Abstract
In this study, the effects of oral administration of different levels of Dunaliella salina (a natural β-carotene source) on growth parameters, immunological and hematological indices, as well as skin carotenoids, of Heros severus were investigated. One hundred and eighty H. severus weighing 27 ± 0.5 g were divided randomly into four groups in triplicate (15 fish in each replicate). Groups 1–4 received food supplemented with 0, 50, 100 and 200 mg kg−1 D. salina powder, respectively. After 6 weeks, the growth parameters were compared among the groups. Blood samples were taken from each group, and hematological parameters including red blood cell count (RBC), white blood cell count (WBC), hematocrit (PCV), hemoglobin (Hb) and immunological indices (serum and mucus lysozyme and bactericidal activity, resistance against Aeromonas hydrophila infection) as well as carotenoid content of skin were evaluated. Results showed that some growth indices increased significantly in fish fed with 100 and 200 mg kg−1 D. salina-supplemented food (P < 0.05). Although serum lysozyme activity was increased in fish fed with food supplemented with 100 and 200 mg kg−1 D. salina (P < 0.05), no significant change was observed in serum and mucus bactericidal activity and mucus lysozyme activity among the groups (P > 0.05). Most of the hematological parameters such as WBC, RBC, PCV and Hb significantly increased in D. salina-treated fish compared with controls (P < 0.05). Mortality induced after challenge with A. hydrophila in 200 mg kg−1 D. salina-treated fish was 36.67 %, which significantly decreased compared with control (P < 0.05). Skin carotenoid content in all D. salina treatments was statistically higher than that of control (P < 0.05). Conclusively, D. salina as a food additive can affect positively the growth, immunological and hematological parameters of H. severus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Present trend of intensification of aquaculture is a major concern for the outbreak of disease as fish are predisposed to stress and subsequent infection by various pathogens (Karunasagar et al. 1997). Various chemotherapeutics have been used to control bacterial infections in fish. As a result, the incidence of antibiotic-resistant bacteria has become a major problem in aquaculture (Yuan et al. 2007). Hence, in recent years, increasing attention has been given to the use of immunostimulants in the fish farms (Alishahi et al. 2010).
The state of immunocompetence is a sensitive indicator of nutritional status, which comprises both immune response and disease resistance of fish (Kumar et al. 2005). Carotenoids are widespread natural fat-soluble pigments that are typified by lycopene and bicyclic β-carotene, which is an important source of vitamin A for animals (Oslon 1989). Carotenoids have received increasing attention in the recent years due to their reported health benefits to human beings (Ashish et al. 2007; Kumar et al. 2005). Effects of carotenoids on aquatic animals are multifaceted: increase the larval growth and survival (Amar et al. 2004), enhance the performance of brood stock (Supamattaya et al. 2005) and larvae (Wyban et al. 1997), increase the resistance to various diseases (Amar et al. 2001; Supamattaya et al. 2005), as well as give the skin the yellow and red colors (Kop and Durmaz 2008).
The use of microalgal biomass has been recently investigated with regard to its potential as a coloring agent as well as immunostimulants (Gourveia et al. 1997; Raymundo et al. 2005; Supamattaya et al. 2005). But the use of synthetic pigment sources is more common because they are easy to obtain (Sales and Janssens 2003).
In some fish species such as ornamental fish and salmonids, skin and fillet color constitutes an important quality parameter (Sigurgisladottir et al. 1997), which improves fish coloration and increases directly the economical value of fish (Cejas et al. 2003). Since fish, like other animals, are unable to synthesize carotenoids de novo (Goodwin 1984), skin color is highly dependent on the carotenoids present in the diet. Dunaliella salina as a natural source of β-carotene can improve skin and fillet pigmentation in fish.
Dunaliella salina is a halotolerant unicellular microalgae—bi-flagellate, naked, green alga (Chlorophyta, Chlorophyceae)—which is widely distributed and found in hypersaline waters (Borowitzka and Borowitzka 1988). Dunaliella spp. are grown as a food source in aquaculture, and D. salina is the richest algal source of β-carotene and glycerol (Ben-Amotz and Avron 1983; Raja et al. 2007). D. salina can produce β-carotene up to 14 % of its dry weight under stress conditions such as high salinity, light and temperature, as well as nutrient limitation.
