Abstract
Glucose 6-phosphate dehydrogenase (G6PD) is a key enzyme catalyzing the first step of the pentose phosphate pathway which generates NADPH for anabolic pathways and protection systems in various organisms, including fish. In the present study, G6PD was purified from grass carp (Ctenopharyngodon idella) hepatopancreas using the methods of 2′,5′-ADP-Sepharose 4B affinity chromatography followed by DEAE Sepharose Fast Flow ion exchange chromatography. The characterization of G6PD and inhibition effects of several metal ions on G6PD activity in vitro were also determined. Grass carp hepatopancreas G6PD, with a specific activity of 18 U/mg protein, was purified 1,066-fold with a yield of 19.5 % and Mr of 71.85 kDa. The enzyme had a temperature optimum of 42 °C, pH optimum of 7.5 and 9.0. The K m values for G6-P and NADP+ were determined to be 0.026, 0.0068 mM, respectively. The V max values for G6-P and NADP+ were 2.20 and 2.27 μM min−1 mg protein−1, respectively. The catalytic efficiency for G6-P and NADP as the substrates was 0.085 and 0.334 × 10−6 min−1 mg protein−1, respectively. Inhibition effects of metal ions on the purified G6PD activity indicated that IC50 values of Zn+2, Mn+2, Al+3, Cu+2, and Cd+2 were 0.42, 0.54, 0.94, 1.20, and 4.17 mM, respectively. The Ki constants of Zn+2, Al+3, Cu+2, and Cd+2 were 0.52, 1.12, 0.26, and 4.8 mM, respectively. Zn+2, Al+3, and Cd+2 showed competitive inhibition, while Cu+2 inhibited the G6PD in a noncompetitive inhibition manner. Our study provided important information about the control of the grass carp liver PPP, the biosynthesis of several important related biomolecules, and the status of detoxification systems in grass carp liver in relation to metabolism.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Glucose 6-phosphate dehydrogenase (G6PD, EC 1.1.1.49) is a key enzyme that initiates the reactions of the pentose phosphate pathway (PPP), catalyzing the conversion of glucose-6-phosphate to 6-phosphogluconate in the presence of nicotinamide adenine dinucleotide phosphate (oxide form, NADP+) (Ciftci et al. 2003). The main physiological function of G6PD is to produce nicotinamide adenine dinucleotide phosphate (reduced form, NADPH), which is essential for synthesis of several biological macromolecules such as nucleic acids and fatty acids (Ciftci et al. 2004, 2007; Senturk et al. 2009). Through the glutathione reductase-peroxidase system and the mixed-function oxidases, NADPH also participates in cell-membrane protection and cell detoxification from xenobiotics (Erdogan et al. 2005; Ulusu and Tandogan 2005; Senturk et al. 2009).
G6PD is widely distributed among microorganisms, plants, and in different animal tissues (Rosemeyer 1987). Its importance in metabolism is well known for many years. Because of the vital importance of this enzyme, G6PD has been characterized from various sources. G6PD was first isolated from human erythrocytes by Yoshida and Huang (1986), and then from Dicentrarchus labrax liver (Bautista et al. 1988), rat brain (Askar et al. 1996), Antarctic fish (Ciardiello et al. 1997), rainbow trout erythrocytes (Ciftci et al. 2004; Senturk et al. 2009), and rat kidney (Adem and Ciftci 2012). Kinetic properties of G6PD purified from different sources such as erythrocytes, liver, kidney, brain, and placenta with some organisms have also been published (Rosemeyer 1987; Levy and Cook 1991; Corpas et al. 1995; Ozer et al. 2001). However, in fish, the related information is available only in the very limited fish species, such as rainbow trout (Ciftci et al. 2004; Senturk et al. 2009).
During the last decades, a dramatic increase in environmental poisoning by pollutants occurs as a consequence of industrial, agricultural, and anthropogenic activities; thus, aquatic organisms are exposed to a significant amount of these pollutants (Heath 1987). Among the pollutants, metals are an interesting subject of research because some of them are required for the function of various enzymes, but become toxic at increased waterborne levels. In China, metal contamination and toxicity in rivers and lakes has posed as a significant environmental hazard for fishery (Qiao et al. 2007; Li and Zhang 2010). For example, Li and Zhang (2010) investigated dissolved trace elements and heavy metals including Al, As, Ba, Cd, Co, Cr, Cu, Fe, Hg, Mn, Ni, Pb, Sb, Se, Si, Sr, and V over a period of 2 years in Han River, China, and indicated serious water contamination in the waters. Yi et al. (2008) reported that the concentrations of heavy metals (Hg, Cd, Pb, Cr, Cu, Zn, and As) in the sediment in some sampling sites in the Yangtze River were up to 0.47, 0.47, 110, 96, 75, 750, and 82 mg/kg dry weight. The sediment in rivers and lakes was considered as the major sink for metal pollution and played a significant role in determining water quality since metals in sediments could be released into the water. The studies carried out on various fishes have shown that metal ions may alter the physiological activities and biochemical parameters in tissues (Liu et al. 2011; Chen et al. 2012). Thus, toxicology studies about the effects of metal ions on various enzyme activities are becoming more and more important (Alici et al. 2008). Ciltas et al. (2003) reported in vitro effects of Chloramine-T and CuSO4 on G6PD enzyme activities of rainbow trout erythrocytes. However, at present, to my knowledge, little information is reported on the in vitro effect of other metal ions on G6PD activity in fish.
