GIP) and growth hormone-releasing hormone (GHRH) (Bongers et al. 1992; Drucker et al. 1997; Grandt et al. 1994; Mentlein et al. 1993). In humans and rats, DPP4 is conveniently located in close proximity to circulating hormones because of a ubiquitous distribution as a membrane-spanning cell-surface aminopeptidase. DPP4 is abundantly expressed within the epithelial lining of the intestine, lung, liver and kidney (Fukasawa et al. 1981; Kenny et al. 1976; McCaughan et al. 1990; Mentzel et al. 1996). In addition, DPP4 is distributed in the endothelial cells of blood vessels and is present as a soluble enzyme in blood plasma (Mentlein 1999).
Several studies have shown that central administration of PYY(1–36) in rats leads to excessive overeating and this appears to be mediated by the Y1 mammalian receptor subtype (Hagan and Moss 1995; Kanatani et al. 2000; Morley et al. 1985). In addition, the coadministration of BIBP 3226, a non-peptide highly selective Y1 receptor antagonist, has been shown to effectively block the stimulation of the Y1 receptor by PYY(1–36) in mammals (Rudolf et al. 1994). Meanwhile, peripheral administration of PYY(1–36) reduces feeding in both rats and goldfish (Chelikani et al. 2004; Gonzalez and Unniappan 2010). This effect is thought to be mediated by the DPP4-mediated generation of PYY(3–36) (Chelikani et al. 2004; Eberlein et al. 1989; Unniappan et al. 2006). Unlike PYY(1–36), both central and peripheral administrations of PYY(3–36) cause a reduction in the food intake of rodents (Batterham et al. 2002; Chelikani et al. 2004, 2005, 2007), monkeys (Moran et al. 2005) and humans (Batterham et al. 2003; Degen et al. 2005; Sloth et al. 2007). However, several research groups were unable to find any effect for PYY(3–36) in regulating feeding in rodents (Tschop et al. 2004) and this lack of effect was possibly due to stress in animals (Abbott et al. 2006). These results indicate that exogenous administration of PYY(1–36) and PYY(3–36) have appetite regulatory effects in mammals that are dependent on several factors including the form of PYY used, mode of administration, dose of peptide provided and whether the animals were habituated to the experimental procedures (Unniappan et al. 2006) or not.
Currently available genome sequences suggest that PYY is present in several species of cartilaginous and bony fish. In addition, several studies have reported abundant expression of PYY in both the central and peripheral nervous system (Cerda-Reverter et al. 2000a, b; Kurokawa and Suzuki 2002; Söderberg et al. 1994, 2000; Sundstrom et al. 2008). Recently, we reported for the first time the peripheral and central injections of PYY(1–36) inhibit food intake in goldfish (Carassius auratus), a non-mammalian vertebrate. However, questions remain about the role of DPP4 in the regulation of PYY-induced reduction in food intake. Unlike in mammals, DPP4-mediated truncation of PYY(1–36) is thought to be significantly attenuated in all teleosts. This is due to a highly conserved N-terminal proline–proline bond instead of the DPP4 target proline–alanine, which would suggest that the ancestral teleost fish could not form PYY(3–36) (Fredriksson et al. 2006). However, whether DPP4 indeed cleaves PYY(1–36) to PYY(3–36) in fish or whether PYY(3–36) has appetite regulatory effects in fish remains unknown.
Is DPP4 able to cleave PYY(1–36) from PYY(3–36) in goldfish? Does central injection of PYY(3–36) have an anorectic role in fish? While abundant speculative answers for these questions solely based on in silico analyses are available, physiological and biochemical results supporting such notions are still lacking. The objectives of this study were to identify the catalytic region of DPP4 from goldfish, determine its tissue distribution, investigate the actions of DPP4 on native PYY(1–36) and to elucidate the effects of exogenous administration of synthetic native PYY(3–36) in regulating feeding of goldfish, a well-characterized model of neuroendocrine physiology. This is the first study to characterize the effects of DPP4 on PYY and the role DPP4 has on the appetite regulatory effects of PYY in a non-mammalian vertebrate. Our novel results indicate strong expression of DPP4 mRNA within the gastrointestinal tract and for the first time show that DPP4 is unable to cleave goldfish PYY(1–36) to PYY(3–36).
Materials and methods
Animals
Both male and female goldfish (C. auratus; common variety), of 20–30 g body weight and ~10 cm in length, were purchased from local suppliers (Ryan’s aquaculture supplies, Toronto, Ontario) and maintained at 20 °C water temperature under a 10-h light and 14-h dark simulated natural short-day photoperiod. Fish were fed a commercial pellet diet (Martin Profishent, Ontario, Canada) once a day at 12:00 p.m. to satiety. Prior to all feeding studies, fish underwent a 2-week acclimation period when food intake was monitored daily. Fish were anesthetized in 0.15 % tricaine methanesulfonate (TMS) (Syndel Laboratories, Vancouver, Canada) prior to intracerebroventricular (ICV), intraperitoneal (IP) injections and dissection of tissues for total RNA extraction. All experimental protocols using fish were approved by the York University/University of Saskatchewan Animal Care Committee, and strictly adhered to the policies of the Canadian Council for Animal Care.