Recently, ornamental fish industry has been increasingly developed in Iran. Heros severus is one of the most important ornamental fish in Iran. Although this species is native of Central America, it has become widespread because of important priority such as bright yellowish color, calm behavior, fast adaptation to environment, comparatively easy reproduction (Morad khany et al. 2008) and associated with densely vegetated areas and feeds on small invertebrates and plant material (lowe-McConnell 1969; Merigoux et al. 1998). Besides, bacterial infections (especially motile Aeromonas infection) are very common health problems in this species in Iran, which lead the aquarists to use large amount of antibiotics. So substances that induce skin pigmentation and stimulate immune response and resistance against bacterial infections of this species can be the best choice as food additive.
Decision on the source of pigments to be used in fish diet is made based on the price and efficiency of them (Baker et al. 2002).
Although carotenoids are known to enhance immune function and disease resistance in higher animal (Bendich and Shapiro 1986; Thompson et al. 1995), little information is available for aquatic animal especially for fish (Amar et al. 2004; Ashish et al. 2007). Therefore, in this study, the effects of different levels of D. salina on skin carotenoids, growth performance, immune responses, hematological parameters and disease resistance of H. severus were evaluated.
Materials and methods
Fish
Severum fish (Heros severus) weighing 23.1 ± 2.3 g (Mean± SD), which have no color on their body, were obtained from an ornamental fish hatchery in Ahvaz, Iran. Fish were transferred to aquarium room of the fish health laboratory in the Faculty of Veterinary Medicine, Shahid Chamran University, Ahvaz, Iran. Fish were maintained, on a commercial diet (Biomar, France), without any supplementation for 1 month to acclimate and ascertain the constant color of fish, so at the initial stage of the study, the mean weight of fish was 27 ± 0.5 g.
Dunaliella salina extract
The native isolate of D. salina coded as Dunaliella M1 was cultivated within a 14-l photobioreactor using the modified Junson medium described by Hejazi et al. (2010). NaCl concentration of the medium was 1.5 M. The photobioreactor was illuminated by Osram lamps (SPOT R80 NATURA E27/ESW) with the average light intensity of 400 μmol m−2 s−1. The temperature and pH were controlled at 25 ± 0.5 and 7.5 ± 0.3, respectively. After 7 days, stress condition was applied for over-production of β-carotene. During the stress stage, NaCl concentration of the medium and the average light was increased to 3 M and 2,000 μmol m−2 s−1, respectively. The cells were harvested after 8 days when the color of the photobioreactor turned to orange (Hejazi et al. 2010). The harvested D. salina was lyophilized and stored in refrigerator until use. For making the experimental foods, D. salina was suspended in sterile water and mixed with distinctive amount of food.
Experimental design
One hundred and eighty severum fish 27 ± 0.5 g (mean ± SD) were randomly divided into four equal groups, each group in triplicate and 15 fish in each group, in 50-l aquaria equipped with external biofilters (Athmann, China), following a completely randomized design (CRD). Groups 1, 2 and 3 were fed with a diet supplemented with 50, 100 and 200 mg kg−1 D. salina powder, respectively, whereas group four (Control group) received the same food without supplementation. Fish sex was not taken into consideration, and feeding trial was conducted for 6 weeks. Fish were hand-fed 1.5 % of biomass and twice a day. The fish were weighed just before the start and at the end of study. Blood samples (300–500 μl) were taken randomly from 6 fish of each replicate of experimental groups after 6 weeks. Sera samples were separated via centrifugation at 3,000 g for 10 min and stored in separated at −20 °C until used. Skin samples (around 0.5 g) of lateral flank near to dorsal fin were taken from fish after bleeding and euthanized with 1 g l−1 MS222 (Choubert and Storebakken 1989). Nine fish in each replicate were challenged with live Aeromonas hydrophila, and cumulative mortality was recorded for 10 days after challenge (Schaperclaus et al. 1991).