Grass carp Ctenopharyngodon idella represented the second largest aquaculture industry in the world inferior to silver carp, constituting 14.7 % of the world aquaculture production, with an average annual increase of 14 % in China, mainly as a source of food (FAO 1999). Meantime, because grass carp feed aggressively on vegetation, they are used widely to control aquatic plant populations in other regions, such as European and Northern America (Opuxzynski and Shireman 1995). The current study aims in purification and characterization of G6PD from grass carp hepatopancreas for the first time and investigated in vitro effects of metal ions (Zn+2, Mn+2, Al+3, Cu+2, and Cd+2) and to examine the mode of inhibition exerted by these metal ions on the pure grass carp liver G6PD enzyme. Our study will provide important information about the control of the grass carp liver PPP, the biosynthesis of several physiologically important biomolecules (such as nucleic acids and fatty acids), and the status of detoxification systems in grass carp liver in relation to metabolism.
Materials and methods
Chemicals
2′, 5′-ADP-Sepharose 4B, DEAE Sepharose Fast Flow, Sephadex G-200 were obtained from Pharmacia Fine Chemicals, Uppsala, Sweden. Glucose-6-phosphate (G6-P), NADP+, protein standards, 2-mercaptoethanol (2-ME), and all other chemicals used were of analytical grade and purchased from Sigma-Aldrich Chemical Co., MO, USA.
Fish husbandry and maintenance
In the present study, grass carp were obtained from a local fish dealer. Prior to the sampling, 60 healthy grass carp (200 ± 15 g) were assigned to three indoors circular fiberglass tanks, with 20 fish for each tank, for 14-day acclimatization. During the acclimatization, they were provided with a commercial Haid® feed at 2 % of body weight daily and with continuous aeration to maintain the dissolved oxygen level above saturation. Fecal matter was removed before feeding. We assure that the experiments performed on animals, animal care, and all protocols followed the ethical guidelines of Huazhong Agricultural University for the care and use of laboratory animals.
The trial was conducted at ambient temperature and natural photoperiod (approximately 12-h light: 12-h dark during the experiment). The water quality parameters were measured in the morning twice a week as dissolved oxygen, >6 mg l−1, pH = 7.3–8.4, and total ammonia–nitrogen 0.05–0.078 mg l−1, water temperature 25 ± 2 °C.
Sampling
At the end of the 2-wk experiment, fish were starved for 24 h before sampling. Then, they were killed by severing of the spinal cord. The hepatopancreas was isolated immediately using sterile forceps in ice, frozen in liquid nitrogen, and stored at −80 °C (not longer than 2 weeks) for subsequent analysis.
Purification of G6PD from grass carp hepatopancreas
In this study, grass carp hepatopancreas G6PD was purified by slight modification of the published procedure (Ulusu and Tandogan 2005, 2006). The purification procedure consisted of two steps after ultracentrifugation: 2′,5′-ADP-Sepharose 4B affinity and DEAE Sepharose Fast Flow anion exchange chromatography steps. All the procedures were carried out at +4 °C.
At first, the hepatopancreas was cut with scissors. Excess blood was removed from the samples after washing with ice-cold saline and homogenized in a glass–Teflon homogenizer with 3 volumes of 10 mM Tris–HCI buffer, containing 1 mM 2-mercaptoethanol and 1 mM EDTA, pH 7.6 (buffer A) on ice. The homogenate was centrifuged at 105,000×g for 60 min at 4 °C. The supernatant obtained was loaded onto 2′,5′-ADP-Sepharose 4B column (1.5 × 6.7 cm) pre-equilibrated with buffer A. The column was washed with the same buffer (the flow rate was 10.8 mL/h) until the absorbance at 280 nm decreased to 0.021 to remove all the non-specifically bound compounds. 6-phosphogluconate dehydrogenase (6-PGD) was not bound to the affinity column and eluted with buffer A. Active G6PD fractions were combined and loaded onto DEAE Sepharose Fast Flow column (1.5 × 7.5 cm) equilibrated with 5 mM potassium phosphate buffer, pH 6.9 (buffer B). The flow rate was maintained at 16.8 ml/h and the column was washed with buffer B until the absorbance at 280 nm decreased to 0.003 O.D. G6PD was eluted by a linear gradient of KCl (175–250 mM) in buffer B. The enzymes were separated from each other. All of the purification procedures were performed at 4 °C. Purification scheme of G6PD from grass carp hepatopancreas was shown in Table 1.