Materials
Goldfish (gf) PYY(1–36) (YPPKPENPGDDAPPEELAKYYTALRHYINLITRQRY-NH2) and gfPYY(3–36) (PKPENPGDDAPPEELAKYYTALRHYINLITRQRY-NH2) were custom-synthesized by Genscript (Piscataway, USA). Purity was confirmed by using electrospray ionization mass spectrometry (ESI–MS) and MALDI-TOF. PYY(1–36) and PYY(3–36) were synthesized with 95 and 98 % purity, respectively, with a single peak confirming the predicted mass. Rat (r) PYY(1–36) and rPYY(3–36) were purchased from Phoenix Pharmaceuticals (Burlingame, USA). BIBP 3226 (Tocris Bioscience, Ellisville, USA), the mammalian Y1 receptor antagonist, was used for feeding studies. Peptides and antagonist were always freshly reconstituted in fish physiological saline (Burnstock 1958).
Determination of DPP4 mRNA sequence encoding the catalytic region
Total RNA was extracted, purified and quantified from goldfish gastrointestinal tract as previously described (Gonzalez and Unniappan 2010). Briefly, total RNA was extracted from goldfish gut using the TRIzol® RNA isolation reagent (Invitrogen, Canada). A Multiskan® Spectrum spectrophotometer (Thermo, Vantaa, Finland) was used to check samples for RNA purity. Only samples with an absorbance ratio >1.7 were used for subsequent cDNA synthesis using the iScriptTM cDNA synthesis kit (BioRad, Canada). The cDNAs were used as templates for reverse transcription PCR (RT-PCR) with two primers based on the zebrafish DPP4 mRNA sequence available on Genbank (NM_001161337.1): DPP4-FA and DPP4-RE (Table 1). The subsequent amplification of goldfish DPP4 cDNA was gel-extracted and purified using a QIAquick Spin kit (QIAGEN, Canada). The template products were validated by DNA sequencing performed with the Applied Biosystems DNA Sequencer (3130xL) using the BigDye® Terminator chemistry and showed the highest similarity to zebrafish DPP4 in a BLAST search.
Identification and phylogenetic analysis of non-mammalian DPP4 sequences
The catalytic region of DPP4 is located in the C-terminal domain of the aminopeptidase (amino acids 511–766). Sequence information on the catalytic DPP4 region for zebrafish (Danio rerio), green pufferfish (Tetraodon nigroviridis), fugu (Takifugu rubripes), medaka (Oryzias latipes), stickleback (Gasterosteus aculeatus), Western clawed frog (Xenopus tropicalis), chicken (Gallus gallus), mouse (Mus musculus), pig, (Sus scrofa) and human (Homo sapiens) were obtained or compiled from the annotated sequences available through the GenBank (http://www.ncbi.nlm.nih.gov/genbank/) and Ensembl genome databases. In addition, two paralogous DPP4 genes, DPP4-1 and DPP4-2, exist due to the teleost-specific whole-genome duplication (Zhou and Irwin 2004). We used zebrafish DPP4-1 and DPP4-2 protein sequence as in silico probes and determined the sequences, which most highly match these two paralogous genes. DPP4 protein sequences were aligned using ClustalW2 general purpose multiple sequence alignment program for DNA or protein (Dereeper et al. 2008; Thompson et al. 1994).
Tissue distribution of DPP4 in goldfish
The tissue-specific expression of DPP4 mRNA in goldfish was determined by the dissection and RNA processing of the following tissues: hindbrain (including vagal lobe and brain stem), midbrain (optic tectum and cerebellum), hypothalamus, telencephalon, olfactory bulbs, pituitary, anterior intestine (J-loop), midgut, posterior intestine (rectum), kidney, spleen, liver, heart, eye, muscle, gill, gall bladder, adipose, ovary, testes and skin. Total RNA from tissues was extracted and cDNAs were synthesized as described previously. Quantification of relative DPP4 gene expression was conducted using species-specific primers for DPP4, DPP4-QRTF1 and DPP4-QRTR1 (Table 1). The gene-specific primers amplified a 160 bp region within the catalytic encoding region. Moreover, gene amplification specificity was determined by (1) the amplicon size from real-time quantitative reverse transcription PCR (qRT-PCR) was confirmed by gel electrophoresis and (2) direct DNA sequencing of amplified products. No double band was observed suggesting a paralogous DPP4 gene was not amplified. Species-specific β-actin primers served as internal controls to normalize cDNA quantity for each tissue sample. Quantification, using qRT-PCR, of DPP4 and β-actin mRNA was performed in triplicate on all samples as previously described (Gonzalez and Unniappan 2010). Briefly, qRT-PCR, of DPP4 and β-actin mRNA, were performed using the iQ™ SYBR® Green Supermix on a Chromo4™ Multicolor Real-Time PCR Detection System (Bio-Rad, Canada). Thermal cycling was conducted as follows: 3 min at 95 °C, then 40 cycles were performed with 95 °C denaturation step for 30 s, an annealing temperature of 60 °C for 30 s and an extension temperature of 73 °C for 30 s. Efficiency of reactions were confirmed using, six twofold dilution standards for the calculation of amplification efficiency for each qRT-PCR assay. The efficiency (E) of the PCR assay was determined from the regression slope of the assay (E = 10 – 1/slope). Relative expression data were obtained after normalization of DPP4 mRNA expression in each sample using β-actin expression from the same sample. Relative quantification was determined using the Livak method as per the manufacturer’s (Bio-Rad) instructions. Purity of each amplicon was confirmed by a melting curve, which consisted of a slow ramp from 55 to 95 °C with SYBR Green readings taken every 1 °C for 40 cycles.