Physico-chemical parameters of water
Water quality parameters viz., temperature, dissolved oxygen, pH, total hardness, ammonia, and nitrate were recorded at 7-day interval during the entire experimental period. Mean ± SD of the parameters such as temperature, pH, total hardness, dissolved oxygen, NH3, NO2 and NO3 were 27 ± 1.3 °C, 8 ± 0.4, 515 ± 45 μz cm−2, 7.9 ± 0.3, >0.01, >0.005 and 0.1 ± 0.02 mg l−1, respectively.
Mucus sample preparation
The fish were placed with the ventral side of the body facing downward, and cutaneous mucus from the dorsal side of the fish was collected by a cell scraper and transferred to a tube with 200 μl, pH 6.0. Samples were kept on ice during transportation to the laboratory and kept frozen to avoid bacterial growth and degradation at −80 °C until used. The mucus centrifuges (3,000 rpm for 10 min) and supernatant were used for lysozyme activity and antibacterial activity of mucus. (Thompson et al. 1995).
Mucus and serum lysozyme activity
Serum lysozyme activity was measured as described by Ellis (1990). Briefly, 10 μl of serum/mucus was mixed with 200 μl of a Micrococcus lysodeichticus (Sigma) suspension at 0.2 mg ml−1 in 0.05 M sodium phosphate buffer (pH 6.2). The mixture was incubated at 27 °C, and its OD was detected after 1 and 6 min at 530 nm using an ELISA (enzyme-linked immunosorbent assay) plate reader (Dynatech, the Netherland). One unit of lysozyme activity was defined as the amount of enzyme that caused a decrease in absorbance of 0.001 min ml−1 serum. Lysozyme concentrations were calculated using a standard curve of lysozyme from hen egg white (Sigma) concentrations.
Mucus and serum bactericidal activity
The method used for serum bactericidal activity was a modified version of that adopted by Kajita et al. (1990) with slight modification. The serum samples were diluted three times with 0.1 % gelatin-veronal buffer (GVB; pH 7.5, containing 0.5 mM ml Mg and 0.15 mM ml Ca), and mucus samples were used without dilution. A. hydrophila (live washed cells) were suspended in the same buffer to make a concentration of 1 × 105 cfu ml−1. The diluted sera and bacteria were mixed at a ratio of 1:1 and incubated for 90 min at 25 °C and continuously agitated. The number of viable bacteria was then calculated by counting the resultant colonies from the incubated mixture on TSA (Tryptic Soy Agar) plates after incubation for 24 h in duplicate.
Growth performance analysis
The average daily weight gain (AWG), specific growth rate (SGR), feed conversion ratio (FCR) and protein efficiency ratio (FER) were calculated according to the following equations:
Total carotenoids
Total carotenoid content of skin of 5 fish in each replicate was determined at the end of the experiment under a spectrophotometer (Suzuki and Katoh 1990). Briefly, 1 g of skin was homogenized in 10 ml ethyl alcohol. Then 1 ml of suspension was picked up and mixed with 1 ml ethyl alcohol 95 % and 3 ml N-hexane, and the yield mixture centrifuged for 10 min at 2,000 rpm. Upper phase was picked up, their optical density was read at 453 nm wavelength on the spectrophotometer, and β-carotene content was determined according to the following formula:
Challenge study with A. hydrophila
After 6 weeks of feeding, 10 fish per group were challenged with virulent A. hydrophila (AH04 obtained from Aquatic Animal Health department, Tehran university, Tehran, Iran). Initially, the pathogenic isolates of A. hydrophila were grown on nutrient broth for 24 h at 30 °C in a BOD incubator and harvested by centrifuging the culture broth at 10,000 rpm for 10 min at 4 °C. The cells were then washed twice in sterile PBS (pH = 7.2), and final concentration was maintained at 108 CFU ml−1. The fish in each experimental group were intraperitoneally injected with 0.2 ml of bacterial suspension. Mortality was recorded for 10 days. Tissues were taken from the dead fish for bacteriological culture to confirm A. hydrophila as the cause of death. The percentage of cumulative mortality in different groups was indicated.
Hematological parameters
Blood samples were immediately analyzed for the estimation of numbers of erythrocytes, hemoglobin (Hb) and hematocrit (Hct) according to Thrall (2004). White blood cell (WBC) was counted as described by Schaperclaus et al. (1991).
