Abstract
Lamnid sharks are regionally endothermic fishes that maintain visceral temperatures elevated above the ambient water temperature. Visceral endothermy is thought to increase rates of digestion and food processing and allow thermal niche expansion. We tested the hypothesis that, at in vivo temperatures, the endothermic shortfin mako shark, Isurus oxyrinchus, has higher specific activities of three digestive enzymes—gastric pepsin and pancreatic trypsin and lipase—than the thresher shark, Alopias vulpinus, and the blue shark, Prionace glauca, neither of which can maintain elevated visceral temperatures. Homogenized stomach or pancreas tissue obtained from sharks collected by pelagic longline was incubated at both 15 and 25 °C, at saturating substrate concentrations, to quantify tissue enzymatic activity. The mako had significantly higher enzyme activities at 25 °C than did the thresher and blue sharks at 15 °C. This difference was not a simple temperature effect, because at 25 °C the mako had higher trypsin activity than the blue shark and higher activities for all enzymes than the thresher shark. We also hypothesized that the thermal coefficient, or Q 10 value, would be higher for the mako shark than for the thresher and blue sharks because of its more stable visceral temperature. However, the mako and thresher sharks had similar Q 10 values for all enzymes, perhaps because of their closer phylogenetic relationship. The higher in vivo digestive enzyme activities in the mako shark should result in higher rates of food processing and may represent a selective advantage of regional visceral endothermy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Although almost all fish species are ectothermic, a small number of regionally endothermic (or spatially heterothermic) fishes can maintain the temperature of specific tissues elevated above ambient water temperature (reviewed in Brill et al. 1994; Dickson and Graham 2004; Goldman et al. 2004). In those fishes, vascular countercurrent heat exchangers, or retia mirabilia, allow metabolic heat generated by the tissues to be retained at its site of production instead of being lost to the environment at the gills (Carey and Teal 1966; Carey et al. 1971, 1981, 1984, 1985). The known regionally endothermic fishes (tunas, billfishes, opahs, lamnid sharks, and thresher sharks) are pelagic predators that swim continuously, migrate over long distances, and encounter large, rapid changes in ambient temperature during repeated dives below the thermocline to pursue prey (e.g., Carey and Lawson 1973; Block et al. 2001; Boustany et al. 2002; Bernal and Sepulveda 2005; Runcie et al. 2009; Boustany et al. 2010). Both niche expansion and increased physiological performance of the warmed tissues have been hypothesized to be selective advantages to explain the convergent evolution of regional endothermy in fishes (reviewed in Block and Finnerty 1994; Dickson and Graham 2004), but limited data exist to test these hypotheses.
The present study focuses on a possible physiological benefit resulting from the ability of sharks in the family Lamnidae to maintain elevated and stable visceral temperatures (Carey et al. 1981; Holts and Bedford 1993; Goldman 1997; Goldman et al. 2004; Sepulveda et al. 2004; Stevens et al. 2010). Among elasmobranch fishes, visceral endothermy occurs only in the five lamnid shark species: shortfin mako, Isurus oxyrinchus (hereafter referred to as the “mako”), longfin mako, Isurus paucus, white, Carcharodon carcharias, porbeagle, Lamna nasus, and salmon, Lamna ditropis (Carey and Teal 1969; Carey et al. 1981, 1985; Goldman 1997; Goldman et al. 2004). The visceral blood supply of lamnid sharks passes through a single large suprahepatic rete mirabile (Carey et al. 1981; Fudge and Stevens 1996). Oxygenated blood from the gills travels posteriorly in large pericardial arteries, which penetrate a hepatic venous sinus located on the ventral surface of the esophagus and repeatedly branch until the venous sinus is filled with small arteries, thus forming the suprahepatic rete. On the posterior end of the rete, the small arteries coalesce into a collecting trunk, which branches off arteries that supply warm blood to the liver, stomach, spleen, and spiral valve intestine (Carey et al. 1981; Fudge and Stevens 1996). The suprahepatic rete allows lamnid sharks to retain the heat generated by digestion and assimilation and to maintain visceral temperatures elevated 4–14 °C above the ambient water temperature (Carey et al. 1981; Goldman et al. 2004; Sepulveda et al. 2004). Large adult lamnid sharks are apparently unique among fishes in that they maintain a stable stomach temperature (Carey et al. 1971; Goldman 1997; Goldman et al. 2004).
Lamnid sharks occupy continental to oceanic habitats in all oceans from tropical to boreal waters and make seasonal horizontal and diel vertical migrations (Carey et al. 1981; Compagno 2001; Boustany et al. 2002; Weng et al. 2005, 2007a, b). Carey et al. (1971) proposed that elevated visceral temperatures would result in higher rates of food processing, which would fuel the high energy demands resulting from the high metabolic rates and activity levels of endothermic fishes. Higher food processing rates would enable lamnid sharks to assimilate prey rapidly and convert food into the lipid reserves necessary for migrations to localized feeding and breeding grounds. With stable visceral temperatures, rates of digestion and assimilation are less affected by rapid changes in ambient temperature during dives. Both effects would contribute to niche expansion because these fishes would be able to exploit colder environments below the thermocline and at higher latitudes (Block and Finnerty 1994; Dickson and Graham 2004; Weng et al. 2005).
