Abstract
Incorporation of a plant blend in the diet can affect growth parameters and metabolism in carnivorous fish. We studied for the first time the long-term (1 year) metabolic response of rainbow trout fed from first feeding with a plant-based diet totally devoid of marine ingredients. Hepatic enzymes were analyzed at enzymatic and molecular levels, at 3, 8 and 24 h after the last meal to study both the short-term effects of the last meal and long-term effects of the diet. The results were compared with those of fish fed a control diet of fish meal and fish oil. Growth, feed intake, feed efficiency and protein retention were lower in the group fed the plant-based diet. Glucokinase and pyruvate kinase activity were lower in the livers of trout fed the plant-based diet which the proportion of starch was lower than in the control diet. Glutamate dehydrogenase was induced by the plant-based diet, suggesting an imbalance of amino acids and a possible link with the lower protein retention observed. Gene expression of delta 6 desaturase was higher in fish fed the plant-based diet, probably linked to a high dietary level of linolenic acid and the absence of long-chain polyunsaturated fatty acids in vegetable oils. Hydroxymethylglutaryl-CoA synthase expression was also induced by plant-based diet because of the low rate of cholesterol in the diet. Changes in regulation mechanisms already identified through short-term nutritional experiments (<12 weeks) suggest that metabolic responses are implemented at short term and remain in the long term.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Global fish catches have been stable since the 1990s; the increasing demand for fish for human consumption has been covered by aquaculture, which has become the animal food production sector with the fastest growth in the two last decades (Food and Agriculture Organisation 2012). Feed used for farmed fish during this period relied heavily on marine resources to cover their nutritional requirements. This dependency threatened the aquaculture industry and led to a decrease in incorporation of fish meal and fish oil which now represents around 20 % for salmonids instead of 45 % in 1995. While aquaculture can be one response to the growth in fish consumption by humans and to the stagnant (or even declining) fishery resources, research into the reduction in the marine feedstuffs is still required to keep the aquaculture industry sustainable. The use of alternative protein and lipid dietary sources has been studied in this context, especially plant sources that are diverse and affordable.
Several studies have evaluated the effects of partial or total fish meal (Kaushik et al. 1995; Martin et al. 2003; Vilhelmsson et al. 2004; Lund et al. 2011; Zhang et al. 2012; Tacchi et al. 2012; Slawski et al. 2011) or fish oil substitution (Richard et al. 2007; Turchini and Francis 2009; Montero et al. 2010; Thanuthong et al. 2011a, b) in fish diets. Substantial replacement of marine resources by plant blends can be achieved without major changes in growth performance in salmonids. However, total replacement by plant ingredients involves technical challenges and only a few studies have tested substitution of both fish oil and fish meal (Panserat et al. 2009; Geay et al. 2011; Le Boucher et al. 2012a, b). Concomitant replacement of fish meal and fish oil affects all major nutrients in the diet (carbohydrates, proteins and lipids) and leads to significant metabolic changes. The three main key factors linked to the replacement of marine resources in feed are the amino acid composition of the diet, unsaturated fatty acid imbalance and the anti-nutritional substances present in plant ingredients such as trypsin inhibitor, lectins and saponins (Krogdahl et al. 1994; Francis et al. 2001). It has been reported that decreased palatability of plant-based diets often led to a decrease in feed intake (Davies et al. 1997; Stickney et al. 1996; Geurden et al. 2005). Lower growth in fish fed a plant diet might be also related to modifications of the hepatic metabolic pathway; energy metabolism, cholesterol biosynthesis and proteolysis activity can be affected by the substitution of marine resources by plant sources (Vilhelmsson et al. 2004). Studies have demonstrated that the decrease in growth with the use of vegetable blends can also have an impact on health in rainbow trout (Onchorynchus mykiss) (Turchini and Francis 2009; Panserat et al. 2009; Le Boucher et al. 2012a). Phytoestrogens supplied by soy flour seem to affect growth negatively in salmonids (Kaushik et al. 1995; Davies et al. 1997). These results have emphasized the need to improve the intake and nutritional value of any plant-based diet. Large-scale approaches such as transcriptomic and proteomic assays have allowed analysis of a wide range of molecules and have highlighted modifications in liver metabolic pathways when fish are fed different diets (Vilhelmsson et al. 2004; Panserat et al. 2008, 2009; Geay et al. 2011). Moreover, feeding trials are often short term (<12 weeks), and the long-term effects of alternative diets on fish metabolism are poorly documented; more information on this topic is still needed, especially when fish are fed a diet entirely comprising plant ingredients, i.e., without marine resources. We aimed at determine whether metabolic hepatic changes described in the literature on the basis of relatively short-term nutritional studies with plant-based diet still remain during long-term trials.
In the study presented here, we investigated, for the first time, the long-term impact of feeding plant-based diet totally devoid of marine ingredients on rainbow trout. A marine resources-based diet was used as a control. In order to study long-term metabolic responses to the experimental diets, fish were fed from first meal until the end of the trial at 355 days after fertilization. Starting the feeding trial at the first feeding avoids the effects of the body reserves due to previous feeding. Several key metabolic enzymes were tested, related to the use of the main macronutrients (carbohydrates, amino acids and lipids) known to be changed with plant-based diets free of marine resources. Three postprandial sampling times were studied to follow the kinetics of the targeted markers. A low throughput analysis scale was chosen to be used as routine in the laboratory; gene expression and enzyme activity were assayed to identify the level of regulation that was affected. The study focused on the liver, which plays central role in intermediary metabolism as the main nutrient supplier to the rest of body.
Materials and methods
Ethical statements
The INRA facilities are authorized for animal experimentation under French regulations (B 29-277-02). The experiments were carried out in accordance with the Guidelines of the National Legislation on Animal Care of the French Ministry of Research (Decree N°2001–464, May 29, 2001), and the scientist in charge of the experimentation had received training and personal authorization (N°B64 10 003).