Statistical analysis
Data were tested for normal distribution with Shapiro–Wilk’s test and for homogeneous variance with Levene’s test. Statistical analysis consisted of one-way ANOVA, using the probability level of 0.05 for rejection of the null hypothesis. After ANOVA, significant differences among means were determined by Duncan’s multiple range test. All statistical analysis was performed using SPSS for Windows software version 16.0.
Results
Growth performance
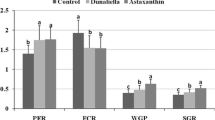
The results of the effect of D. salina on H. severus were shown in Fig. 1. Although a deep influence of D. salina (100 and 200 mg kg−1 food) was indicated in the growth performance of H. severus (P < 0.05), no significant differences were seen in growth factors of fish fed with food including 50 mg kg−1 D. salina (P > 0.05).
Effect of D. salina on growth parameters [specific growth ratio (SGR), food conversion ratio (FCR), average daily weight gain (AWG) and protein efficiency ratio (FER)] of H. severus fed for 6 weeks. G2, G3 and G4 fish groups fed with commercial basal diet containing 50, 100 and 200 mg kg−1 D. salina, respectively. G1 commercial basal diet free from D. salina. Mean ± SD from 18 fish. Groups with different letter on SD bar were significantly different (P < 0.05)
Mucus and serum lysozyme activity
The serum lysozyme activity was significantly increased in 100 and 200 D. salina-treated fish compared with the control group (Fig. 2). Mucus lysozyme activity not varied markedly among treatments (P > 0.05).
Effect of D. salina on serum and mucus lysozyme activity of H. severus. G2, G3 and G4 fish groups fed with commercial basal diet containing 50, 100 and 200 mg kg−1 D. salina, respectively. G1 group fed with commercial basal diet free from D. salina. Mean ± SD from 18 fish. Groups with different letter on SD bar were significantly different (P < 0.05)
Serum and mucus bactericidal activity
Serum and mucus bactericidal activity of fish fed different levels of D. salina were shown in Fig. 3. Not only serum but also mucus bactericidal activity was not affected by the oral administration of D. salina-treated food (P > 0.05).
Effect of D. salina on serum and mucus bactericidal activity of H. severus. G2, G3 and G4 fish groups fed with commercial basal diet containing 50, 100 and 200 mg kg−1 D. salina, respectively. G1 group fed with commercial basal diet free from D. salina. Mean ± SD from 18 fish. Groups with different letter on SD bar were significantly different (P < 0.05)
Skin carotenoid rate
Supplementation of the food with 100 and 200 mg kg−1 D. salina for 6 weeks significantly (P < 0.05) increased the skin carotenoid rate of H. severus (Fig. 4).
Skin carotenoid rate of H. severus fed different levels of D. salina for 6 weeks. G2, G3 and G4 fish groups fed with commercial basal diet containing 50, 100 and 200 mg kg−1 D. salina, respectively. G1 group fed with commercial basal diet free from D. salina. Mean ± SD from 18 fish. Groups with different letter on SD bar were significantly different (P < 0.05)
Hematological parameters
Supplementation of the food with 100 and 200 mg kg−1 D. Salina for 6 weeks significantly (P < 0.05) increases the hematological indices rate of H. severus (Table 1). Hematocrit (Hb), RBC and WBC significantly increased in G3 and G4 (P < 0.05), whereas no change was seen in G2 when compared with control groups (P > 0.05).
Challenge study with A. hydrophila
Percentage cumulative mortality of H. severus after challenging with A. hydrophila in different experimental groups was represented in Fig. 5. The lowest mortality (36.67 % ± 5.78) was recorded in fish fed with 200 mg kg−1 D. salina. Post-challenge mortality in G2 (80 ± 10) and G3 (60 ± 10) was not significantly changed when compared with control (70 ± 5.78). The clinical signs observed in fish were as follows: swollen abdomen, reddish vent exophthalmia and loss of equilibrium.
Discussion
Natural β-carotene sources have been reported to be potent immunostimulants in fish (Gourveia et al. 1997; Raymundo et al. 2005; Supamattaya et al. 2005).