In order to isolate visceral endothermy as a variable and test for physiological benefits of visceral endothermy, we compared the mako, I. oxyrinchus Rafinesque, to the thresher, Alopias vulpinus (Bonnaterre), and blue shark, Prionace glauca (Linnaeus), two pelagic species that are related to the lamnids but are unable to maintain elevated visceral temperatures and that were locally available and possible to sample. The thresher shark is closely related to the lamnid sharks [Order Lamniformes: Family Alopiidae (Compagno 1990; Naylor et al. 1997; Shimada 2005; Velez-Zuazo and Agnarsson 2011)], does not elevate visceral temperatures (C. Sepulveda pers. comm.), displays diel diving behavior (Stevens et al. 2010), and occasionally co-occurs with the mako in the Southern California Bight during the summer (Compagno 1984; Preti et al. 2001; Sepulveda et al. 2005). Because other lamniform sharks were not available, we also sampled the blue shark (Order Carcharhiniformes: Family Carcharhinidae) because the requiem sharks (Order Carcharhiniformes) and the carpet sharks (Order Orectolobiformes) comprise the sister taxon to the Order Lamniformes (Velez-Zuazo and Agnarsson 2011). The blue shark cannot elevate visceral temperatures, occupies the same pelagic habitat as the mako, and makes regular deep dives (Sciarrotta and Nelson 1977; Carey and Scharold 1990; Stevens et al. 2010). These three predators also have similar diets, which, for the mako shark, include jumbo squid, saury, sardine, mackerel, mullet, swordfish, bonito, tuna, yellowtail, market squid, and other sharks (Mannan et al. 1961; Maia et al. 2006; Clark et al. 2010; Preti et al. 2012). The thresher shark feeds upon sardines, anchovies, hake, mackerel, market squid, saury, rockfish, and pelagic crabs (Preti et al. 2001, 2012). The diet of the blue shark includes jumbo squid, armhook squid, pelagic octopuses, jewel squid, herring, salmon, mackerel, lumpfish, dogfish, cod, and, occasionally, seals (McChord and Campana 2003; Preti et al. 2012).
Food processing rates depend upon many factors, including prey acquisition, mastication, gut pH and temperature, digestive enzyme activity, microbial processing, and nutrient absorption (Clements and Raubenheimer 2006). Because we were unable to conduct studies on live sharks, we quantified the activity of three digestive enzymes—gastric pepsin and pancreatic trypsin and lipase—based on the high protein and lipid content of the carnivorous diets of the three shark species studied, and because these enzymes are known to be stable in frozen tissue samples (e.g., Chan et al. 2004). We hypothesized that the mako shark would have higher digestive enzyme activities than the thresher shark and the blue shark at in vivo visceral temperatures. If so, then our results would provide support for the idea that visceral endothermy conveys a potential selective advantage to regionally endothermic sharks by increasing digestive enzyme activities and presumably food processing rates. We compared enzyme activities at 25 °C in the mako with those at 15 °C in the thresher and blue sharks, approximating the average in vivo temperatures of the three species. The mako shark encounters water temperatures of 5–24 °C during dives while maintaining a stable visceral temperature of approximately 25 °C (Carey et al. 1981; Stevens et al. 2010; Abascal et al. 2011). Thresher and blue sharks experience water temperatures of 9–27 and 5–27 °C during dives, respectively (Weng et al. 2005; Cartamil et al. 2010a; Stevens et al. 2010; Patterson et al. 2011). In the northeastern Pacific these two species spend most of their time at ambient temperatures of 14–18 °C (Weng et al. 2005; Cartamil et al. 2010a, b).
We also hypothesized that the Q 10 value, or the ratio between the enzyme activities at 25 and 15 °C, would be higher for the mako shark than for the thresher shark or the blue shark. We predicted that, if the digestive enzymes of endothermic fishes are modified to operate over a narrow range of temperatures, then reducing the assay temperature by 10 °C to a temperature outside of the in vivo temperature range of the mako would alter the enzyme activities by a greater degree than in the two ectothermic species, resulting in higher Q 10 values (Angilletta 2009).
Methods
Experimental procedures
Tissue samples were collected from individual mako, thresher, and blue sharks during annual abundance surveys conducted from 2008 to 2011 in the Southern California Bight by National Marine Fisheries Service personnel of the Southwest Fisheries Science Center, La Jolla, CA. Samples (~5 g) of stomach, pancreas, and intestine (the latter used as a negative control) from each individual [mako (N = 16, 90–193 cm FL, 9–85 kg), blue (N = 16, 72–197 cm FL, 2–44 kg), and thresher (N = 6, 80–177 cm FL, 9–92 kg)] were dissected from sharks that were dead for less than 4 h, frozen in liquid nitrogen, then transported to California State University Fullerton on dry ice, and stored at −80 °C for up to 36 months. Subsamples (~0.5 g) of each tissue were homogenized in a tenfold dilution of 50 mM Tris–HCl buffer (pH 7.8 at 25 °C) using a chilled Kontes Duall ground-glass homogenizer. The homogenate was centrifuged at 0 °C for 10 min at 12,000 g, after which an aliquot (100 μL) of the supernatant was stored at −80 °C for later determination of soluble protein concentration. The remaining supernatant was stored on ice until the appropriate assay for pepsin, trypsin, or lipase was run. The extracted enzymes were activated, if necessary, and then incubated in triplicate at 15 and 25 °C with the appropriate substrate at saturating concentrations (as determined by preliminary assays). Digestive enzyme activities are reported in μmol of substrate converted to product per minute (international units, U) per gram of tissue wet mass, or U g−1 tissue.
Pepsin Assay [modified from Anson (1938)]
Pepsin is a gastric proteolytic enzyme that cleaves peptide bonds between hydrophobic and aromatic amino acids within proteins. In this endpoint assay, pepsin from shark stomach tissue cleaves tyrosine and tryptophan from the hemoglobin substrate. The tyrosine released was quantified by measuring absorbance at 280 nm (A 280) in a diode-array spectrophotometer (Hewlett-Packard 8452A, Palo Alto, CA, USA). A mixture of 100 µL of homogenized tissue supernatant and 100 µL of 2 % horse hemoglobin (0.155 mM final concentration) in 60 mM HCl (pH 2) was incubated at either 15 or 25 °C for 10 min. The reaction was stopped by adding 1000 µL of 5 % trichloroacetic acid, after which the mixture was centrifuged at 6000 g for 6 min at 4 °C, and A 280 of the supernatant measured. The change in A 280 of the sample (relative to a negative control with 50 mM Tris–HCl homogenization buffer substituted for tissue supernatant) during the 10-min incubation period, along with the extinction coefficient of the end product tyrosine (ε tyrosine) of 1.25 cm2 μmol−1 at 280 nm, was used to calculate the units of pepsin activity. One unit (U) of pepsin activity = one μmol of tyrosine liberated min−1.