Fish and diets
The experiment was conducted with the INRA synthetic strain of rainbow trout, Oncorhynchus mykiss, a domesticated population maintained since the early 1980s and fed standard commercial diets containing both fish meal and fish oil. Fish were maintained in flow-through tanks in INRA experimental facilities (PEIMA, Sizun, France) under natural light, and temperature was daily monitored (13.9 °C ± 2.2). Three tanks seeded with 600 eggs were used during the whole experiment for each dietary treatment: marine resources-based diet (M) and plant-based diet (PB), respectively (Table 1). The M diet consisted mainly of fish meal and fish oil, extruded wheat was added as a binder. The PB diet was totally devoid of marine ingredients which had been replaced by a blend of plant products. The plant ingredients used to develop the PB diet were chosen to satisfy trout needs in fatty acids, amino acids, vitamins and minerals (National Research Council U. S. 2011). Linseed oil was supplied as precursor for n–3 long-chain fatty acid. Soy lecithin, lysine and calcium phosphate were added to the PB diet to adjust the phospholipid, essential amino acid and available phosphorus content. Diets were formulated to be isoproteic, isolipidic and isoenergetic (Table 2) and were given to fish as the first meal (41 dpf). The proportion of ingredients was modified at 236 dpf for the M diet and at 280 dpf for the PB diet to adapt protein and lipid content to fish nutritional requirements according to their size (National Research Council U. S. 2011). Analytical composition of the experimental diets given to fish until the end of the study (355 dpf) is presented in Table 2. The experimental diets were produced in INRA facilities (Donzacq, France). Pellet size was adjusted according to the mean body weight of fish. Rations were adjusted for biomass differences according to expected growth so that fish can eat ad libitum.
Growth and feed intake
For growth measures, six random samplings per diet (two per tank) were performed about once a month during the whole experiment. At each sampling, 50 fish were weighted to estimate the mean body weight of the six experimental tanks.
Daily consumption was recorded for 64 days during the last period of the trial from 291 to 355 dpf when the feed pellets were of sufficient size for an accurate monitoring of feed intake. Net intake was estimated taking into account the unfed remaining pellets which were collected from the tanks after each distribution and weighed. Feed efficiency was then calculated according to the net intake of dry matter and the gain in fish biomass (i.e., the difference between fish biomass measured on days 355 dpf and day 291 dpf).
Sampling
Fish were fasted for 48 h before the end of trial. They were re-fed on the day of sampling, except for ten fish that were sampled for whole-body analysis. This fasting period was applied to homogenize the feed intake of fish on the day of sampling and the metabolic response of the fish within each group. At 355 dpf, fish were anesthetized in a bath with 2-phenoxyethanol (0.05 %) solution and killed by severing the spinal cord behind the head. Fish were sampled at 3, 8 and 24 h after the last meal. Eight individual samples per experimental diet per hour were used for analysis as biological replicates. Blood was removed from the caudal vein into heparinized syringes and centrifuged (3000×g, 5 min); the plasma recovered was immediately frozen and kept at −20 °C until analysis. The livers were excised, divided in two parts and immediately frozen in liquid nitrogen and then kept at −80 °C until analysis.
Analysis of whole fish and chemical composition of diet
Fish sampled for body composition analysis were ground, and a sample was freeze-dried. The chemical composition of samples of freeze-dried whole body and diets was analyzed in the INRA laboratory using the following procedures: dry matter after drying at 105 °C for 24 h, protein content (N × 6.25) by the Kjeldahl method after acid digestion and gross energy in an adiabatic bomb calorimeter (IKA, Heitersheim Gribheimer, Germany). InVivo labs (Vannes, France) performed the analysis of starch content (enzymatic hydrolysis) and amino acid profiles (by HPLC) of diets. Total lipids were extracted from diet samples according to the Folch method (Folch et al. 1957). Fatty acid composition of the diet was analyzed from total lipid extract with prior preparation of methyl ester. Fatty acid identification was performed by gas chromatography as described elsewhere (Richard et al. 2007).
Plasma analysis
Plasma metabolite levels were measured using commercial kits: glucose (Glucose RTU, bioMérieux, Marcy l’Etoile, France), triglycerides (PAP 150, bioMérieux) and cholesterol (CHOD-PAP, Sobioda).
Enzyme activity
For the assessment of enzyme activity, the livers were ground in 10 volumes of ice-cold buffer at pH 7.4 (50 mmol/l TRIS, 5 mmol/l EDTA, 2 mmol/l DTT and a protease inhibitor cocktail (P2714, purchased from Sigma, St Louis, MO)). After homogenization, 1 min of sonic disruption was applied to the samples kept on ice. Homogenates were then centrifuged at 4 °C, and the supernatants were used immediately for enzyme assays. The enzymes assayed were : glucokinase (GK; EC 2.7.1.2) as described by Panserat et al. (2000a), pyruvate kinase (PK; EC 2.7.1.40) following the protocol of Kirchner et al. (2003), glucose 6 phosphatase (G6Pase; EC 3.1.3.9) from Alegre et al. (1988), glutamate dehydrogenase (GDH; EC 1.4.1.2) as described by Gómez-Requeni et al. (2003), aspartate aminotransferase (ASAT; EC 2.6.1.1) and alanine aminotransferase (ALAT; EC 2.6.1.2) following the protocol of the commercial kit for both enzymes (Enzyme line, bioMérieux), citrate synthase (CS; E.C. 2.3.3.1) following the protocol of Singer et al. (1990), fatty acid synthase (FAS; EC 2.3.1.85) according to Chang et al. (1967) and modified by Chakrabarty and Leveille (1969) and 3-hydroxyacyl-CoA dehydrogenase (HOAD; EC 1.1.1.35) according to Kobayashi et al. (1996). Enzyme activity was measured at 30 °C in duplicate following variation of absorbance of NAD(P)(H) at 340 nm except for citrate synthase where DTNB [5,5′ dithiobis (2-nitrobenzoic acid)] was used to follow the reaction at 412 nm. The reactions were started by adding the specific substrate; a Power Wave X (BioTek Instrument, Inc.) plate reader was used. A blank with water instead of the substrate was run for each sample. One unit of enzyme activity was defined as the amount of enzyme that catalyzed the transformation of 1 μmol of substrate per min at 30 °C, except for FAS which was expressed as the amount of enzyme oxidizing 1 µmol of NADPH at 30 °C. Enzyme activity was expressed per mg of soluble protein. Protein concentration was measured in triplicate according to Bradford (Bradford 1976), using a protein assay kit (Bio Rad, München, Germany) with bovine serum albumin as a standard.