In vitro effects of DPP4 on gfPYY(1–36)
The effect of purified porcine DPP4 (Sigma-Aldrich, Canada) on gfPYY(1–36) was tested with MALDI-TOF mass spectrometry. Parallel studies using rPYY(1–36) were also conducted to serve as a positive control for both the incubation time and ratio of enzyme to sample. Samples (20 µL) were incubated at 37 °C for 180 min with 0.1 M Tris buffer (pH 7.6) containing peptide alone, or peptide plus DPP4 in a 1:1 ratio. Samples were collected after 180 min, and the reaction was stopped with equal volumes of 0.1 % trifluoroacetic acid (TFA). Subsequently, mass spectrometric analyses were conducted at the York University Proteomics Facility, Centre for Research in Mass Spectrometry using a Q-STAR XL with an o-MALDI source (MDS SCIEX/Applied Biosystems Inc.).
Effects of IP injections of PYY(3–36) and PYY(1–36) on food intake of goldfish
To conduct an examination on the effects of peripheral administration of gfPYY(3–36) on food intake in goldfish, eight groups of six (n = 1 fish/tank), weight-matched goldfish (BW 34.30 ± 5.31 g) were housed in ~60 L aquariums. We chose 0, 10, 100 and 1000 ng/g BW of gfPYY(3–36) and gfPYY(1–36) based on our previously reported food intake-reducing effects of gfPYY(1–36) (Gonzalez and Unniappan 2010). IP injections studies were conducted as previously described (Gonzalez and Unniappan 2010). Briefly, goldfish were acclimated to tanks for several weeks prior to the study to reduce the effects of stress. The fish were fed at a scheduled feeding time (12:00 h) to satiation once a day with 4 % BW ration per fish. Quantification of food intake was determined daily 1 h post-feeding by removing, drying, and measuring the dry weight of uneaten food pellets. On the day of the study, goldfish were anesthetized and injected with either 100 µL saline alone or peptide/drug using a 1-mL syringe and 25(5/8) G needle (BD, Oakville, Canada). Subsequently, fish were returned to aquariums and fed at 5 min post-injection, at which time all fish were active and swimming. In all remaining studies, the same acclimation and feeding protocols were used.
Effects of ICV injections of PYY(3–36) on food intake of goldfish
To determine whether direct ICV injections of gfPYY(3–36) into the brain would regulate food intake, three groups of six, weight-matched goldfish (BW of 30.00 ± 6.79 g) were used for central injections. Doses of saline (control), 0, 5 and 50 ng/g of gfPYY(3–36) were used based on our previously reported food intake-reducing effects of gfPYY(1–36) ICV injections (Gonzalez and Unniappan 2010). ICV injections were conducted as previously described (Peter and Gill 1975). Briefly, under anesthesia a dorsal flap was cutout from the goldfish frontal bones with the aid of a dentist drill equipped with a circular saw. A 2 µL solution (hormone or vehicle) was stereotaxically microinjected into the posterior preoptic region of the brain using a 10-µL Hamilton microsyringe (Hamilton Company, Reno, NV, USA). Following injection, suturing and recovery, fish were given a 6 % BW ration of food. Fish were allowed to feed for 1 h, and unfed pellets were recovered, dried and measured as described above. Delivery of the material to the brain using the stereotaxic apparatus was verified using injections of India ink.
Antagonistic effects of IP injections of PYY(1–36) and BIBP 3226
In order to identify the Y receptor that mediates the effects of PYY on food intake, BIBP 3226, a Y1 mammalian receptor antagonist, was coinjected with gfPYY(1–36). Three groups of six, weight-matched goldfish (BW 59.2 ± 15.8 g) were used for IP injections. Fish were coinjected with both BIBP 3226 at doses of 500, 50, 5 or 0 ng/g and gfYY(1–36) at 10 ng/g, and food intake was quantified. In addition, we wanted to determine whether injections of BIBP 3226 alone could alter food intake. For this study, four groups of six, weight-matched goldfish (BW 43.9 ± 9.2 g) were used for IP injections of BIBP 3226 alone at doses of 500, 50, 5 and 0 ng/g.
Antagonistic effects of ICV injections of PYY(1–36) and BIBP 3226
This experiment was conducted to test whether the coadministration BIBP 3226 with PYY(1–36) can influence the effects of PYY(1–36) on food intake when applied centrally. Fish were injected with saline alone or PYY(1–36) at 10 ng/g BW, or with both BIBP 3226 and gfPYY(1–36) at 10 ng/g BW. Four groups of six, weight-matched goldfish (BW of 27.70 ± 3.81 g) were used for ICV injections.
Results
Identification of teleost DPP4 genes
The catalytic region (amino acids 511–766) of DPP4 consists of a catalytic triad, which is made up of an active site Ser630, located in the sequence Gly-Trp-Ser-Tyr-Gly, and the two other residues, Asp708 and His 740 (Fig. 1). The catalytic region of DPP4, DPP4-1 and DPP4-2 is highly conserved among the teleosts and other vertebrates examined. The sequence encoding the catalytic region was purified and amplified from goldfish intestinal tissue extracts (GenBank accession number: JF760209, Supplemental Figure 1). The goldfish DPP4 sequence obtained contains the catalytic region of DPP4, which is responsible for its aminopeptidase activity. Moreover, the goldfish DPP4 gene conforms more closely with the DPP4-1 gene identified in other teleost species. The partial goldfish DPP4 mRNA sequence displays a highly conserved catalytic region with a high percent similarity to the homologous DPP4-1 regions found in other species including zebrafish (D. rerio; 96 %), medaka (O. latipes; 84 %), stickleback (G. aculeatus; 84 %), fugu (T. rubripes; 81 %), green pufferfish (T. nigroviridis; 81 %), Western clawed frog (X. tropicalis; 76 %), chicken (G. gallus; 76 %), pig (S. scrofa; 78 %), mouse (M. musculus; 77 %) and human (H. sapiens; 77 %) (Fig. 1). A phylogenetic analysis (Fig. 2) also found clustering of fish DPP4 sequences that displayed very high sequence similarity.