Trypsin Assay [modified from Preiser et al. (1975) and German et al. (2004)]
Trypsin is a pancreatic serine protease that cleaves peptide bonds after arginine and lysine residues. In this endpoint assay, trypsin from shark pancreas tissue cleaves p-nitroaniline from the substrate N-alpha-benzoyl-l-arginine p-nitroanilide, releasing p-nitroaniline. The assay sensitivity was increased using the Bratton Marshall reaction where a naphthyl group binds to the p-nitroaniline, producing p-nitroaniline-N-1-naphthyl which absorbs light at 550 nm. Trypsin is stored intracellularly in an inactive zymogen form. Therefore, it was first activated by incubating 300 µL of homogenized pancreas supernatant with 45 µL of enterokinase (4 U mL−1 in 40 mM succinate buffer, pH 5.6) for 15 min at room temperature. Next, 25 µL of the activated trypsin supernatant was mixed with 175 µL of 2 mM N-alpha-benzoyl-l-arginine p-nitroanilide (1.75 mM final concentration) suspended in 100 mM Tris buffer (pH 7.8 at 25 °C) and incubated at either 15 or 25 °C for 60 min. The reaction was stopped by adding 200 µL of 0.2 N HCl to the mixture, and then, the following three reagents were added at 3-min intervals: 50 µL of 0.1 % sodium nitrite, 50 µL of 0.5 % ammonium sulfamate, and 50 µL of 0.05 % N-1-naphthylethylenediamine in 95 % ethanol. The reaction proceeded at room temperature for 5 min, after which 100 µL of the supernatant was added to a Falcon #3075 flat-bottom 96-well microplate to measure the change in absorbance of the sample (relative to a negative control using 50 mM Tris–HCl homogenization buffer substituted for tissue supernatant) in a SPECTRAmax 190 microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). We determined that the change in A 550 over time was linear over the entire 60-min reaction period by incubating the substrate (1.75 mM final concentration) with 25 µL of activated pancreas supernatant and stopping the reaction at 10-min intervals for 60 min. The extinction coefficient (ε p-nitroaniline-N-1-naphthyl) × pathlength value of 3.86 mM−1 was calculated from a standard curve and used to convert the change in A 550 during the 60-min incubation period to units of trypsin activity. One unit of trypsin activity = one μmol of p-nitroaniline liberated min−1.
Lipase Assay [modified from Iijima et al. (1998) and German et al. (2004)]
Bile-salt-activated lipase is a carboxylic esterase that cleaves ester bonds to produce an alcohol and a carboxylic acid anion. In this endpoint assay, lipase from shark pancreas tissue cleaves p-nitrophenol from the synthetic lipid substrate p-nitrophenol-myristate. Bile salts must be present to act as a cofactor to emulsify the substrate. Therefore, 20 µL of homogenized pancreas supernatant was added to a solution of 5.3 mM sodium cholate and 0.25 mM 2-methoxyethanol in 250 mM Tris buffer (pH 9.0 at 25 °C) and the mixture was incubated at either 15 or 25 °C for 15 min. Next, p-nitrophenol-myristate (0.54 mM final concentration) was added and incubated for 30 min. The reaction was stopped by adding 5:2 v/v acetone/heptane, and the mixture was centrifuged at 5350 g for 2 min. The lower aqueous layer was placed into a Falcon #3075 flat-bottom 96-well microplate, and the absorbance at 405 nm was measured in a SPECTRAmax 190 microplate spectrophotometer. The extinction coefficient (ε p-nitrophenol) × pathlength value of 2.02 mM−1 was calculated from a standard curve and used to convert the change in A405 of the sample (relative to a negative control using 50 mM Tris–HCl homogenization buffer substituted for tissue supernatant) over the 15-min incubation period to units of lipase activity. One unit of lipase activity = one μmol of p-nitrophenol liberated min−1.
Q 10 measurements
In order to quantify the effects of temperature on the activity of each digestive enzyme in each species, the Q 10 for each of the three enzymes was calculated for the temperature range of 15–25 °C using the equation Q 10 = (R 25 °C/R 15 °C), where R = enzyme activity at the indicated temperature. Because the enzyme activities at the two temperatures were not independent, a Q 10 value was obtained for each enzyme in each individual shark, and then, species means were calculated.
Protein assay
The protein concentration was determined for each tissue sample (Bradford method, Bio-Rad kit # 500-0001, Hercules, CA, USA) and used to test for correlations between digestive enzyme activity and the amount of protein in the supernatant, which would indicate inconsistent tissue sample quality or errors in sample preparation or enzyme assay procedures. Tissue supernatant was serially diluted up to 100-fold in 50 mM Tris buffer (pH 7.8 at 25 °C). Then, 10 µL of each diluted homogenate was mixed with 200 µL of Bio-Rad dye reagent, incubated at room temperature for 5 min, and A 595 was recorded using a SPECTRAmax 190 microplate spectrophotometer. Only the absorbance values that fell within the linear range (from 0 to 1.0 mg ml−1) of the bovine gamma globulin standard curve were used to determine the sample protein concentration (mg protein g−1 tissue).
Statistical analysis
In order to meet the assumptions of normality and homoscedasticity, data were log-, inverse-, or reciprocal-transformed for the statistical tests. We used Pearson’s correlation coefficients to test for significant correlations between the digestive enzyme activity and protein concentration in each tissue type of each species, and between digestive enzyme activity and shark mass for each species. Because no significant size or protein concentration effects were found within species, we tested for effects of species, temperature, and their interaction for each of the three enzyme activities (U g−1) using a two-way mixed-model analysis of variance (ANOVA) and Student’s t test post hoc tests using a Bonferroni correction in SAS (v. 9.1.3; Cary, NC, USA). Mean Q 10 values for each enzyme were tested for interspecific differences using ANOVA and Student’s t test post hoc tests with a Bonferroni correction. Differences were considered significant if P < 0.05 for ANOVA tests, P < 0.0033 for enzyme activity post hoc tests, and P < 0.025 for Q 10 post hoc tests.