mRNA level analysis
Total RNA was extracted from livers using the TRIzol reagent method (Invitrogen, Carlsbad, CA): 1 µg of total RNA was reverse transcribed in duplicate with SuperScript III Reverse Transcriptase (Invitrogen) and random primer according to the manufacturer’s instructions. mRNA expression was assayed using LightCycler 480 SYBR Green I Master (Roche Diagnosis, Indianapolis, IN, USA), 0.4 mM final of each primer, 2 µl of cDNA in a total volume of 6 µl. A lightCycler 480 II thermocycler (Roche) was used. The genes assayed and the primer sequences used in this study are presented in Table 3; they correspond to the enzymes whose activity was measured, plus two other enzymes, i.e., delta(6) fatty acid desaturase (D6D; EC 1.14.49) involved in fatty acid bioconversion, and hydroxymethylglutaryl-CoA synthase (HCMGS; EC 2.3.3.10), a key enzyme of cholesterol biosynthesis. Relative quantifications of target genes transcripts were normalized using Elongation Factor 1α (EF1α) as reference gene transcript following the Pfaffl method (Pfaffl 2001).
Statistical analysis
Parameters related to growth, feed intake, feed efficiency, hepatosomatic index and nutrient retention were compared between diets using the t test (Student). All data related to enzymes were tested for homogeneity of variances by Leven’s tests. The effects of diet, of time and of diet × time interactions were then tested by two-way ANOVA. In cases of significant interaction, one-way ANOVA was applied to analyze differences between the two diets and the three sampling times. Post hoc tests were performed using Tukey’s multiple mean comparisons. All statistical calculations were made using R software (The R Project for Statistical Computing 2013). Differences were considered statistically significant at P < 0.05.
Results
Body weight measured during the whole experiment showed that significant difference between diets appeared soon, after 1.5 month (Fig. 1). Fish fed M diet were bigger than fish fed PD diet. The difference observed was conserved significantly during the long-term study.
Growth performance of fish fed marine resources (M) or plant-based diet (PB). Fourteen successive weight measurements were carried out during the first year of life. Each value is the mean of six random samplings for each date and each diet. Stars denote the significance difference between diets for body weight (***P = 0.01)
During the 64-day feeding trial (from day 291 to the end of the study (355 dpf)), body weight gain, net intake and feed efficiency were found significantly higher for fish fed the M diet with 2.5-, 2- and 1.3-fold increases for each parameter, respectively. The hepatosomatic index score was also slightly higher in fish fed the M diet than in those fed the V diet, but the difference was not significant. In terms of nutrient and energy retention, only protein retention was significantly affected, retention being 1.4-fold less in the PB diet-fed group (Table 4).
Plasma metabolites
Plasma triglycerides and cholesterol levels were strongly affected by the dietary treatment, with lower values recorded in fish fed PB diet compared to fish fed the M diet (Fig. 2). In terms of plasma glucose, a significant interaction was found between diet and postprandial time. Significantly lower glycaemia was observed in fish fed PB diet at 3 h and 8 h after a meal, but no longer at 24 h (Fig. 2). No significant postprandial change was observed in the glucose and cholesterol plasma levels for either dietary treatment. Plasma triglyceride levels increased significantly with sampling time for both diets.
Plasma metabolite levels of rainbow trout fed marine resources (M) or plant-based diet (PB). Each value is the mean ± SE of n = 8 fish/group. Two-way ANOVA results are presented in the upper right corner of the figure. Letters indicate differences between groups after one-way ANOVA analysis when the interaction between diet and time was significant. Differences were considered statistically significant when P < 0.05, NS nonsignificant
Liver metabolism: transcript expression and activity of enzymes
Glucose metabolism was studied through measurement of gene expression and enzyme activity for three key enzymes: GK, PK and G6Pase (Fig. 3). At 24 h after the last meal, GK gene expression was significantly different between the two diets, with a sevenfold induction of mRNA levels for fish fed the M diet. Significant diet and time effects were also observed for GK activity, which was higher in fish fed the M diet at all time points. No differential expression of PK mRNA was observed for either condition, whereas PK activity was significantly higher in fish fed the M diet (P < 10−5; two-way ANOVA). The G6Pase was chosen as a key enzyme of hepatic glucose production. G6Pase transcript level was significantly higher in fish fed the M diet, but the activity remained unchanged. Significant effect of time was observed on gene expression and enzymatic activity for both diets with a gradual decrease or increase of mRNA levels and activity, respectively, with time.
Enzymes involved in glucose metabolism in the liver of rainbow trout fed marine resources (M) or plant-based diet (PB). Each value is the mean ± SE of n = 8 fish/group. Two-way ANOVA results are presented in the upper right corner of the figure. Letters indicate differences between groups after one-way ANOVA analysis when the interaction between diet and time was significant. Differences were considered statistically significant when P < 0.05, NS nonsignificant
ASAT, ALAT and GDH (Fig. 4) were studied to determine the potential of hepatic amino acid catabolism. ASAT gene expression did not change with the dietary treatment or postprandial time except for the ASAT3, the expression of which was about twofold lower in fish fed the PB diet. Significant effects of diet and postprandial timing were observed for ASAT activity with particularly lower activity in fish fed the PB diet that could be linked to the lower ASAT3 gene expression. No molecular effect was detected for ALAT enzyme. However, ALAT activity was dependent on postprandial timing and diet, but interaction occurred and activities were higher in fish fed the PB diet at 24 h. GDH expression and activity were higher in fish fed the PB diet except for GDH1 gene expression. GDH1 gene expression level decreased with postprandial time.