Goldfish DPP4 sequence alignment with tetrapods and teleost species. The amino acid sequence alignment of the catalytic region of DPP4 (tetrapods), DPP4-1 and DPP4-2 (teleost). The alignment of DPP4 from bony fish, amphibians, birds and mammals. The name of the species is given on the left side of the alignment and the number of amino acids is provided. The colored amino acids highlight the differences in conservation of the amino acids between species within the catalytic region of DPP4. In addition, the amino acids which form the catalytic triad are indicated by the yellow boxes. Species names used in the alignment were as follows: goldfish (C. auratus), zebrafish (D. rerio), medaka (O. latipes), stickleback (G. aculeatus), fugu (T. rubripes), green pufferfish (T. nigroviridis), Western clawed frog (X. tropicalis), chicken (G. gallus), pig (S. scrofa), mouse (M. musculus) and human (H. sapiens). Sequences obtained from GenBank and Ensembl databases were aligned using ClustalW. GenBank accession numbers of the sequences were as follows; C. auratus (DPP4-1, JF760209) D. rerio (DPP4-1, NP_001154809.1; DPP4-2, ENSDARP00000118769), O. latipes (DPP4-1, ENSORLP00000003424; DPP4-2, ENSORLP00000021120), G. aculeatus (DPP4-1, ENSGACP00000018353; DPP4-2, ENSGACP00000007372), T. rubripes (DPP4-1, ENSTRUP00000022072; DPP4-2, ENSTRUP00000003859), T. nigroviridis (DPP4-1, ENSTNIP00000016265; DPP4-2, CAG09338.1), X. tropicalis, (DPP4, AAI27359.1), G. gallus (DPP4, NP_001026426.1), S. scrofa (DPP4, NP_999422.1), M. musculus (DPP4, NP_034204.1), H. sapiens (DPP4, AAA52308.1). (Color figure online)
Phylogenetic analysis of catalytic region of DPP4 amino acid sequences. DPP4 (tetrapods), DPP4-1 and DPP4-2 (teleost). Each node has a bootstrap value which was obtained for 500 replicates. GenBank accession numbers or Ensembl identification numbers are as follows; H. sapiens (DPP4, AAA52308.1), S. scrofa (DPP4, NP_999422.1), M. musculus (DPP4, NP_034204.1), G. gallus (DPP4, NP_001026426.1), X. tropicalis, (DPP4, AAI27359.1), T. nigroviridis (DPP4-1, ENSTNIP00000016265; DPP4-2, CAG09338.1), T. rubripes (DPP4-1, ENSTRUP00000022072; DPP4-2, ENSTRUP00000003859), G. aculeatus (DPP4-1, ENSGACP00000018353; DPP4-2, ENSGACP00000007372), O. latipes (DPP4-1, ENSORLP00000003424; DPP4-2, ENSORLP00000021120), D. rerio (DPP4-1, NP_001154809.1; DPP4-2, ENSDARP00000118769 ), C. auratus (DPP4-1, JF760209)
Tissue distribution of DPP4 in goldfish
In goldfish, qualitative measurements of DPP4 mRNA expression using qRT-PCR detected high levels of DPP4 expression in the gastrointestinal tract of goldfish (Fig. 3). The highest levels of DPP4 expression were detected in the J-loop, midgut, rectum and kidney. In addition, lower but detectable levels were found within the olfactory bulb and telencephalon (Fig. 3). The lowest levels of DPP4 mRNA expression were observed in the gall bladder and heart (Fig. 3).
Tissue distribution of DPP4 mRNA expression in C. auratus. Differential expression of DPP4 mRNA in goldfish tissues using precise real-time quantitative reverse transcription PCR. The results were normalized to β-actin, which served as a control to verify the amount and quality of samples (n = 5 fish)
In vitro effects of DPP4 on gfPYY(1–36)
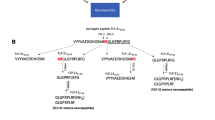
A MALDI-TOF analysis of synthetic samples confirms the purity of the peptides used in these studies (Fig. 4). Synthetic gfPYY(1–36) and gfPYY(3–36) correspond precisely with predicted molecular weights of 4290.1 and 4029.0 g/mol−1, respectively (Fig. 4a, b). In the presence of DPP4, gfPYY(1–36) did not show any indication of cleavage over 180-min incubation period (Fig. 4c). Rat PYY(1–36) and rat PYY(3–36) standards ran as described by the manufacturer at 4241.0 and 3980.9 g/mol−1, respectively (Fig. 4d, e). However, in the presence of DPP4 over a 180-min incubation period rat PYY(1–36), our positive control was completely digested to produce a single peak which corresponded precisely to that of the rat PYY(3–36) standard (Fig. 4f).