Results
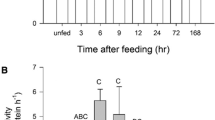
Enzyme activities (U g−1) and Q 10 values are summarized in Fig. 1 and Table 1, respectively. The values of digestive enzyme activities for each species at its estimated average in vivo temperature are indicated by solid bars (Fig. 1). Enzyme activity in each tissue, for each species, was independent of shark mass for the size range of sharks used in this study. Enzyme activity did not correlate with protein concentration for any tissue or species. Intestine tissue was used as a negative control in all digestive enzyme assays and yielded no detectable activities (data not shown).
Mean (± s.e.m.) pepsin (a), trypsin (b), and lipase (c) activity (units per gram wet tissue weight) in the shortfin mako shark (N = 16), the thresher shark (N = 6), and the blue shark (N = 16) at 15 °C (blue) and 25 °C (red). Solid bars indicate activity at the estimated mean in vivo temperatures of the three species, and letters a–d denote means that differ significantly for each enzyme (Student’s t test post hoc tests on log-transformed data with a Bonferroni correction, P < 0.0033). (Color figure online)
Pepsin
The effect of temperature on pepsin activity differed among the three species studied based on the significant interaction between temperature and species (F 1,2 = 8.18, P = 0.0012). Pepsin activity in all three species studied was significantly greater at 25 °C than at 15 °C (F 1 = 646.57, P < 0.0001). Pepsin activities in the mako and blue shark were significantly greater than in the thresher shark at both 25 °C (t 2 = 21.25, P < 0.0001, and t 2 = 23.29, P < 0.0001, respectively) and 15 °C (t 2 = 21.49, P < 0.0001, and t 2 = 27.38, P < 0.0001, respectively). Pepsin activity at 15 °C was significantly greater in the blue shark than in the mako shark (t 2 = 7.98, P < 0.0001). At in vivo temperatures, pepsin activity (Fig. 1a) in the mako at 25 °C was significantly greater than that in the thresher at 15 °C (t 2 = 36.22, P < 0.0001) and in the blue shark at 15 °C (t 2 = 11.96, P < 0.0001). The pepsin Q 10 value (Table 1) for the mako did not differ significantly from that of the thresher shark, but in both species it was significantly greater than in the blue shark (t 2 = 3.64, P = 0.0005 and t 2 = 3.10, P = 0.0027, respectively).
Trypsin
There was a significant interaction between temperature and species on trypsin activity (F 1,2 = 7.63, P = 0.0018). Trypsin activity in the mako, thresher, and blue sharks was significantly greater at 25 °C than at 15 °C (F 1 = 115.26, P < 0.0001). Trypsin activity was significantly greater in the mako than in the thresher and blue sharks at 25 °C (t 2 = 6.22, P < 0.0001 and t 2 = 6.66, P < 0.0001, respectively), but did not differ significantly among the three species at 15 °C. At in vivo temperatures (Fig. 1b), trypsin activity in the mako at 25 °C was significantly greater than in the thresher shark at 15 °C (t 15 = 10.31, P < 0.0001) and the blue shark at 15 °C (t 15 = 13.06, P < 0.0001). The Q 10 value for trypsin in the mako did not differ from that in the thresher or blue sharks, but Q 10 in the thresher was significantly greater than in the blue shark (t 2 = 3.19, P = 0.0021; Table 1). The reciprocal-transformed trypsin Q 10 values failed Levene’s test (P = 0.0011), but passed Welch’s test for homogeneity of variance (F 2 = 3.30, P = 0.0521). Because all three species had very low trypsin activities at 15 °C, all trypsin Q 10 values were high (Table 1).
Lipase
There was no significant interaction between temperature and species on lipase activity. Lipase activity in the mako and blue sharks, but not the thresher shark, was significantly greater at 25 °C than at 15 °C (F 1 = 91.62, P < 0.0001). There was a significant effect of species on lipase activity (F 2 = 287.00, P < 0.0001). Lipase activity at 25 °C did not differ significantly between the mako and blue shark, but in both species it was significantly greater than in the thresher shark (t 2 = 17.23, P < 0.0001 and t 2 = 16.03, P < 0.0001, respectively). Lipase activity at 15 °C was significantly greater in the mako than in the blue shark (t 2 = 3.66, P = 0.0008), which was significantly greater than in the thresher shark (t 2 = 13.12, P < 0.0001). Post hoc tests indicated that lipase activity in the mako was higher than in the thresher and blue sharks at in vivo temperatures (Fig. 1c). Lipase activity was significantly greater in the mako at 25 °C than in the thresher shark at 15 °C (t 15 = 20.88, P < 0.0001) and the blue shark at 15 °C (t 15 = 10.51, P < 0.0001). There was a significant interspecific difference in the lipase Q 10 values (F 2 = 37.46, P < 0.0001; Table 1). The lipase Q 10 did not differ between the lamniform mako and thresher sharks, but those values were significantly lower than in the blue shark (t 2 = 7.86, P < 0.0001; and t 2 = 8.21, P < 0.0001, respectively).
Discussion
Interspecific comparisons of digestive enzyme activities
This is the first study to test for physiological benefits of visceral endothermy in elasmobranchs by comparing closely related species that can and cannot elevate visceral temperatures above ambient water temperature. At in vivo temperatures, the mako shark had significantly higher activities of all three digestive enzymes studied than did the thresher and blue sharks, supporting the hypothesis that regional endothermy increases the physiological performance of the warmed visceral organs in this species (Carey and Teal 1966; Carey et al. 1981, 1982, 1984; Stevens and McLeese 1984; Block and Carey 1985; Block and Finnerty 1994; Goldman 1997). At the estimated in vivo temperatures, mean pepsin activity in the mako shark was 6 times higher than in the thresher and 1.7 times higher than in the blue shark; trypsin activity in the mako was 27 times higher than in the thresher and 12 times higher than in the blue shark; and lipase activity in the mako was 3.2 times higher than in the thresher and 1.6 times higher than in the blue shark. All enzyme activities for a given species were higher at 25 °C than at 15 °C, except lipase in the thresher shark, possibly due to the small sample size for thresher sharks in this study. The within-species temperature effects were expected, given that increasing temperature increases molecular motion resulting in higher reaction rates. Nevertheless, when compared at 25 °C, all enzyme activities in the mako were significantly higher than in the thresher shark, and trypsin activity was higher in the mako than in the blue shark, which indicates that the high digestive enzyme activities in the mako shark result from more than a simple temperature effect.