Enzymes involved in amino acid catabolism in the liver of rainbow trout fed marine resources (M) or plant-based diet (PB). Each value is the mean ± SE of n = 8 fish/group. Two-way ANOVA results are presented in the upper right corner of the figure. Letters indicate differences between groups after one-way ANOVA analysis when interaction between diet and time was significant. Differences were considered statistically significant when P < 0.05, NS nonsignificant
CS expression and activity were assessed to study energy metabolism (Fig. 5). CS mRNA level and activity increased with time. An effect of diet was observed only at the molecular level, with higher CS gene expression in fish fed the PB diet (P < 10−7, two-way ANOVA).
Gene expression and enzyme activity of citrate synthase (energy metabolism marker) in the liver of rainbow trout fed marine resources (M) or plant-based diet (PB). Each value is the mean ± SE of n = 8 fish/group. Two-way ANOVA results are presented in the upper right corner of the figure. Letters indicate differences between groups after one-way ANOVA analysis when the interaction between diet and time was significant. Differences were considered statistically significant when P < 0.05, NS nonsignificant
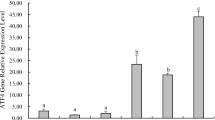
Lipid metabolism was studied through the key enzymes: FAS, HOAD, D6D and HMGCS (Fig. 6). Expression of FAS, a marker of fatty acid synthesis, was induced by the PB diet (P < 10−5, two-way ANOVA). A strong effect of the postprandial time (more than threefold increase) was also observed (P < 10−10, two-way ANOVA) with FAS mRNA level in the PB-fed group at 24 h after the last meal. No effect of diet or time on FAS activity was detected. Expression and activity of HOAD, that is involved in lipid β-oxidation, were significantly higher in fish fed the PB diet. A strong increase in level of activities was observed with time (P < 10−10, two-way ANOVA). D6D, that is required for the bioconversion of fatty acids into highly unsaturated fatty acids, did not vary with time and was consistently higher in fish fed the PB diet (3.5-fold, P < 10−5, two-way ANOVA). HMGCS involved in the first step of cholesterol synthesis was also expressed more strongly in fish fed the PB diet (P < 10−5, two-way ANOVA), but the difference was significant only 24 h after the last meal (significant time × diet interaction) as for FAS mRNA.
Enzymes involved in lipid metabolism in the liver of rainbow trout fed marine resources (M) or plant-based diet (PB). Each value is the mean ± SE of n = 8 fish/group. Two-way ANOVA results are presented in the upper right corner of the figure. Letters indicate differences between groups after one-way ANOVA analysis when the interaction between diet and time was significant. Differences were considered statistically significant when P < 0.05, NS nonsignificant
Discussion
The aim of this study was to determine for the first time the long-term metabolic responses at biochemical and molecular levels in the livers of rainbow trout fed a 100 % plant-based diet from first feeding to 1 year of age. Growth and nutrient retention, and certain plasma metabolites and enzymes of the main intermediary metabolic pathways in the liver, were studied in order to know whether change observed at short-term trials with plant-based diet still remains at long term. We found that the total replacement of fish meal and fish oil led to a significant effect on fish growth, trout fed the M diet being more than twice as big as fish fed the PB diet at the end of the trial. Growth difference appeared soon, but there was no increase in the relative difference over time (Le Boucher et al. 2011). The lower growth performance of fish fed the PB diet can be explained by the combination of a lower feed intake, lower feed efficiency and lower protein retention. Poor palatability, the presence of anti-nutritional factors, variation in amino acid and fatty acid profiles in plant ingredients might have had a negative effect on growth, as previously described with other PB diets and fish populations (Turchini and Francis 2009; Montero et al. 2010; Torstensen et al. 2008; Alami-Durante et al. 2010; Slawski et al. 2011). Only a few studies (Panserat et al. 2009; Geay et al. 2011; Le Boucher et al. 2012a) have assessed the effects of total substitution of both fish meal and fish oil by plant ingredients, but all have reported negative effects on growth with a 100 % PB diet. However these works were done during few weeks only, we described here the long-term metabolic responses of fish fed such a diet.
Long-term effect of plant-based diet devoid of marine ingredients on glucose metabolism
In the present study, the M diet was higher in starch content (12 %) than the PB diet (8.5 %) because wheat was added to the M diet to act as binder, whereas the plant-based diet was mainly composed of vegetable oils and plant proteins extracts. A positive relationship was found between glycaemia, levels of GK and PK activity and also with GK mRNA levels but only at 24 h, suggesting a long-term effect of feeding. In the group fed the M diet, higher level of activity was associated with a higher level of carbohydrate intake and consequently higher glycaemia was observed than with the PB diet. GK, known as hexokinase IV, plays a key role in intermediary metabolism by phosphorylating glucose for glycolysis and for storage of glucose in the liver as glycogen. GK activity has previously been described as a reliable indicator of the dietary starch content in rainbow trout and Atlantic salmon (Borrebaek et al. 1993; Panserat et al. 2000a; Capilla et al. 2003). Moreover, hepatic GK expression is generally well correlated with glycaemia and decreased with food deprivation (Panserat et al. 2000a; Capilla et al. 2003; Metón et al. 2004; Soengas et al. 2006). For the first time, our study demonstrated that GK responds to carbohydrate intake on long-term dietary treatment. PK catalyzes the last step of the glycolytic pathway. Although no variation in PK gene expression was detected, PK activity was affected positively by the starch content of the diet at all the postprandial sampling times. This confirmed our previous findings after feeding juvenile fish during 10 weeks (Panserat et al. 2001) for which no specific induction of PK mRNA expression at 6 and 24 h with a carbohydrate-rich diet was detected, suggesting control of the activity of this enzyme at the posttranscriptional level in contrast to mammals (Pilkis and Granner 1992). Overall, glycaemia, GK and PK enzymes were induced by long-term feeding high levels of dietary carbohydrates confirming short-term studies results.