Supplementation of food with D. salina (100 and 200 mg kg−1) significantly affected growth performance index, including specific growth ratio, food conversion ratio, average daily weight gain and protein efficiency ratio (P < 0.05). Contrary results were reported regarding the effect of oral administration of carotenoids on growth parameters: Nevejana et al. (2003) reported the growth stimulation following administration of D. salina as a natural source of β-carotene in Argopecten purpuratus. However, Amar et al. (2001), (2004) and Choubert et al. (2006) reported no change in growth indices following administration of food supplemented with not only natural but also synthetic carotenoid sources in rainbow trout.
In fish, the effectiveness of carotenoid source in terms of deposition and physiological function is species-specific. In addition, all fish species not possess the same pathways for the metabolism of carotenoids; therefore, there is no universal transformation of carotenoids in fish tissues (Kop and Durmaz 2008).
Contrary to those in mammals, there are few studies examining the role of carotenoids in immunity in fish. (Thompson et al. 1995). In this study, serum lysozyme activity and resistance against bacterial infection were significantly increased followed by administration of D. salina-supplemented food (P < 0.05). The lysozyme activity is an important indicator of the immune defense in both invertebrates and vertebrates (Ellis 1990).
Serum lysozyme activity increased with the addition of D. salina at 100 and 200 mg kg−1. β-carotene is a vitamin A precursor and a quencher of singlet oxygen and as such could affect the immune system through both the antioxidant and retinoid pathways. D. salina also contains large amounts of 9-cis β-carotene, which has been shown to be more potent than all-trans β-carotene in enhancing cell–cell interaction through gap junctions (Zhang et al. 1992). Lysozyme is secreted by leukocytes and is a marker of leukocyte activity, increasing concomitantly with phagocytic activity (Paulsen et al. 2001). The augmentation of lysozyme activity by β-carotene could be via its stimulation of phagocytic cells as is the case with most immunostimulants. The increased lysozyme activity has been reported in rainbow trout fed with astaxanthin and D. salina-supplemented food (Amar et al. 2001, 2004).
Results also showed that supplemented diet with D. salina increased the serum bactericidal activity, but not to a significant extent (P > 0.05). Divyagnaneswari et al. (2007) in tilapia and Kajita et al. (1990) in rainbow trout reported an increase in serum bactericidal activity after administration of biological immune stimulants.
Bactericidal activity is due to various antimicrobial peptides such as lysozyme, complement component and so on. Carotenoid-supplemented diets affect bio-defense mechanisms in rainbow trout and found that serum lysozyme and complement activity in β-carotene supplement fed groups were significantly higher than those of control fish (Amar et al. 2001).
In this study, the skin carotenoids significantly increased in all D. salina-treated groups (P < 0.05); meanwhile, skin carotenoids rate correlates with D. salina concentration in food. It can be due to deposition of carotenoids in skin which can change the color of skin as well. Tejera et al. (2007) found that different carotenoid sources can affect skin carotenoids of red porgy and its rate correlate with the concentration of astaxanthin. Similar reports confirmed increased skin carotenoids following oral administration of different carotenoid sources (Ha et al. 1993; Rehulka 2000).
It is known that food component can affect hematological parameters, such as erythrocyte number, Hb amount, hematocrit value and hemoglobin indexes (Schaperclaus et al. 1991). In this study, significant increase (P < 0.05) in PCV, RBC, hemoglobin and WBC was observed in fish fed with food that contains 100 and 200 mg kg−1 D. salina. PCV and RBC are general indicators for fish health and help describe abnormalities caused by immunostimulants (Selvaraj et al. 2005). The increase in erythrocyte number and hemoglobin content observed in this study may be due to the stimulating effect of the D. salina extract component on the erythropoietic tissue as a result of which the viability of the cells might be affected (Tavares et al. 1999). The differential leukocytic count is an indicator of health in fish (Magnado’ttir 2006). Probably, the increase in the leukocyte count might have resulted in the enhancement of the nonspecific defense, because leukocytes are the key elements in the immune system and are the major affecter and effectors cells on which D. salina exerts its activities.