Enzyme-specific activity is the product of the substrate turnover rate of a single enzyme molecule and the number of molecules present. If the three-dimensional structure and substrate turnover rates for each of the three enzymes are conserved among species, then differences in enzyme activity at a given temperature should represent differences in the amount of enzyme present in the digestive tract at the time of sampling. The consistently low pepsin, trypsin, and lipase activities in the thresher shark would then mean that this species had fewer digestive enzymes per gram of tissue than did the mako and blue sharks sampled. The quantity of pepsin, trypsin, and lipase in the tissues at the time of sampling is a function of how much enzyme is released during feeding and the rates of enzyme replenishment within the digestive tissues, which are unknown in sharks (Secor 2009). If an individual shark had fed just prior to sampling, it is likely that the digestive enzyme activities measured would be lower than in a shark that had not recently fed. All of the sharks used in this study were caught on baited hooks, implying that their stomachs were not full prior to capture. For the few individual sharks for which stomach fullness data were also obtained (A. Preti pers. comm.), there was no correlation between enzyme activity and stomach fullness. The measured digestive enzyme activities may also have been influenced by the recent diet of individual sharks, if the sharks adjust the relative amounts of digestive enzymes that they synthesize and secrete to digest a particular prey species most effectively (Matus de la Parra et al. 2007; Secor 2009). Such differences among individuals may explain the large variation in enzyme activities within species found in this and other studies of fish digestive enzyme activities (e.g., Chan et al. 2004; Matus de la Parra et al. 2007).
It is possible that the pepsin and lipase activities are similar in the mako and blue sharks, but differ in the thresher shark, because the feeding ecology of these three species has a greater influence on digestive enzyme activity than their phylogenetic relationship. The consistently lower digestive enzyme activities in the thresher shark could be explained if prey species of the thresher were easier to digest than prey of the mako and blue sharks, for example because of lower lipid content or lower amount of indigestible material such as bone. Stomach content data for sharks collected during the longline surveys in which the individuals used in this study were collected indicate that: The local thresher shark population eats primarily anchovy and sardine, the blue shark eats mostly cephalopods, and the mako shark consumes nearly equal amounts of cephalopods and pelagic teleosts (Preti et al. 2001, 2012). Clark et al. (2010) determined that market squid, Loligo opalescens, has a lower nutritional content (~2 % lipid, 12 % protein, 1.5 % ash) and is easier to digest than Pacific sardine, Sardinops sagax (~15 % lipid, 17 % protein, 2.5 % ash). More information is needed on other factors that contribute to food processing to assess the potential interacting effects of temperature, diet, absorption rate, and assimilation efficiency in these sharks.
We could find no previous data for the activities of pepsin, trypsin, or lipase in any elasmobranch species with which to compare the enzyme activities measured in this study, but those values are within an order of magnitude of values measured in several teleost fishes using similar methods (e.g., Chan et al. 2004; German et al. 2004; Neumann 2009; Odedeyi and Fagbenro 2010). However, digestive enzyme activities reported for the Pacific bluefin tuna, Thunnus orientalis, a teleost that has evolved visceral endothermy, are two to three orders of magnitude higher than measured in the sharks, but increase similarly with temperature (Matus de la Parra et al. 2007). Stevens and McLeese (1984) found that trypsin and chymotrypsin activities increased threefold over the range of visceral temperatures (10–30 °C) measured in the endothermic Atlantic bluefin tuna, Thunnus thynnus. In albacore, Thunnus alalunga, and yellowfin, T. albacares, tunas, eastern Pacific bonito, Sarda chiliensis, and chub mackerel, Scomber japonicus, Neumann (2009) measured higher pepsin, trypsin, and lipase activities at higher temperatures (15–25 °C). However, unlike the sharks in the present study, the activities for the albacore, a viscerally endothermic species, were not always higher than those for the ectothermic scombrid species at in vivo temperatures.
Although the ectothermic sharks cannot elevate visceral temperatures above ambient temperature, they could increase food processing rates by moving into warmer waters and thermoregulating behaviorally. Based on the results of the present study, the blue shark could achieve the same pepsin and lipase activities as the mako shark if it remained in 25 °C water, but its trypsin activity would still be less than half that of the mako shark. The thresher could achieve activities similar to the mako shark only by remaining in 25 °C water and synthesizing 2.5 times as many enzymes within the digestive tract. Acoustic tracking data show that thresher and blue sharks spend up to 15 % of their time in waters below 10 °C, whereas endothermic white, porbeagle, and salmon sharks spend up to 30, 80, and 90 % of their time, respectively, in waters below 10 °C (Carey and Scharold 1990; Weng et al. 2005, 2007a, b; Nasby-Lucas et al. 2009; Pade et al. 2009; Cartamil et al. 2010a). Therefore, visceral endothermy should allow lamnid sharks to maintain high food processing rates to a greater degree than possible by behavioral thermoregulation alone and thus to spend more time in cold waters without compromising digestive system function.
Thermal sensitivity of digestive enzymes
We expected that, among the three species studied, the endothermic mako shark would have the highest Q 10 values because its digestive enzymes should be optimized to operate at a stable temperature. Lower Q 10 values were expected in the two ectothermic sharks because their enzymes should be adapted to operate effectively over a wider range of temperatures (Angilletta 2009). Instead, we found that the mako and thresher sharks have higher Q 10 values than the blue shark for pepsin and trypsin and that these two lamniform species have lower Q 10 values than the blue shark for lipase. The similar Q 10 values in the mako and thresher sharks, which differ from those in the blue shark, could reflect the phylogenetic relationships among the three species (Velez-Zuazo and Agnarsson 2011). The larger genetic distance between the blue shark and the two lamniform sharks may be reflected in the blue shark having digestive enzymes with a different amino acid sequence, conferring a different thermal sensitivity, than the two lamniform species. Alternatively, the 10 °C temperature range over which Q 10 values were estimated in this study may not be large enough to reveal the predicted interspecific effects.