MALDI-TOF mass spectrometry analysis of DPP4 actions on gfPYY(1–36). MALDI-TOF mass spectrometry analysis of goldfish gfPYY (a–c) and rPYY (d–f) in the presence or absence of DPP4. Synthetic standards for the full length gfPYY(1–36) (4290 g/mol−1) (a) and rPYY(1–36) (4241 g/mol−1) (d) are present as one peak per sample. In addition, standards for gfPYY(3–36) (4029 g/mol−1) (b) and rPYY(3–36) (3980.9 g/mol−1) (e) are shown as one peak per sample. When synthetic gfPYY(1–36) was incubated in the presence of purified porcine DPP4, gfPYY(1–36) did not show cleavage of the full-length peptide (c), while rPYY(1–36) underwent complete conversion to rPYY(3–36) under identical conditions (f) (n = 3/group)
Effects of IP injections of PYY(3–36) and PYY(1–36) on food intake of goldfish
Similar to previous reports, IP injections of 10 ng/g BW of PYY(1–36) reduces food intake in goldfish by 26.07 % after 1 h (Gonzalez and Unniappan 2010). However, increased IP dosing of PYY(1–36) at 100 and 1000 ng/g did not further reduce food intake beyond what was previously observed (Fig. 5a). IP injections of gfPYY(3–36) at doses of 10, 100 and 1000 ng/g BW did not alter the food intake of goldfish, compared to saline-treated fish over a 1-h period (Fig. 5b). No significant differences were observed in food intake between study groups in the weeks prior to the feeding study and on the day of the study between saline-injected fish and those subjected to anesthesia alone during the first hour post-injection (data not shown).
Effects of Peripheral Injections of PYY(1–36) and PYY(3–36) in C. auratus. Effects of intraperitoneal injections of synthetic gfPYY(1–36) and gfPYY(3–36) on food intake over 1 h. a. Intraperitoneal injections of gfPYY(1–36) (10, 100, and 1000 ng/g) significantly reduced food intake. b. Intraperitoneal injections of gfPYY(3–36) (10, 100, and 1000 ng/g) did not alter food intake at any of the doses tested. Same letters denote values that are not statistically different. One-way ANOVA, Newman–Keuls multiple comparison test (n = 6 fish/group)
Effects of ICV injections of PYY(3–36) on food intake of goldfish
Similar to the observation of fish that received IP injections of gfPYY(3–36), ICV injections of the truncated peptide at 5 and 50 ng/g BW did not reduce food intake compared to saline controls (Fig. 6).
Antagonistic effects of IP injections of PYY(1–36) and BIBP 3226
Intraperitoneal coadministration of the mammalian Y1 receptor antagonist, BIBP 3226, with goldfish PYY(1–36) blunted the anorectic effects of PYY (Fig. 7a), and no significant reduction in food was seen, when compared to the control animals. When injected on its own, BIBP 3226 did not significantly alter food intake at doses of 5, 50 and 500 ng/g BW compared to saline controls (Fig. 7b).
BIBP322 attenuates gfPYY(1–36) inhibition of food intake in C. auratus peripherally. Effects of intraperitoneal injections of synthetic gfPYY(1–36) on food intake over 1 h. a. Effects of coadministration of BIBP 3226 and gfPYY(1–36). The effects of BIBP 3226 significantly blocked the actions of PYY(1–36). b. Effects of intraperitoneal injections of BIBP 3226 alone. Same letters denote values that are not statistically different. One-way ANOVA, Newman–Keuls multiple comparison test (n = 6 fish/group)
Antagonistic effects of ICV injections of PYY(1–36) and BIBP 3226
A central injection of 10 ng/g BW BIBP 3226 attenuated the inhibitory effects of gfPYY(1–36) on food intake in goldfish (Fig. 8). No significant difference in feeding was found when compared to saline-treated controls (Fig. 8). As reported previously [3], central injections of PYY(1–36) in the absence of the Y1 receptor antagonist inhibited feeding by 33 % compared to the saline control group (Fig. 8).
BIBP322 attenuates gfPYY(1–36) inhibition of food intake in C. auratus centrally. Saline alone or PYY(1–36) at 10 ng/g BW, or both BIBP 3226 and gfPYY(1–36) at 10 ng/g BW were administered. The effects of centrally injected gfPYY(1–36) were significantly disrupted by the antagonistic effects of the Y1 receptor blocker BIBP 3226. Same letters denote values that are not statistically different. One-way ANOVA, Newman–Keuls multiple comparison test (n = 6 fish/group)
Discussion
We have employed both molecular, biochemical and in vivo physiological studies to help characterize the role of DPP4 in the appetite regulatory actions of PYY in goldfish. The DPP4 mRNA sequence analyses conform the catalytic region of DPP4 found in both mammalian and non-mammalian vertebrates. Both the annotated DPP4 sequences available on GenBank and the previously unannotated sequences we identified within the Ensembl databases suggest that DPP4 is well conserved among bony fishes and tetrapods. DPP4 is a serine-type peptidase named after the presence of Ser630 within the catalytic core. Other critical residues forming the catalytic core include Asp708 and His740 which along with Ser630 form the catalytic triad of DPP4 (Lambeir et al. 2003). The presence of duplicated DPP4 genes among teleosts is due to a well-documented third round whole-genome duplication (3R-WGD) that occurred approximately 350 million years ago (Christoffels et al. 2004; Jaillon et al. 2004). An alignment of the 256 amino acid catalytic region of DPP4 from several species shows high-degree sequence conservation with goldfish DPP4 mRNA sequence. Moreover, we found a complete conservation of the catalytic triad across all species and paralogs examined. Goldfish DPP4-1 sequence contains all the three of the purported amino acids, which comprise the catalytic triad. The conservation across species suggests that the triad is likely conserved in many other vertebrate species as well.