Activity determination
G6PD activity assays were run at 25 °C according to Beutler’s (1984) methods, which depended on the reduction of NADP+ by G6PD, in the presence of glucose 6-phosphate. For the spectrophotometric measurements, the reaction mixture (for routine activity determinations) contained 10 mM MgCl2, 0.2 mM NADP+, and 0.6 mM G6-P in 100 mM Tris–HCl buffer, pH 7.5, and a suitable amount of the enzyme. The conversion of NADP+ to NADPH was followed by monitoring the change in absorbance at 340 nm. Assays were carried out in duplicates. One unit of enzyme (U) activity, defined as the amount of enzyme that reduced 1 μmol NADP+ per minute, was expressed as units per mg of hepatic soluble protein. The protein content was quantified according to Bradford’s method (Bradford 1976), using bovine serum albumin as standard.
SDS polyacrylamide gel electrophoresis (SDS-PAGE)
To control the enzyme purity and determine the subunit molecular weight, SDS-PAGE was performed using Laemmli’s procedure (Laemmli 1970). Rabbit phosphatase B (97,200), bovine albumin (66,409), chicken ovalbumin (44,287), bovine carbonic anhydrase (29,000), and trypsin inhibitor (20,100) were used as standards. The acrylamide concentrations were 3 and 10 %, containing 10 % sodium dodecyl sulfate (SDS) for stacking gel and running gel, respectively. The gel was stabilized in a solution containing 50 % propanol, 10 % trichloroacetic acid, 40 % distilled water for 30 min. Gel was stained for 2 h in a solution of 0.1 % Coomassie Brilliant Blue R-250 containing 50 % methanol, 10 % acetic acid, and 40 % distilled water. The washing was carried out in the same solution without the dye until protein bands were cleared (Ciltas et al. 2003). The electrophoretic pattern was photographed.
Optimum pH determination
For the optimal pH determination, the enzyme activity was measured in 100 mM Tris–HCl and phosphate buffers within the pH range of 5.0–10.0, respectively.
In the present study, the enzyme activity was also determined in 100 mM Tris–HCl buffer at pH of 7.0, 8.0, and 9.0. When beginning the experiment, the equal volumes of buffer and enzyme solutions were mixed and kept in a refrigerator (4 °C). Activity determinations were made within an interval of 8–24 h (Yilmaz et al. 2002; Ciftci et al. 2003).
Optimum temperature determination
For the determination of optimum temperature, enzyme activity was assayed between 15 and 60 °C at optimal pH for this purpose.
Kinetic studies
Substrate kinetics were determined at 25 °C in optimal pH (0.1 M Tris–HCl, pH 7.5), and various concentrations of NADP and G6-P. For Michaelis–Menten constants (K m) and V max evaluation, Lineweaver–Burk curves were used (Lineweaver and Burk 1934), which were obtained in five different concentrations of NADP+ (0.01, 0.02, 0.05, 0.1, and 0.16 mM) with a fixed concentration of G6-P (0.6 mM), and the same experiments were done for G6-P (at five different concentrations of G6-P: 0.1, 0.15, 0.25, 0.3, and 0.5 mM, respectively, and at the constant NADP+ concentration: 0.2 mM).
In vitro effects of metal ions
Cd+2 (2–8 mM), Cu+2 (1–5 mM), Al+3 (0.4–1.5 mM), Mn+2 (0.1–0.5 mM), and Zn+2 (0.1–0.7 mM) were used as inhibitors. Assays were carried out under standard conditions with varying concentration of each metal ions. The inhibition of the enzyme by Cd, Cu, Al, Mn, and Zn was further examined by varying G6-P concentration at a fixed NADP concentration and at six different constant concentrations of each metal ion. The activity of control cuvette in the absence of an inhibitor was taken as 100 %. All compounds were tested in triplicates at each concentration used. For each inhibitor, an activity %-[Inhibitor] graph was drawn. Metal ions concentrations that produced 50 % inhibition (IC50) were calculated from the regression graphs.
To determine K i values, three different inhibitor concentrations (Cu: 1.5, 2 and 3 mM; Zn: 0.1, 0.2 and 0.3 mM; Cd: 4, 6 and 7 mM; Al: 0.4, 0.8 and 1 mM, respectively) were tested for each metal ion. In these experiments, G6-P was used as substrate at four different concentrations (0.2, 0.25, 0.3, and 1.0 mM, respectively). Inhibitor (metal ions) solutions were added to the reaction medium, resulting in three different fixed concentrations of inhibitors in 200 μl of total reaction volume. All assays were repeated three times. Lineweaver–Burk graphs were drawn by using [S]/V versus [S] values. K i constant and the inhibitor type were calculated from these graphs (Lineweaver and Burk 1934).