Benefits of visceral endothermy
The results of this study support the hypothesis that visceral endothermy enhances physiological performance of the digestive tract in lamnid sharks. Proteases such as pepsin, trypsin, and chymotrypsin hydrolyze the peptide bonds within proteins, and this process is thought to be the rate-limiting step in protein degradation and absorption during digestion (reviewed in Secor 2009). Therefore, by increasing the activity of digestive enzymes, particularly trypsin which had the highest Q 10 value, visceral endothermy should increase the food processing rate in the mako shark without incurring the additional costs associated with synthesizing more enzymes or remaining in warm waters. Higher digestive enzyme activities would allow the mako shark, and presumably other lamnid sharks, to digest more proteins and lipids per day than can individuals from closely related species (e.g., the thresher shark) or sympatric ectothermic species (e.g., the blue shark). Consequently, lamnid sharks could have more nutrients available to fuel their high metabolic rates, and excess nutrients could be assimilated into somatic tissues, lipid reserves for migrations, or yolk for oophagous pups in pregnant females (Carey et al. 1981; Gilmore 1993). A similar benefit has been proposed for the Pacific bluefin tuna, which, like other endothermic tunas, has a higher standard metabolic rate than do similarly sized ectothermic species at the same water temperature (Sepulveda and Dickson 2000; Sepulveda et al. 2003; Blank et al. 2007). Although no metabolic rate data exist for thresher or blue sharks, the standard metabolic rate of the mako shark is greater than that of any other shark measured to date, including ectothermic lemon, Negaprion brevirostris, scalloped hammerhead, Sphyrna lewini, and leopard sharks (Graham et al. 1990; reviewed in Bernal et al. 2001; Sepulveda et al. 2007).
It is possible that regional endothermy was important for the evolution and radiation of the ancestral lamnid sharks during the Miocene epoch approximately 25 million years ago (Purdy 1996; Dickson and Graham 2004). During that time, the Tethys Sea, a large tropical habitat that had existed for approximately 70 million years, was greatly reduced, and oceanic cooling at high latitudes created a latitudinal temperature gradient, causing a shift in wind patterns and the formation of oceanic gyres (Purdy 1996; Martin 2003; Lyle et al. 2008). The upwelling of cold nutrient-rich water into warm habitats resulted in localized areas of high primary productivity (Purdy 1996; Martin 2003; Lyle et al. 2008). The reduction in tropical habitat and ensuing shifts in prey availability may have forced the ancestral lamnid sharks that had evolved in tropical waters (e.g., Carcharodon megalodon and Isurus hastalis) to pursue their prey into temperate waters and either adapt to decreased temperatures or use physiological and behavioral strategies to modulate changes in tissue temperatures (Purdy 1996; Dickson and Graham 2004; Lyle et al. 2008). In sharks with the ability to retain heat within the viscera, changes in the temperature and physiological function of the digestive tract would have been minimized during extended excursions into cold water, while a competing ectothermic shark that was reliant upon behavioral thermoregulation would have had to return to warmer water to increase its rate of digestion. Thus, visceral endothermy would have freed ancestral lamnid sharks from the constraint of returning to warm water to digest prey rapidly after foraging, allowing them to exploit cold habitats and expand their thermal niche.
Abbreviations
- A xxx :
-
Absorbance at a specific wavelength of XXXnm
- ANOVA:
-
Analysis of variance
- ε :
-
Extinction coefficient
- FL:
-
Fork length
- Q 10 :
-
Temperature coefficient = (enzyme activity25 °C/enzyme activity15 °C)
- U :
-
Unit of enzyme activity = μmol of substrate converted to product min−1
- s.e.m.:
-
Standard error of the mean
References
Abascal FJ, Quintans M, Ramos-Cartelle A, Mejuto J (2011) Movement and environmental preferences of the shortfin mako, Isurus oxyrinchus, in the southeastern Pacific Ocean. Mar Biol 158:1175–1184
Angilletta M (2009) Thermal adaptation: a theoretical and empirical synthesis. Oxford University Press, Oxford
Anson ML (1938) The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J Gen Physiol 22:79–89
Bernal D, Sepulveda CA (2005) Evidence for temperature elevation in the aerobic musculature of the common thresher shark, Alopias vulpinus. Copeia 2005:146–151
Bernal D, Sepulveda CA, Graham JB (2001) Water-tunnel studies of heat balance in swimming mako sharks. J Exp Biol 204:4043–4054
Blank JM, Morrissette JM, Farwell CJ, Price M, Schallert RJ, Block BA (2007) Temperature effects on metabolic rate of juvenile Pacific bluefin tuna Thunnus orientalis. J Exp Biol 210:4254–4261
Block BA, Carey FG (1985) Warm brain and eye temperatures in sharks. J Comp Physiol B 156:229–236
Block BA, Finnerty JR (1994) Endothermy in fishes: a phylogenetic analysis of constraints, predispositions, and selection pressures. Environ Biol Fish 40:283–302
Block BA, Dewar H, Blackwell SB, Williams TD, Prince ED, Farwell CJ, Boustany A, Teo SLH, Seitz A, Walli A, Fudge D (2001) Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science 293:1310–1314
Boustany AM, Davis SF, Pyle P, Anderson SD, LeBoeuf BJ, Block BA (2002) Expanded niche for white sharks. Nature 415:35–36
Boustany AM, Matteson R, Castleton M, Farwell C, Block BA (2010) Movements of Pacific bluefin tuna (Thunnus orientalis) in the eastern North Pacific revealed with archival tags. Prog Oceanogr 86:94–104