The phylogenetic analysis reveals a strong independent clustering of the two paralogous DPP4 genes (DPP4-1 and DPP4-2) across the teleost species examined. In the cyprinid species, goldfish and zebrafish, DPP4-1 sequences are most closely aligned together. A similar trend is observed among the DPP4-1 and DPP4-2 sequences of the pufferfish species, T. rubripes and T. nigroviridis. The presence of a 3R-WGD would suggest that the goldfish genome contains an additional DPP4-2 gene. Moreover, the possibility of a duplicate DPP4 gene may suggest a redundant and/or sub-specialized role for this aminopeptidase. Interestingly, both DPP4-1 and DPP4-2 paralogous genes in teleosts have retained a highly conserved catalytic region and a completely conserved catalytic triad. However, to date no functional evidence exists for a role of DPP4-1 and DPP4-2 in fishes.
Both the distribution and expression levels of DPP4 differ greatly across various tissues in rats and humans. DPP4 is abundantly expressed in the brush-border membranes of the small intestine of humans and in the exosomes secreted by intestinal epithelial cells (Fukasawa et al. 1981; Sterchi 1981; van Niel et al. 2001). In the kidney, DPP4 is strongly expressed in the Bowman’s capsule and the brush-border microvilli of the proximal tubuli (Kenny et al. 1976). In the mammalian brain, DPP4 is also found on the endothelium of the blood–brain barrier and in the cerebrospinal fluid (Bernstein et al. 1987; Kato et al. 1979). Our current studies further these previous reports by describing the tissue distribution of DPP4 within a non-mammalian vertebrate. In goldfish, DPP4 mRNA is abundantly expressed within the tissues of the gastrointestinal tract, with the highest levels found in the J-loop, midgut and rectum. Detectable levels of DPP4 were also found in the kidney, olfactory bulb and telencephalon of goldfish. Our results expand previous mRNA expression studies by confirming the presence of DPP4 within the intestine, kidney and brain. Furthermore, the gut–brain expression pattern of PYY mRNA in teleost is similar to that seen in mammals (Cerda-Reverter et al. 2000a, b; Murashita et al. 2009; Söderberg et al. 2000; Sundstrom et al. 2008). The presence of DPP4 within the teleostean gastrointestinal tract suggests that DPP4 may play an important role in the regulation of bioactive peptides other than PYY.
Considering that the catalytic triad of DPP-4 in fish and mammals is completely conserved and that DPP-4 is extensively expressed in fish tissues, we then aimed to determine whether DPP-4 cleaves PYY(1–36) in fish. One issue we faced is that the lack of fish DPP-4 available for such studies. Therefore, we used porcine DPP-4 that shares a common catalytic triad with goldfish for our in vitro studies to address the above question. We also employed rat PYY(1–36) as a positive control to display the activity of porcine DPP-4 in cleaving PYY(1–36). Our results indicate that while porcine DPP-4 cleaves rat PYY(1–36) to PYY(3–36), this DPP-4 that shares a common catalytic triad with goldfish DPP-4 is unable to cleave synthetic goldfish PYY(1–36) to PYY(3–36). These results, for the first time, provide biochemical evidence for the time told concept that goldfish PYY(1–36) is resistant to DPP-4 cleavage due to the lack of cleavage sites in its sequence. The proline–alanine residues that are critical for DPP4 cleavage are absent from the N-terminal region of teleost PYY(1–36) (Fredriksson et al. 2006), including that of goldfish PYY (Gonzalez and Unniappan 2010). The conserved N-terminal proline–proline residues found in fish PYY are thought to significantly attenuate DPP4 activity (Fredriksson et al. 2006). In support of this notion, our MALDI-TOF mass spectrometry analysis found that DPP4 did not cleave gfPYY(1–36) to generate gfPYY(3–36) in vitro. This is the first study to show the lack of DPP4 activity on PYY(1–36) in a non-mammalian vertebrate. Prior studies have also shown that the zebrafish Y2 receptor has a ninefold reduced affinity for NPY(3–36) compared to full length NPY, which would suggest that zebrafish Y receptors are more sensitive to the truncation of NPY, and presumably PYY(1–36) as well, as compared to their mammalian counterparts (Fredriksson et al. 2006). This reduction in affinity for truncated forms of the peptide is of particular importance because of the studies that have implicated PYY(3–36) as an anorectic peptide in mammals. The ability to generate PYY(3–36) and its role in inhibiting feeding may be unique to the mammalian lineage as other non-mammals including frog, tortoise and python all have proline–proline bonds present at their N-terminus (Fredriksson et al. 2006; Larhammar 1996).
We then continued our research in vivo, to address the second question: is PYY(3–36), a cleavage product of PYY(1–36) unlikely found endogenously in fish, an anorexigen in fish? Both intraperitoneal and intracerebroventricular injections of synthetic goldfish PYY(3–36) at the doses tested had no effects on food intake of goldfish. The lack of anorectic activity for PYY(3–36) seen in our studies is in agreement with the fact that PYY(1–36) is not cleaved to PYY(3–36) in fish. Exogenous administration of PYY(3–36) that caused a fivefold increase in circulating levels of PYY(3–36) induced approximately 30 % decrease in food intake in normal weight humans (Batterham et al. 2002, 2003). In mammals this effect is mediated by Y2 receptors as the effects of peripheral injections of PYY(3–36) in rats can be significantly reduced by the coadministration of a Y2 receptor antagonist BIIE0246 (Abbott et al. 2005; Batterham et al. 2002). However, the results of our in vivo studies on PYY(1–36) are in agreement with our previous report on the appetite regulatory effects of PYY(1–36) in fish. Both intraperitoneal and intracerebroventricular injections of PYY(1–36) inhibited food intake by approximately 26 and 33 %, respectively. In mammals, a similar reduction in food intake has been observed with peripheral injections of PYY(1–36) (Chelikani et al. 2005; Unniappan et al. 2006). The anorectic effects of PYY(1–36), at least partly, are believed to be attributed by the conversion of PYY(1–36) to its truncated form PYY(3–36) by DPP4 (Chelikani et al. 2004; Sloth et al. 2007; Unniappan et al. 2006). Furthermore, the coadministration of a Y1 receptor antagonist, BIBP 3226, abolished the anorectic effects of goldfish PYY(1–36). Our results provide evidence for a Y1-like receptor-mediated anorectic actions of PYY(1–36) in fish. BIBP 3226 has been shown to effectively and selectively block the stimulation of the Y1 receptor by NPY and PYY(1–36) in mammals (Rudolf et al. 1994). As in mammals, there are several Y receptors present in fish. Whether other receptors also mediate the anorectic actions of PYY(1–36), as well as the specificity of fish ligands to NPY family of receptors, warrant further investigation.