In contrast to the glycolytic pathway, G6Pase was analyzed because it catalyzes the last reaction of hepatic glucose production. Both G6Pase isoforms indicated that fish fed the PB diet (lower starch level) had lower mRNA levels. Although no effects on level of activity were measured, the decreased G6Pase gene expression in fish fed lower levels of starch may appear paradoxical, but the absence of regulation of hepatic glucose production when fish are fed with carbohydrates has been shown previously (Moon 2001; Panserat et al. 2000b; Polakof et al. 2012) and could explain the non-glucose-tolerant phenotype of rainbow trout. Other neoglucogenic enzymes such as fructose biphosphatase and phosphoenolcarboxykinase have been previously described as ‘non-inhibited’ by dietary carbohydrates (Panserat et al. 2001). Thus here, the long-term trial did not clearly modify the apparent nutritional non-regulated trait of G6Pase.
Long-term effect of plant-based diet devoid of marine ingredients on nitrogen metabolism
The M and PB diets were formulated to contain similar amount of protein, with levels of essential amino acids well above trout requirements (National Research Council U. S. 2011) except for methionine in the PB diet which was just below the requirements. Despite this, a reduction in protein retention was observed in fish fed the PB diet. As the proteins originated from different sources, amino acid profile was not exactly identical in the two diets (Table 2). These variations in amino acid profile (Torstensen et al. 2008; Gómez-Requeni et al. 2003) might have had an impact on protein retention. Moreover, the presence of anti-trypsic factors or phytic acid in plant sources, especially soybean meal (Krogdahl et al. 1994; Francis et al. 2001; Lund et al. 2011; Krogdahl et al. 1994) can have a negative effect on digestion. In addition, in fish fed the PB diet, the GDH enzyme, one of the most active hepatic enzymes for deamination, was stimulated at enzymatic and molecular levels (at least for GDH2 and GDH3 isoforms) by intake of PB diet which could have led to the lower amino acid retention. The increased level of hepatic GDH activity might have been related to the higher glutamate level in the PB diet (twofold compared to the M diet), as has been previously reported in rainbow trout fed a high glutamate diet (Moyano et al. 1991). A negative correlation between specific activity of GDH and protein retention has also previously been reported (Gómez-Requeni et al. 2003). The other two amino acid catabolic enzymes studied here, ASAT and ALAT, showed slightly different levels of activity between fish fed the two diets with a weak effect at molecular level (only ASAT3 varied). Martin et al. (2003) reported increase in GDH and ASAT activities in rainbow trout fed with high incorporation of soy meal during 12 weeks. Previous study (8 weeks trial) also described higher GDH activity level with incorporation of plant protein in diet, whereas ASAT and ALAT gave no clear response (Moyano et al. 1991). GDH, localized at the end of amino acid catabolic pathway, is more informative than the two transaminases because it reflects the global amino acid utilization. In the present study, GDH remains induced at long-term confirming shorter trials and observations in liver (Martin et al. 2003; Moyano et al. 1991).
Long-term effect of plant-based diet devoid of marine ingredients on lipid metabolism
As fish oil was totally replaced by a blend of rapeseed, linseed and palm oil in the PB diet, fatty acid composition was strongly modified compared to the M diet (Table 2). These changes did not significantly affect lipid retention, but did impact on regulation of lipid metabolism. mRNA levels of FAS, HOAD, D6D and HMGCS were significantly higher in fish fed the PB diet, whereas levels of plasma triglycerides and cholesterol were reduced with the PB diet.
FAS is known to be down-regulated by the amount and composition of the fatty acids and amino acids (Gómez-Requeni et al. 2003). By contrast, a previous short-term study in trout reported a strong repression of hepatic FAS gene expression by vegetable oil at 24 h after last meal (Panserat et al. 2008); this was putatively explained by the high concentration of linolenic acid (ALA) in vegetable oil compared to fish oil. Alvarez et al. (2000) also reported that ALA had an inhibitory effect on FAS activity in hepatocytes. In another study, fish oil replacement by vegetable oils did not modify FAS expression or activity (Richard et al. 2007). Our data show strong induction of gene expression at 24 h by plant-based diet but without effect on activity. Does the long-term experiment affect FAS expression and induce it? For the moment, the reasons for the discrepancies between these findings regarding FAS are not known. Moreover, FAS mRNA levels and activity in our study were not correlated, as has already been observed in trout (Panserat et al. 2008), thus suggesting a discordance between FAS expression and activity. Based on these findings, more researches on fish lipid metabolism should be lead to better understand FAS complex regulation on long term in trout liver.
In terms of lipid catabolism, the activity of the HOAD enzyme reflects (at least partially) the potential of fatty acid oxidation to produce energy through the Krebs cycle with CS as a key enzyme. In this study, both mRNA levels and HOAD activity were higher in fish fed the PB diet, evoking increased lipid oxidation. The same tendency was also observed for CS gene expression in fish fed the PB diet. Such a link between HOAD and CS, when lipid oxidation provides a substrate for the citric acid cycle, has been previously described in fish (Kolditz et al. 2008; Pérez-Jiménez et al. 2012).
The D6D enzyme participates in the production of long-chain polyunsaturated fatty acids (LC-PUFA) using LA and ALA (found in high proportions in vegetable oil blends) as initial substrates and can be a key enzyme in maintaining flesh quality by optimizing EPA and DHA production. In this study, we found a strong inducing effect of the PB diet, probably resulting from the high amount of ALA in vegetable oils and the absence of LC-PUFA in the PB diet. In agreement with this, previous studies during few weeks trials have reported transcriptional regulation of genes encoding the enzymes involved in LC-PUFA biosynthesis related to the presence or absence of dietary LC-PUFA (Seiliez et al. 2001; Panserat et al. 2009; Vagner and Santigosa 2011; Thanuthong et al. 2011a, b). However, the results regarding the fatty acid composition of trout showed that those fed the PB diet had a very much lower proportion of total n–3 LC-PUFA than those fed the marine resources-based diet (159.2 and 281.4 mg g−1 lipids for PB and M groups, respectively, P < 0.01(Le Boucher, personal communication), confirming the limited capacity of trout fed the PB diet to produce LC-PUFA even though there was induction of D6D gene expression. Also, here, we observed the gene stimulation is maintained up to 1 year.