Brill RW, Dewar H, Graham JB (1994) Basic concepts relevant to heat transfer in fishes, and their use in measuring the physiological thermoregulatory abilities of tunas. Environ Biol Fish 40:109–124
Carey FG, Lawson KD (1973) Temperature regulation in free-swimming bluefin tuna. Comp Biochem Physiol A 44:375–392
Carey FG, Scharold JV (1990) Movement of blue sharks (Prionace glauca) in depth and course. Mar Biol 106:329–342
Carey FG, Teal JM (1966) Heat conservation in tuna fish muscle. Proc Natl Acad Sci USA 56:1464–1469
Carey FG, Teal JM (1969) Mako and porbeagle: warm-bodied sharks. Comp Biochem Physiol A 28:199–204
Carey FG, Teal JM, Kanwisher JW, Lawson KD, Beckett KS (1971) Warm-bodied fish. Am Zool 11:137–145
Carey FG, Teal JM, Kanwisher JW (1981) The visceral temperatures of mackerel sharks (Lamnidae). Physiol Zool 54:334–344
Carey FG, Kanwisher JW, Brazier O, Gabrielson G, Casey JG, Pratt HL (1982) Temperature and activities of a white shark, Carcharodon carcharias. Copeia 1982:254–260
Carey FG, Kanwisher JW, Stevens ED (1984) Bluefin tuna warm their viscera during digestion. J Exp Biol 109:1–20
Carey FG, Casey JG, Pratt HL, Urquhart D, McCosker JE (1985) Temperature, heat production and heat exchange in lamnid sharks. Mem S Calif Acad Sci 9:92–108
Cartamil DP, Wegner NC, Aalbers S, Sepulveda CA, Baquero A, Graham JB (2010a) Diel movement patterns and habitat preferences of the common thresher shark (Alopias vulpinus) in the Southern California Bight. Mar Fresh Res 61:596–604
Cartamil DP, Wegner NC, Kacev D, Ben-Aderet N, Kohin S, Graham JB (2010b) Movement patterns and nursery habitat preferences of juvenile thresher sharks (Alopias vulpinus) in the Southern California Bight. Mar Ecol Prog Ser 404:249–258
Chan A, Horn MH, Dickson KA, Gawlicka A (2004) Digestive enzyme activities in carnivores and herbivores: comparisons among four closely related prickleback fishes (Teleostei: Stichaeidae) from a California rocky intertidal habitat. J Fish Biol 65:848–858
Clark TD, Brandt WT, Nogueira J, Rodriguez LE, Price M, Farwell CJ, Block BA (2010) Postprandial metabolism of Pacific bluefin tuna (Thunnus orientalis). J Exp Biol 213:2379–2385
Clements KD, Raubenheimer D (2006) Feeding and Nutrition. In: Evans DH, Claiborne JB (eds) The physiology of fishes, 3rd edn. CRC Press, Boca Raton, pp 47–82
Compagno LJV (1984) Sharks of the world: an annotated and illustrated catalogue of shark species known to date. Food and agriculture organization of the United Nations species catalogue, vol 4, part 2, Carcharhiniformes. FAO Fish Syn 125:251–265
Compagno LJV (1990) Relationships of the megamouth shark, Megachasma pelagios (Lamniformes: Megachasmidae), with comments on its feeding habits. NOAA Tech Rep NMFS 90:357–379
Compagno LJV (2001) Sharks of the world: an annotated and illustrated catalogue of shark species known to date. Food and agriculture organization of the united nations species catalogue, vol 2. bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes, Orectolobiformes). FAO Fish Syn 1:51–125
Dickson KA, Graham JB (2004) Evolution and consequences of endothermy in fishes. Physiol Biochem Zool 77:998–1018
Fudge DS, Stevens ED (1996) The visceral retia mirabilia of tuna and sharks: an annotated translation and discussion of the Eschricht and Muller 1835 paper and related papers. Guelph Ichthyol Rev 4:1–328
German DP, Horn MH, Gawlicka A (2004) Digestive enzyme activities in herbivorous and carnivorous prickleback fishes (Teleostei: Stichaeidae): ontogenetic, dietary, and phylogenetic effects. Physiol Biochem Zool 77:789–804
Gilmore GR (1993) Reproductive biology of lamnoid sharks. Environ Biol Fish 38:95–114
Goldman KJ (1997) Regulation of body temperature in the white shark, Carcharodon carcharias. J Comp Physiol B 167:423–429
Goldman KJ, Anderson SD, Latour RJ, Musick JA (2004) Homeothermy in adult salmon sharks, Lamna ditropis. Environ Biol Fish 71:403–411
Graham JB, Dewar H, Lai NC, Lowell WR, Arce SM (1990) Aspects of shark swimming performance determined using a large water tunnel. J Exp Biol 151:175–192
Holts DB, Bedford DW (1993) Horizontal and vertical movements of the shortfin mako shark, Isurus oxyrinchus, in the Southern California Bight. Aust J Mar Fresh Res 44:901–909
Iijima N, Tanaka S, Ota Y (1998) Purification and characterization of bile salt-activated lipase from the hepatopancreas of red sea bream, Pagrus major. Fish Physiol Biochem 18:59–69
Lyle M, Barron J, Bralower TJ, Huber M, Olivarez-Lyle A, Ravelo AC, Rea DK, Wilson PA (2008) Pacific Ocean and Cenozoic evolution of climate. Rev Geophys 46:1–47
Maia A, Quieroz N, Corriea J, Cabral H (2006) Food habits of the shortfin mako shark, Isurus oxyrinchus, off the southwest coast of Portugal. Environ Biol Fish 77:57–167
Mannan A, Fraser DI, Dyer WJ (1961) Proximate composition of Canadian Atlantic fish. II. Mackerel, tuna and swordfish. J Fish Res Bd Can 18:495–499
Martin RA (2003) Paleoecology of Megalodon and the White Shark. World Wide Web Publication. http://www.elasmo-research.org/education/evolution/paleoecology.htm. Accessed 15 March 2012
Matus de la Parra A, Rosas A, Lazo JP, Viana MT (2007) Partial characterization of the digestive enzymes of Pacific bluefin tuna Thunnus orientalis under culture conditions. Fish Physiol Biochem 33:223–231
McChord ME, Campana SE (2003) A quantitative assessment of the diet of the blue shark (Prionace glauca) off Nova Scotia, Canada. J NW Atl Fish Sci 32:57–63
Nasby-Lucas N, Dewar H, Lam CH, Goldman KJ, Domeier ML (2009) White shark offshore habitat: a behavioral and environmental characterization of the eastern Pacific shared offshore foraging area. PLoS ONE 4:1–14