In conclusion, this is the first report on the characterization of lack of DPP4-mediated regulation of PYY in a non-mammalian vertebrate. Although, GHRH and several gastrointestinal hormones (PYY, GLP-1, GLP-2, GIP) are cleaved by DPP4 in mammals, DPP4-mediated truncation of endogenous PYY is likely significantly attenuated in teleosts. Our physiological experiments show that peripheral and central injections of PYY(1–36) reduces food intake in goldfish. Contrary to what is observed in mammals, we did not observe any effects for central or peripheral administration of synthetic native PYY(3–36) on food intake in goldfish. The results described here also provide evidence for the lack of endogenous PYY(3–36) and its anorexigenic actions in fish.
References
Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, Bloom SR (2005) Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3–36) on food intake. Brain Res 1043:139–144
Abbott CR et al (2006) The importance of acclimatisation and habituation to experimental conditions when investigating the anorectic effects of gastrointestinal hormones in the rat. Int J Obes 30:288–292
Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR (1985) Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology 89:1070–1077
Batterham RL et al (2002) Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418:650–654
Batterham RL et al (2003) Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 88:3989–3992
Bernstein HG, Schon E, Ansorge S, Rose I, Dorn A (1987) Immunolocalization of dipeptidyl aminopeptidase (DAP IV) in the developing human brain. Int J Dev Neurosci 5:237–242
Bongers J, Lambros T, Ahmad M, Heimer EP (1992) Kinetics of dipeptidyl peptidase IV proteolysis of growth hormone-releasing factor and analogs. Biochim Biophys Acta 1122:147–153
Burnstock G (1958) Reversible inactivation of nervous activity in a fish gut. J Physiol 141:35–45
Cerda-Reverter JM, Martinez-Rodriguez G, Anglade I, Kah O, Zanuy S (2000a) Peptide YY (PYY) and fish pancreatic peptide Y (PY) expression in the brain of the sea bass (Dicentrarchus labrax) as revealed by in situ hybridization. J Comp Neurol 426:197–208
Cerda-Reverter JM, Martinez-Rodriguez G, Zanuy S, Carrillo M, Larhammar D (2000b) Molecular evolution of the neuropeptide Y (NPY) family of peptides: cloning of three NPY-related peptides from the sea bass (Dicentrarchus labrax). Regul Pept 95:25–34
Chelikani PK, Haver AC, Reidelberger RD (2004) Comparison of the inhibitory effects of PYY(3–36) and PYY(1–36) on gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol 287:R1064–R1070
Chelikani PK, Haver AC, Reidelberger RD (2005) Intravenous infusion of peptide YY(3–36) potently inhibits food intake in rats. Endocrinology 146:879–888
Chelikani PK, Haver AC, Reidelberger RD (2007) Intermittent intraperitoneal infusion of peptide YY(3–36) reduces daily food intake and adiposity in obese rats. Am J Physiol Regul Integr Comp Physiol 293:R39–R46
Christoffels A, Koh EG, Chia JM, Brenner S, Aparicio S, Venkatesh B (2004) Fugu genome analysis provides evidence for a whole-genome duplication early during the evolution of ray-finned fishes. Mol Biol Evol 21:1146–1151
Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C (2005) Effect of peptide YY3–36 on food intake in humans. Gastroenterology 129:1430–1436
Dereeper A et al (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res 36:W465–W469
Drucker DJ et al (1997) Regulation of the biological activity of glucagon-like peptide 2 in vivo by dipeptidyl peptidase IV. Nat Biotechnol 15:673–677
Eberlein GA et al (1989) A new molecular form of PYY: structural characterization of human PYY(3–36) and PYY(1–36). Peptides 10:797–803
Fredriksson R, Sjodin P, Larson ET, Conlon JM, Larhammar D (2006) Cloning and characterization of a zebrafish Y2 receptor. Regul Pept 133:32–40
Fukasawa KM, Fukasawa K, Sahara N, Harada M, Kondo Y, Nagatsu I (1981) Immunohistochemical localization of dipeptidyl aminopeptidase IV in rat kidney, liver, and salivary glands. J Histochem Cytochem 29:337–343
Gonzalez R, Unniappan S (2010) Molecular characterization, appetite regulatory effects and feeding related changes of peptide YY in goldfish. Gen Comp Endocrinol 166:273–279
Grandt D, Schimiczek M, Struk K, Shively J, Eysselein VE, Goebell H, Reeve JR Jr (1994) Characterization of two forms of peptide YY, PYY(1–36) and PYY(3–36), in the rabbit. Peptides 15:815–820
Hagan MM, Moss DE (1995) Effect of peptide YY (PYY) on food-associated conflict. Physiol Behav 58:731–735
Jaillon O et al (2004) Genome duplication in the teleost fish Tetraodon nigroviridis reveals the early vertebrate proto-karyotype. Nature 431:946–957
Kanatani A et al (2000) Role of the Y1 receptor in the regulation of neuropeptide Y-mediated feeding: comparison of wild-type, Y1 receptor-deficient, and Y5 receptor-deficient mice. Endocrinology 141:1011–1016