There are various reports of elevated PCV, Hb, RBC and WBC value after administration of some herbal immunostimulants in fish (Harikrishnan et al. 2003; Alishahi et al. 2010). Contrary results were observed among previous studies on interaction of carotenoids and hematological parameters in fish. Nakano et al. (1995) reported that hematological parameters increased in carotenoids-treated fish. But Rehulka (2000) reported that rainbow trout fed diet containing astaxanthin had significantly lower levels of RBC, PCV and hemoglobin. This finding shows that dietary carotenoids influenced blood components. These controversial results could be explained by the different species of fish, the route and dose of administration and physiological situation.
Supplementation of dried algal cells caused retardation of growth in black tiger shrimp (Liao et al. 1993) and striped jack (Watanabe et al. 1990).
It is important to estimate bacterial infection resistance of treated fish to determine the efficiency of an immunostimulant. In the present study, the lowest mortality after challenge with A. hydrophila was registered in fish treated with 200 mg kg−1 D. salina, which was significantly lower than that of control group (P < 0.05). Then, food supplementation with D. salina had a positive influence on the survival of H. severus by increasing its resistance against A. hydrophila infection. Probably, carotenoid contents or unknown ingredients in the D. salina enhanced resistance against bacterial infection in fish. Decreased mortalities after challenge with A. hydrophila were reported not only in Cyprinus carpio, fed on Aloe vera and viscum album extracts (Alishahi et al. 2010, 2012) and Chinese herbs (Astragalus radix and Ganoderma lucidum) (Yin et al. 2008), but also in Indian major carp, using tuftsin Yin et al. (2008).
Based on the results, it can be concluded that incorporation of D. salina powder in food (100 and 200 mg kg−1) in H. severus may lead to an increase in growth parameters, stimulate some hematological and immunological parameters and resistance against bacterial infection. However, the mechanism of immunostimulation in fish is still not completely clear, and we suggest to do further studies on mechanism of D. salina function. Also, other effects of D. salina on fish as well as other routs of administration should be investigated.
References
Alishahi M, Ranjbar M, Ghorbanpor M, Peyghan R, Mesbah M, Razijalali M (2010) Effects of dietary on specific and nonspecific immunity in the common carp Aloe vera (Cyprinus carpi). Int J Vet Res 4:189–195
Alishahi M, Ghorbanpour M, Peyghan R (2012) Effects of Viscum album and Nigella sativa extracts on some immune responses of common carp (Cyprinus carpio). J Asian Fish Sci 25:15–28
Amar EC, Kiron V, Satoh S, Watanabe T (2001) Influence of various dietary synthetic carotenoids on bio-defense mechanisms in rainbow trout (Oncorhynchus mykiss). Aquac Res 32:162–173
Amar EC, Kirona V, Satoha S, Watanabea T (2004) Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish Shellfish Immunol 16:527–537
Ashish KJ, Pal AK, Sahu NP, Shivendra K, Mukherjee SC (2007) Haemato-immunological responses to dietary yeast RNA, u-3 fatty acid and b-carotene in Catla catla juveniles. Fish Shellfish Immunol 23:917–927
Baker RTM, Pfeiffer AM, Schöner FJ, Smith-Lemmon L (2002) Pigmenting efficacy of astaxanthin and canthaxanthin in fresh-water reared Atlantic salmon, Salmo salar. Anim Feed Sci Technol 99:97–106
Ben-Amotz A, Avron M (1983) On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol 72:593–597
Bendich A, Shapiro SS (1986) Effect of h-carotene and canthaxanthin on the immune responses of the rat. Nutrition 116:2254–2262
Borowitzka MA, Borowitzka LJ (1988) Dunaliella. In: Borowitzka MA, Borowitzka LJ (eds) Microalgal biotechnology. Cambridge University Press, New York, pp 456–465