In the present study, cholesterol concentrations in the blood (4.9 g/l ± 0.2 with M diet and 2.6 g/l ± 0.1 with PB diet) were in the standard range of values expected for trout (1.6 and 5.8 g/l) (Řehulka and Minařík 2012). The twofold decrease observed in the PB-fed trout can be related to the high rate of cholesterol in animal material compared to vegetable blend as previously shown (Turchini and Francis 2009). As suggested by Richard et al. (2007), LA and ALA present in plants could also contribute to the hypocholesterolemic effect, as previously observed in mammals. Probably related to the lower cholesterolemia in fish fed the PB diet, strong induction of HMGCS expression, the enzyme which catalyzes the first stage of cholesterol synthesis, was observed. Previous studies on short-term experiments testing total substitution of marine resources by plant ingredients also reported higher gene expression and synthesis of enzymes involved in cholesterol production (Vilhelmsson et al. 2004; Panserat et al. 2009). This highlights the ability of rainbow trout to respond to long-term feeding with a low cholesterol diet and to maintain a standard plasma cholesterol level on long term.
Plasma triglycerides and cholesterol, HOAD (enzymatic and gene expression), D6D and HMGCS (gene expression), demonstrate that lipid metabolism is modified in relation to nutrient intake. Long-term feeding confirms previous observations obtain during shorter trials such as D6D and HMGCS expression. But our finding concerning FAS expression was at the opposite of what has been observed previously suggesting an effect of long-term experiment or a bad comprehension of FAS complex regulation.
Conclusion
We studied for the first time the long-term metabolic response of rainbow trout fed a 100 % PB diet. The aim of this study was to determine whether the effects on plasma metabolites and liver metabolism observed at short term with PB diet were remained at long term. Our results showed that changes in macronutrients linked to the PB diet free of marine resources lead to a decrease in growth performance, feed intake and feed efficiency. We confirmed at long term the impact of starch amount on GK and PK as previously shown with short-term studies. GDH, HOAD, HMGCS and D6D were induced up to 1 year in fish fed plant-based diet in accordance with short-term trials. Within the context of increased of fish meal prices and endangered natural resources, the use of ingredients other than marine resources such as plant ingredients would be a more sustainable solution to respond to the growing demands of the aquaculture industry. The enzymes tested here could be used as biomarkers to assess the effects of feeding fish with alternatives to fish meal and fish oil and to help the development of new promising strategies in fish nutrition such as nutritional programming projects.
Abbreviations
- M diet:
-
Marine resources-based diet
- PB diet:
-
Plant-based diet
- GK:
-
Glucokinase
- PK:
-
Pyruvate kinase
- G6Pase:
-
Glucose 6 phosphatase
- GDH:
-
Glutamate dehydrogenase
- ASAT:
-
Aspartate aminotransferase
- ALAT:
-
Alanine aminotransferase
- CS:
-
Citrate synthase
- FAS:
-
Fatty acid synthase
- HOAD:
-
3-Hydroxyacyl-CoA dehydrogenase
- D6D:
-
Delta6desaturase
- HMGCS:
-
Hydroxymethylglutaryl-CoA synthase
References
Alami-Durante H, Médale F, Cluzeaud M, Kaushik SJ (2010) Skeletal muscle growth dynamics and expression of related genes in white and red muscles of rainbow trout fed diets with graded levels of a mixture of plant protein sources as substitutes for fishmeal. Aquaculture 303:50–58. doi:10.1016/j.aquaculture.2010.03.012
Alegre M, Ciudad CJ, Fillat C, Guinovart JJ (1988) Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal Biochem 173:185–189. doi:10.1016/0003-2697(88)90176-5
Alvarez MJ, Díez A, López-Bote C et al (2000) Short-term modulation of lipogenesis by macronutrients in rainbow trout (Oncorhynchus mykiss) hepatocytes. Br J Nutr 84:619–628
Borrebaek B, Waagbø R, Christophersen B et al (1993) Adaptable hexokinase with low affinity for glucose in the liver of atlantic salmon (Salmo salar). Comp Biochem Physiol Part B Comp Biochem 106:833–836. doi:10.1016/0305-0491(93)90038-7
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Capilla E, Médale F, Navarro I et al (2003) Muscle insulin binding and plasma levels in relation to liver glucokinase activity, glucose metabolism and dietary carbohydrates in rainbow trout. Regul Pept 110:123–132
Chakrabarty K, Leveille GA (1969) Acetyl CoA carboxylase and fatty acid synthetase activities in liver and adipose tissue of meal-fed rats. Exp Biol Med 131:1051–1054
Chang HC, Seidman I, Teebor G, Lane MD (1967) Liver acetyl CoA carboxylase and fatty acid synthetase: relative activities in the normal state and in hereditary obesity. Biochem Biophys Res Commun 28:682–686
Davies SJ, Morris PC, Baker RTM (1997) Partial substitution of fish meal and full-fat soya bean meal with wheat gluten and influence of lysine supplementation in diets for rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac Res 28:317–328. doi:10.1046/j.1365-2109.1997.t01-1-00861.x
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Food and Agriculture Organisation (2012) The state of world fisheries and aquaculture. http://www.fao.org/docrep/016/i2727e/i2727e00.htm
Francis G, Makkar HP, Becker K (2001) Antinutritional factors present in plant-derived alternate fish feed ingredients and their effects in fish. Aquaculture 199:197–227. doi:10.1016/S0044-8486(01)00526-9