Kato T, Hama T, Nagatsu T, Kuzuya H, Sakakibara S (1979) Changes of X-prolyl dipeptidyl-aminopeptidase activity in developing rat brain. Experientia 35:1329–1330
Kenny AJ, Booth AG, George SG, Ingram J, Kershaw D, Wood EJ, Young AR (1976) Dipeptidyl peptidase IV, a kidney brush-border serine peptidase. Biochem J 157:169–182
Kurokawa T, Suzuki T (2002) Development of neuropeptide Y-related peptides in the digestive organs during the larval stage of Japanese flounder, Paralichthys olivaceus. Gen Comp Endocrinol 126:30–38
Lambeir AM, Durinx C, Scharpe S, De Meester I (2003) Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 40:209–294
Larhammar D (1996) Structural diversity of receptors for neuropeptide Y, peptide YY and pancreatic polypeptide. Regul Pept 65:165–174
McCaughan GW, Wickson J, Gorrell MD (1990) Characterization of the rat CD26 antigen. Transplant Proc 22:2103–2104
Mentlein R (1999) Dipeptidyl-peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul Pept 85:9–24
Mentlein R, Dahms P, Grandt D, Kruger R (1993) Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul Pept 49:133–144
Mentzel S, Dijkman HB, Van Son JP, Koene RA, Assmann KJ (1996) Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem 44:445–461
Moran TH, Smedh U, Kinzig KP, Scott KA, Knipp S, Ladenheim EE (2005) Peptide YY(3–36) inhibits gastric emptying and produces acute reductions in food intake in rhesus monkeys. Am J Physiol Regul Integr Comp Physiol 288:R384–R388
Morley JE, Levine AS, Grace M, Kneip J (1985) Peptide YY (PYY), a potent orexigenic agent. Brain Res 341:200–203
Murashita K, Kurokawa T, Nilsen TO, Ronnestad I (2009) Ghrelin, cholecystokinin, and peptide YY in Atlantic salmon (Salmo salar): molecular cloning and tissue expression. Gen Comp Endocrinol 160:223–235
Peter RE, Gill VE (1975) A stereotaxic atlas and technique for forebrain nuclei of the goldfish, Carassius auratus. J Comp Neurol 159:69–101
Rudolf K et al (1994) The first highly potent and selective non-peptide neuropeptide Y Y1 receptor antagonist: BIBP3226. Eur J Pharmacol 271:R11–R13
Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A (2007) Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab 292:E1062–E1068
Söderberg C, Pieribone VA, Dahlstrand J, Brodin L, Larhammar D (1994) Neuropeptide role of both peptide YY and neuropeptide Y in vertebrates suggested by abundant expression of their mRNAs in a cyclostome brain. J Neurosci Res 37:633–640
Söderberg C, Wraith A, Ringvall M, Yan YL, Postlethwait JH, Brodin L, Larhammar D (2000) Zebrafish genes for neuropeptide Y and peptide YY reveal origin by chromosome duplication from an ancestral gene linked to the homeobox cluster. J Neurochem 75:908–918
Sterchi EE (1981) The distribution of brush border peptidases along the small intestine of the adult human. Pediatr Res 15:884–885
Sundstrom G, Larsson TA, Brenner S, Venkatesh B, Larhammar D (2008) Evolution of the neuropeptide Y family: new genes by chromosome duplications in early vertebrates and in teleost fishes. Gen Comp Endocrinol 155:705–716
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tschop M et al (2004) Physiology: does gut hormone PYY3–36 decrease food intake in rodents? Nature 430:1
Unniappan S, McIntosh CH, Demuth HU, Heiser U, Wolf R, Kieffer TJ (2006) Effects of dipeptidyl peptidase IV on the satiety actions of peptide YY. Diabetologia 49:1915–1923
van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M (2001) Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology 121:337–349
Volkoff H, Canosa LF, Unniappan S, Cerda-Reverter JM, Bernier NJ, Kelly SP, Peter RE (2005) Neuropeptides and the control of food intake in fish. Gen Comp Endocrinol 142:3–19
Zhou L, Irwin DM (2004) Fish proglucagon genes have differing coding potential. Comp Biochem Physiol B Biochem Mol Biol 137:255–264
Acknowledgments
This work was funded by the Natural Sciences and Engineering Research Council (NSERC) of Canada through a Discovery grant, and a Discovery Accelerator Supplement. Infrastructure support for our laboratory is provided by the John R. Evans Leaders Fund from the Canada Foundation for Innovation, and an Establishment grant from the Saskatchewan Health Research Foundation to SU. SU is a recipient of the Canadian Institutes of Health Research New Investigator Salary Award. We thank Naresh Ramesh for assistance with reference list formatting.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gonzalez, R., Unniappan, S. Mass spectrometry-assisted confirmation of the inability of dipeptidyl peptidase-4 to cleave goldfish peptide YY(1–36) and the lack of anorexigenic effects of peptide YY(3–36) in goldfish (Carassius auratus). Fish Physiol Biochem 42, 831–844 (2016). https://doi.org/10.1007/s10695-015-0178-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0178-y