Cejas J, Almansa E, Tejera N, Jerez S, Bolaños A, Lorenzo A (2003) Effect of dietary supplementation with shrimp on skin pigmentation and lipid composition of red porgy (Pagrus pagrus) alevins. Aquaculture 218:457–469
Choubert G, Storebakken T (1989) Dose response to astaxanthin and canthaxanthin pigmentation of rainbow trout (Oncorhynchus mykiss) fed various dietary carotenoids concentrations. Aquaculture 81:69–77
Choubert G, Mendes-Pinto MM, Morais R (2006) Pigmenting efficacy of astaxanthin fed to rainbow trout (Oncorhynchus mykiss): effect of dietary astaxanthin and lipid sources. Aquaculture 257:429–436
Divyagnaneswari M, Christybapita D, Dinakaran Michael R (2007) Enhancement of nonspecific immunity and disease resistance in Oreochromis mossambicus by Solanum trilobatum leaf fractions. Fish Shellfish Immunol 23:249–259
Ellis AE (1990) Lysozyme assay. In: Stolen JS, Fletcher DP, Anderson BS, Robertson BS (eds) Techniques in fish immunology. SOS Publication, Fair Haven, pp 101–103
Goodwin TW (1984) The biochemistry of carotenoids, vol. II. Animals, 2nd edn. Chapman & Hall, London, p 224
Gourveia L, Gomes E, Empis J (1997) Use of Chlorella vulgaris in diets for rainbow trout (Oncorhynchus mykiss) to enhance pigmentation of muscle. Aquaculture 7:61–70
Ha BS, Kang DS, Kim JH, Choi OS, Ryu HY (1993) Metabolism of dietary carotenoids and effects to improve the body color of cultured flounder and red sea bream. Bull Korean Fish Soc 26:91–101
Harikrishnan R, Nisha MR, Balasundaram C (2003) Hematological and biochemical parameters in common carp (Cyprinus carpio) following herbal treatment for Aeromonas hydrophila infection. Aquaculture 221:41–50
Hejazi MA, Barzegari A, Hosseinzadeh A, Hejazi MS (2010) Introduction of a novel 18S rRNA gene arrangement along with distinct ITS region in the saline water microalga Dunaliella, Salina systems. Saline Syst 6:4
Kajita Y, Sakai M, Atsuta S, Kobayash M (1990) The immunomodulatory effects of levamisole on rainbow trout (Oncorhynchus mykiss). Fish Pathol 25:93–98
Karunasagar I, Ali A, Otta SK (1997) Immunization with bacterial antigen: infection with motile Aeromonas. In: Gudding R, Lillehaug A, Midtlyng J (eds). Fish Vaccinol Dev Biol Stand 90: 1–7
Kop A, Durmaz Y (2008) The effect of synthetic and natural pigments on the colour of the cichlids (Cichlasoma severum sp., Heckel 1840). Aquaculture 16:117–122
Kumar S, Sahu NP, Pal AK, Choudhury D, Yengkokpam S, Mukherjee SC (2005) Effect of dietary carbohydrate on haematology, respiratory burst activity and histological changes in Lebeo rohita juveniles. Fish Shellfish Immunol 19:331–344
Liao WL, Nur-E-Borhan S, Okada S, Matsui T, Yamaguchi K (1993) Pigmentation of cultured black tiger prawn by feeding with a Spirulina supplemented diet. 59: 165–169
Lowe-McConnell RH (1969) The cichlid fishes of Guyana, South America, with notes on their ecology and breeding behavior. Zool J Linn Soc 48:255–302
Magnado’ttir B (2006) Innate immunity of fish (overview). Fish Shellfish Immunol 20:137–151
Merigoux S, Ponton D, De Merona B (1998) Fish richness and species-habitat relationships in two coastal streams of French Guiana, South American. Environ Biol Fish 51:25–39
Morad khany Z, Matinfar A, Soltani M, Mosavy H (2008) Effect of using enriched Artemia urmiana with highly unsaturated fatty acid and vitamin C on the Cichlasoma severum). Fishery 2:15–22
Nakano T, Tosa M, Takeuchi M (1995) Improvement of biochemical features in fish health by red yeast and synthetic astaxanthin. Agric Food Chem 43:1570–1573
Nevejana N, Saeza I, Gajardoa G, Sorgeloos P (2003) Supplementation of EPA and DHA emulsions to a Dunaliella tertiolecta diet: effect on growth and lipid composition of scallop larvae, Argopecten purpuratus. Aquaculture 217:613–632
Oslon JA (1989) Provitamin, a function of carotenoids: the conversion of b-carotene into vitamin A. Nutrition 119:105–118