Naylor GJP, Martin AP, Mattison EG, Brown WM (1997) Interrelationships of lamniform sharks: Testing phylogenetic hypotheses with sequence data. In: Kocher TD, Stepien CA (eds) Molecular systematics of fishes. Academic Press, San Diego, pp 199–218
Neumann D (2009) A comparative study of digestive enzyme activities in tunas and other scombrid fishes. Master’s Thesis, California State University, Fullerton
Odedeyi DO, Fagbenro OA (2010) Feeding habits and digestive enzymes in the gut of Mormyrus rume (Valenciennes 1846) (Osteichthyes Mormyridae). Trop Zool 23:75–89
Pade NG, Quieroz N, Humphries NE, Witt MJ, Jones CS, Noble LR, Sims DW (2009) First results from satellite-linked archival tagging of porbeagle shark, Lamna nasus: area fidelity, wide-scale movements and plasticity in diel depth changes. J Exp Mar Biol Ecol 370:64–74
Patterson JC, Sepulveda CA, Bernal D (2011) The vascular morphology and in vivo muscle temperatures of thresher sharks (Alopiidae). J Morph 272:1353–1364
Preiser H, Schmitz J, Maestracci D, Crane RK (1975) Modification of an assay for trypsin and its application for the estimation of endopeptidase. Clin Chim Acta 59:169–175
Preti A, Smith SE, Ramon DA (2001) Feeding habits of the common thresher shark (Alopias vulpinus) from the California-based drift gill net fishery, 1998–1999. Cal Coop Ocean Fish Invest Rep 42:145–152
Preti A, Soykan CA, Dewar H, Wells RJD, Spear N, Kohin S (2012) Comparative feeding ecology of shortfin mako, blue and thresher sharks in the California current. Environ Biol Fish 95:127–146
Purdy RW (1996) Paleoecology of fossil white sharks. In: Klimley AP, Ainley DG (eds) Great white sharks: the biology of Carcharodon carcharias. Academic Press, San Diego, pp 67–78
Runcie RM, Dewar H, Hawn DR, Frank LR, Dickson KA (2009) Evidence for cranial endothermy in the opah (Lampris guttatus). J Exp Biol 212:461–470
Sciarrotta TR, Nelson DR (1977) Diel behavior of the blue shark, Prionace glauca, near Santa Catalina Island, California. Fish Bull US 57:519–528
Secor SM (2009) Specific dynamic action: a review of the postprandial metabolic response. J Comp Physiol B 179:1–56
Sepulveda CA, Dickson KA (2000) Maximum sustainable speeds and cost of swimming in juvenile kawakawa tuna, Euthynnus affinis, and chub mackerel, Scomber japonicus. J Exp Biol 203:3089–3101
Sepulveda CA, Dickson KA, Graham JB (2003) Swimming performance studies on the eastern Pacific bonito Sarda chiliensis, a close relative of the tunas (family Scombridae) I. Energetics. J Exp Biol 206:2739–2748
Sepulveda CA, Kohin S, Chan C, Vetter R, Graham JB (2004) Movement patterns, depth preferences, and stomach temperatures of free-swimming juvenile mako sharks, Isurus oxyrinchus, in the Southern California Bight. Mar Biol 145:191–199
Sepulveda CA, Wegner NC, Bernal D, Graham JB (2005) The red muscle morphology of the thresher sharks (family Alopiidae). J Exp Biol 208:4255–4261
Sepulveda CA, Graham JB, Bernal B (2007) Aerobic metabolic rates of swimming juvenile mako sharks, Isurus oxyrinchus. Mar Biol 152:1087–1094
Shimada K (2005) Phylogeny of lamniform sharks (Chondrichthyes: Elasmobranchii) and the contribution of dental characteristics to lamniform systematics. Paleontol Res 9:55–72
Stevens ED, McLeese JM (1984) Why bluefin tuna have warm tummies: temperature effects on trypsin and chymotrypsin. Am J Physiol 246:R487–R494
Stevens JD, Bradford RW, West GJ (2010) Satellite tagging of blue sharks (Prionace glauca) and other pelagic sharks off eastern Australia: depth, behaviour, temperature experience and movements. Mar Biol 157:575–591
Velez-Zuazo X, Agnarsson I (2011) Shark tales: a molecular species-level phylogeny of sharks (Selachimorpha, Chondrichthyes). Mol Phylogenet Evol 58:207–217
Weng KC, Castilho PC, Morrissette JM, Landeira-Fernandez AM, Holts DB, Schallert RJ, Goldman KJ, Block BA (2005) Satellite tagging and cardiac physiology reveal niche expansion in salmon sharks. Science 310:104–106
Weng KC, Boustany AM, Pyle P, Anderson SA, Brown A, Block BA (2007a) Migration and habitat of white sharks (Carcharodon carcharias) in the eastern Pacific Ocean. Mar Biol 152:877–894
Weng KC, O’Sullivan JB, Lowe CG, Winkler CE, Dewar HE, Block BA (2007b) Movements, behavior and habitat preferences of juvenile white sharks Carcharodon carcharias in the eastern Pacific. Mar Ecol Prog Ser 338:211–224
Acknowledgments
This research was supported by a California State University Fullerton (CSUF) Department of Biological Science graduate student research grant (K.C.N.) and a CSUF Senior Faculty Research Award (K.A.D.). We thank Christopher Kehrier and Scott Holtz for help with collecting tissue samples, CSUF undergraduate students Ali Forghani and Ramtin Khanipour who collected preliminary data, the captains and crews of the F/V Ventura II and the F/V Outer Banks, and Suzanne Kohin of the National Marine Fisheries Service Southwest Fisheries Science Center, for allowing K.C.N. to participate in the annual juvenile shark abundance surveys in the Southern California Bight. We thank Sean Walker for assistance with the statistical analyses.
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal rights statement
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in this study were approved by the CSUF Institutional Animal Care and Use Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Newton, K.C., Wraith, J. & Dickson, K.A. Digestive enzyme activities are higher in the shortfin mako shark, Isurus oxyrinchus, than in ectothermic sharks as a result of visceral endothermy. Fish Physiol Biochem 41, 887–898 (2015). https://doi.org/10.1007/s10695-015-0055-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-015-0055-8