Geay F, Ferraresso S, Zambonino-Infante JL et al (2011) Effects of the total replacement of fish-based diet with plant-based diet on the hepatic transcriptome of two European sea bass (Dicentrarchus labrax) half-sibfamilies showing different growth rates with the plant-based diet. BMC Genom 12:522. doi:10.1186/1471-2164-12-522
Geurden I, Cuvier A, Gondouin E et al (2005) Rainbow trout can discriminate between feeds with different oil sources. Physiol Behav 85:107–114. doi:10.1016/j.physbeh.2005.03.010
Gómez-Requeni P, Mingarro M, Kirchner S et al (2003) Effects of dietary amino acid profile on growth performance, key metabolic enzymes and somatotropic axis responsiveness of gilthead sea bream (Sparus aurata). Aquaculture 220:749–767. doi:10.1016/S0044-8486(02)00654-3
Kaushik SJ, Cravedi JP, Lalles JP et al (1995) Partial or total replacement of fish meal by soybean protein on growth, protein utilization, potential estrogenic or antigenic effects, cholesterolemia and flesh quality in rainbow trout, Oncorhynchus mykiss. Aquaculture 133:257–274. doi:10.1016/0044-8486(94)00403-B
Kirchner S, Kaushik S, Panserat S (2003) Low protein intake is associated with reduced hepatic gluconeogenic enzyme expression in rainbow trout (Oncorhynchus mykiss). J Nutr 133:2561–2564
Kobayashi A, Jiang LL, Hashimoto T (1996) Two mitochondrial 3-hydroxyacyl-CoA dehydrogenases in bovine liver. J Biochem (Tokyo) 119:775–782
Kolditz C, Borthaire M, Richard N et al (2008) Liver and muscle metabolic changes induced by dietary energy content and genetic selection in rainbow trout (Oncorhynchus mykiss). AJP Regul Integr Comp Physiol 294:R1154–R1164. doi:10.1152/ajpregu.00766.2007
Krogdahl Å, Lea TB, Olli JJ (1994) Soybean proteinase inhibitors affect intestinal trypsin activities and amino acid digestibilities in rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A Physiol 107:215–219. doi:10.1016/0300-9629(94)90296-8
Le Boucher R, Quillet E, Vandeputte M et al (2011) Plant-based diet in rainbow trout (Oncorhynchus mykiss Walbaum): are there genotype-diet interactions for main production traits when fish are fed marine versus plant-based diets from the first meal? Aquaculture 321(1–2):41–48. doi:10.1016/j.aquaculture.2011.08.010
Le Boucher R, Dupont-Nivet M, Vandeputte M et al (2012a) Selection for adaptation to dietary shifts: towards sustainable breeding of carnivorous fish. PLoS ONE 7:e44898. doi:10.1371/journal.pone.0044898
Le Boucher R, Vandeputte M, Dupont-Nivet M et al (2012b) Genotype by diet interactions in European sea bass (Dicentrarchus labrax L.): nutritional challenge with totally plant-based diets. J Anim Sci 91:44–56. doi:10.2527/jas.2012-5311
Lund I, Dalsgaard J, Rasmussen HT et al (2011) Replacement of fish meal with a matrix of organic plant proteins in organic trout (Oncorhynchus mykiss) feed, and the effects on nutrient utilization and fish performance. Aquaculture 321:259–266. doi:10.1016/j.aquaculture.2011.09.028
Martin SAM, Vilhelmsson O, Médale F et al (2003) Proteomic sensitivity to dietary manipulations in rainbow trout. Biochim Biophys Acta BBA Proteins Proteomics 1651:17–29. doi:10.1016/S1570-9639(03)00231-0
Metón I, Caseras A, Fernández F, Baanante IV (2004) Molecular cloning of hepatic glucose-6-phosphatase catalytic subunit from gilthead sea bream (Sparus aurata): response of its mRNA levels and glucokinase expression to refeeding and diet composition. Comp Biochem Physiol B: Biochem Mol Biol 138:145–153. doi:10.1016/j.cbpc.2004.03.004
Montero D, Mathlouthi F, Tort L et al (2010) Replacement of dietary fish oil by vegetable oils affects humoral immunity and expression of pro-inflammatory cytokines genes in gilthead sea bream Sparus aurata. Fish Shellfish Immunol 29:1073–1081. doi:10.1016/j.fsi.2010.08.024
Moon TW (2001) Glucose intolerance in teleost fish: fact or fiction? Comp Biochem Physiol B: Biochem Mol Biol 129:243–249
Moyano F, Cardenete G, De la Higuera M (1991) Nutritive and metabolic utilization of proteins with high glutamic acid content by the rainbow trout (Oncorhynchus mykiss). Comp Biochem Physiol A Physiol 100:759–762. doi:10.1016/0300-9629(91)90404-Z
National Research Council (U. S.) (2011) Nutrient requirements of fish and shrimp. National Academies Press, Washington
Panserat S, Médale F, Blin C et al (2000a) Hepatic glucokinase is induced by dietary carbohydrates in rainbow trout, gilthead seabream, and common carp. Am J Physiol Regul Integr Comp Physiol 278:R1164–R1170
Panserat S, Médale F, Brèque J et al (2000b) Lack of significant long-term effect of dietary carbohydrates on hepatic glucose-6-phosphatase expression in rainbow trout (Oncorhynchus mykiss). J Nutr Biochem 11:22–29
Panserat S, Plagnes-Juan E, Kaushik S (2001) Nutritional regulation and tissue specificity of gene expression for proteins involved in hepatic glucose metabolism in rainbow trout (Oncorhynchus mykiss). J Exp Biol 204:2351–2360
Panserat S, Kolditz C, Richard N et al (2008) Hepatic gene expression profiles in juvenile rainbow trout (Oncorhynchus mykiss) fed fishmeal or fish oil-free diets. Br J Nutr 100:953. doi:10.1017/S0007114508981411
Panserat S, Hortopan GA, Plagnes-Juan E et al (2009) Differential gene expression after total replacement of dietary fish meal and fish oil by plant products in rainbow trout (Oncorhynchus mykiss) liver. Aquaculture 294:123–131. doi:10.1016/j.aquaculture.2009.05.013