Paulsen SM, Engstad RE, Robertsen B (2001) Enhanced lysozyme production in Atlantic salmon (Salmo salar) macrophages treated with yeast beta glucan and bacterial lipopolysaccharide. Fish Shellfish Immunol 11:23–37
Raja R, Hemaiswarya S, Rengasamy R (2007) Exploitation of Dunaliella for β-carotene production. Appl Microbiol Biotechnol 74:517–523
Raymundo A, Gouveida L, Batisa AP, Empis J, Sousa L (2005) Fat mimetic capacity of chlorella vulgaris biomass in oil-in-water food emulsions stabilized by pea protein. Food Res Int 38:961–965
Rehulka J (2000) Influence of astaxanthin on growth rate, condition, and some blood indices of rainbow trout (Oncorhynchus mykiss). Aquaculture 190:27–47
Sales J, Janssens PX (2003) Nutrient requirements of ornamental fish. Aquatic Living Resour 16:533–540
Schaperclaus W, Kulow H, Schreckenbach K (1991) Hematological, and serological technique. In: Kothekar VS (ed) Fish disease. 2nd ed. vol. 1. New Delhi: Gulab primlani, Oxonian press Pvt. Ltd, 71–108
Selvaraj V, Sampath K, Sekar V (2005) Administration of yeast glucan enhances survival and some non-specific and specific immune parameters in carp (Cyprinus carpio) infected with Aeromonas hydrophila. Fish Shellfish Immunol 19:293–306
Sigurgisladottir S, Torrissen O, Lie Ø, Thomassen M, Hafsteinsson H (1997) Salmon quality: methods to determine the quality parameters. Rev Fish Sci 5:223–252
Supamattaya K, Kiriratnikom S, Boonyaratpalin M (2005) Effect of a Dunaliella extract on growth performance, health condition, immune response and disease resistance in black tiger shrimp (Penaeus monodon). Aquaculture 248:207–216
Suzuki J, Katoh N (1990) A simple and cheap method for measuring serum vitamin a in cattle using only a spectrophotometer. Vet Sci 52(6):1281–1283
Tavares DM, Martins ML, Nascimento KS (1999) Evaluation of the hematological parameters in Piaractus mesopotamicus with Argulus sp. infestation and treatment with organophosphate. Revista Brasileria de Zoologia 16:553–555
Tejera N, Cejas JR, Rodríguez C, Bjerkeng B, Jerez S, Bolaños A, Lorenzo A (2007) Pigmentation, carotenoids, lipid peroxides and lipid composition of skin of red porgy (Pagrus pagrus) fed diets supplemented with different astaxanthin sources. Aquaculture 270:218–230
Thompson I, Choubert G, Houlihan DF, Secombes CJ (1995) The effect of dietary vitamin A and astaxanthin on the immunocompetence of rainbow trout (Oncorhynchus mykiss). Aquaculture 133:91–102
Thrall MA (2004) Veterinary hematology and clinical chemistry. Lippincott Williams & Wilkins: USA 241: 277–288
Watanabe T, Liao w, Takeuchi T, Yamamoto H (1990) Effect of dietary Spirulina supplementation on growth performance and flesh lipids of cultured striped jack. J Tokyo Univ Fish 77:231–239
Wyban J, Martinez G, Sweeney J (1997) Adding paprika to Penaeus vannamei maturation diet improves nauplii quality. World Aquac 28:59–62
Yin G, Ardo L, Thompson KD, Adams A, Jeney Z, Jeney G (2008) Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp (Cyprinus carpio) and protection against Aeromonas hydrophila. Fish Shellfish Immunol 25:140–145
Yuan C, Li D, Chen W, Sun F (2007) Administration of a herbal immunoregulation mixture enhances some immune parameters in carp (Cyprinus carpio). Fish Physiol Biochem 10:1007–1120
Zhang LX, Cooney RV, Bertram JS (1992) Carotenoids up-regulate Connexin 43 gene expression independent of their provitamin A or antioxidant properties. Cancer Res 52:5707–5712
Acknowledgments
This work was financially supported by the Research Council of Shahid Chamran University, Ahvaz, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Alishahi, M., Karamifar, M., Mesbah, M. et al. Hemato-immunological responses of Heros severus fed diets supplemented with different levels of Dunaliella salina . Fish Physiol Biochem 40, 57–65 (2014). https://doi.org/10.1007/s10695-013-9823-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-013-9823-5