Pérez-Jiménez A, Cardenete G, Hidalgo MC et al (2012) Metabolic adjustments of Dentex dentex to prolonged starvation and refeeding. Fish Physiol Biochem 38:1145–1157. doi:10.1007/s10695-011-9600-2
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Pilkis SJ, Granner DK (1992) Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol 54:885–909. doi:10.1146/annurev.ph.54.030192.004321
Polakof S, Panserat S, Soengas JL, Moon TW (2012) Glucose metabolism in fish: a review. J Comp Physiol 182:1015–1045. doi:10.1007/s00360-012-0658-7
Řehulka J, Minařík B (2012) Cholesterolaemia and triacylglycerolaemia in farmed rainbow trout, Oncorhynchus mykiss. Aquac Res 43:1651–1659. doi:10.1111/j.1365-2109.2011.02971.x
Richard N, Kaushik S, Larroquet L et al (2007) Replacing dietary fish oil by vegetable oils has little effect on lipogenesis, lipid transport and tissue lipid uptake in rainbow trout (Oncorhynchus mykiss). Br J Nutr 96:299. doi:10.1079/BJN20061821
Seiliez I, Panserat S, Kaushik S, Bergot P (2001) Cloning, tissue distribution and nutritional regulation of a Δ6-desaturase-like enzyme in rainbow trout. Comp Biochem Physiol B: Biochem Mol Biol 130:83–93. doi:10.1016/S1096-4959(01)00410-9
Singer TD, Mahadevappa VG, Ballantyne JS (1990) Aspects of the energy metabolism of lake sturgeon, Acipenser fulvescens, with special emphasis on lipid and ketone body metabolism. Can J Fish Aquat Sci 47:873–881. doi:10.1139/f90-100
Slawski H, Adem H, Tressel R-P et al (2011) Total fish meal replacement with rapeseed protein concentrate in diets fed to rainbow trout (Oncorhynchus mykiss Walbaum). Aquac Int 20:443–453. doi:10.1007/s10499-011-9476-2
Soengas JL, Polakof S, Chen X et al (2006) Glucokinase and hexokinase expression and activities in rainbow trout tissues: changes with food deprivation and refeeding. Am J Physiol Regul Integr Comp Physiol 291:R810–R821. doi:10.1152/ajpregu.00115.2006
Stickney RR, Hardy RW, Koch K et al (1996) The effects of substituting selected oilseed protein concentrates for fish meal in rainbow trout Oncorhynchus mykiss diets. J World Aquac Soc 27:57–63. doi:10.1111/j.1749-7345.1996.tb00594.x
Tacchi L, Secombes CJ, Bickerdike R et al (2012) Transcriptomic and physiological responses to fishmeal substitution with plant proteins in formulated feed in farmed Atlantic salmon (Salmo salar). BMC Genom 13:363. doi:10.1186/1471-2164-13-363
Thanuthong T, Francis DS, Manickam E et al (2011a) Fish oil replacement in rainbow trout diets and total dietary PUFA content: II Effects on fatty acid metabolism and in vivo fatty acid bioconversion. Aquaculture 322–323:99–108. doi:10.1016/j.aquaculture.2011.09.026
Thanuthong T, Francis DS, Senadheera SD et al (2011b) Fish oil replacement in rainbow trout diets and total dietary PUFA content: I Effects on feed efficiency, fat deposition and the efficiency of a finishing strategy. Aquaculture 320:82–90. doi:10.1016/j.aquaculture.2011.08.007
The R Project for Statistical Computing (2013) http://www.r-project.org/. Accessed 3 Dec 2013
Torstensen BE, Espe M, Sanden M et al (2008) Novel production of Atlantic salmon (Salmo salar) protein based on combined replacement of fish meal and fish oil with plant meal and vegetable oil blends. Aquaculture 285:193–200. doi:10.1016/j.aquaculture.2008.08.025
Turchini GM, Francis DS (2009) Fatty acid metabolism (desaturation, elongation and β-oxidation) in rainbow trout fed fish oil- or linseed oil-based diets. Br J Nutr 102:69. doi:10.1017/S0007114508137874
Vagner M, Santigosa E (2011) Characterization and modulation of gene expression and enzymatic activity of delta-6 desaturase in teleosts: a review. Aquaculture 315:131–143. doi:10.1016/j.aquaculture.2010.11.031
Vilhelmsson OT, Martin SAM, Médale F et al (2004) Dietary plant-protein substitution affects hepatic metabolism in rainbow trout (Oncorhynchus mykiss). Br J Nutr 92:71. doi:10.1079/BJN20041176
Zhang Y, Øverland M, Shearer KD et al (2012) Optimizing plant protein combinations in fish meal-free diets for rainbow trout (Oncorhynchus mykiss) by a mixture model. Aquaculture 360–361:25–36. doi:10.1016/j.aquaculture.2012.07.003
Acknowledgments
We thank Thierry Kerneis who managed the trial at the PEIMA experimental fish farm, Frederic Terrier and Frank Sandres for the preparation of the two experimental diets in Donzacq INRA experimental facilities, and Alexandre Herman for plasma analysis.
Funding
This research was funded by the FUI (Fond Unique Interministériel) Vege-Aqua (2009–2012) and by the European Commission (European project FP7-KBBE-2001 N°288925 ARRAINA for Advanced Research Initiatives for Nutrition and Aquaculture).
Author contributions
The contributions of the authors to the study were as follow: R.L.B., M.D.N., E.Q. and F.M. conceived and designed the experiments. L.L. supervised the in vivo trial. F.M., R.L.B. and L.L. designed and performed samplings. V.V. and R.L.B. performed all the analysis. V.V. and RLB. analyzed the data. V.V., S.P. and F.M. wrote the paper. All authors read and approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
No competing interests to declare.
Rights and permissions
About this article
Cite this article
Véron, V., Panserat, S., Le Boucher, R. et al. Long-term feeding a plant-based diet devoid of marine ingredients strongly affects certain key metabolic enzymes in the rainbow trout liver. Fish Physiol Biochem 42, 771–785 (2016). https://doi.org/10.1007/s10695-015-0174-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0174-2