Results
Properties of G6PD
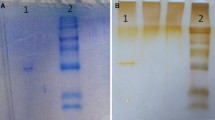
In the present study, a rapid procedure to purify G6PD from grass carp hepatopancreas was presented. The steps used were 2′,5′-ADP-Sepharose 4B affinity chromatography followed by DEAE Sepharose Fast Flow ion exchange chromatography. After the two-step purification, the specific activity of the enzyme was determined to be 18 U/mg protein, and 1,066-fold increase in the purity was obtained. The overall yield was about 19.5 % (Table 1). The SDS-PAGE result indicated that a high purity was obtained for the enzyme (Fig. 1). For the standard proteins and G6PD, R f values were calculated and R f − log MW graph (Fig. 2) was obtained according to Laemmli procedure (Laemmli 1970), showing that the molecular weight (Mr) of G6PD was 71.85 kDa.
SDS-PAGE photograph of G6PD. (Lane 1 homogenate. Lane 2 standard proteins, rabbit phosphatase B (97,200), bovine albumin (66,409), chicken ovalbumin (44,287), bovine carbonic anhydrase (29,000), trypsin inhibitor (20,100); lane 3 and 4 G6PD). SDS-PAGEs were performed on five different pools of animals with consistent results
To obtain the optimum temperature, the activities of the enzyme were measured between 15 and 60 °C (Fig. 3). Optimum temperature was found to be 42 °C from the graph. But in our experiments, we prefer to study at physiological temperature, 25 °C.
For the optimal pH determination, the enzyme activity was determined at 100 mM Tris–HCl buffer between pH 5.0 and 10.0. As shown in Fig. 4a, enzyme activity versus pH curve had more than one maximum value (at pH 7.5 and pH 9.0). This type of curve might be seen for diprotic systems (Segel 1975) and indicated that the active site of the enzyme might contain several ionizable groups. The enzyme was found to be not stable at all three tested pH values (7.0, 8.0 and 9.0) (Fig. 4b).
Kinetic behavior of G6PD
Lineweaver–Burk double-reciprocal plots obtained for G6-P as varied substrate at different fixed NADP+ concentrations were shown in Fig. 5a. 1/v versus NADP+ plots at different constants G6-P concentrations were seen in Fig. 5b. In the figures, the intersection points of the family of the lines were above the horizonal axis, indicating that the reaction catalyzed by G6PD from grass carp pancreas proceeded by a sequential mechanism.
a Double-reciprocal plot of initial velocity against G6-P as varied substrate at different fixed NADP+ concentrations for the reaction catalyzed by G6PD from grass carp hepatopancreas. The velocities were determined at 25 °C in 100 mM Tris–HCl buffer, pH 7.4. b Double-reciprocal plot of initial velocity against NADP+ as varied substrate at different fixed G6-P concentrations for the reaction catalyzed G6PD from grass carp hepatopancreas. The velocities were determined at 25 °C in 100 mM Tris/HCl buffer, pH 7.4
K m and V m values were calculated from the Hanes–Woolf graphs (Fig. 6). The K m values for G6-P and NADP+ and Vm were determined to be 0.026, 0.0068 mM, 2.20 and 2.27 μM min−1 mg protein−1. The catalytic efficiency for G6-P and NADP was 0.085 and 0.33 × 10−6 min−1 mg protein−1, respectively (Table 2).
In vitro inhibition assays
IC50 values of Zn+2, Mn+2, Al+3, Cu+2, and Cd+2 were 0.42, 0.54, 0.94, 1.20, and 4.17 mM, respectively. The K i constants of Zn+2, Al+3, Cu+2, and Cd+2, calculated from Hanes–Woolf graphs, were 0.52, 1.12, 0.26, and 4.8 mM, respectively (Table 3). Zn+2, Al+3, and Cd+2 showed competitive inhibition, while Cu+2 inhibited the G6PD in a noncompetitive inhibition manner (Figs. 7, 8).
Discussion
In the present study, G6PD was purified from grass carp hepatopancreas using 2′,5′-ADP-Sepharose 4B affinity chromatography and DEAE Sepharose Fast Flow ion exchange chromatography. G6PD was separated well from 6PGD at the end of the purification procedure. Also, 6PGD was not bind to the affinity column presumably because this enzyme had a low affinity to the 2′,5′-ADP-Sepharose 4B column as compared G6PD. The method had also been used in other studies with success (Levy 1979; Yoshida and Huang 1986; Sahin et al. 2010). In the present study, the specific activity of the enzyme was determined to be 18 U/mg protein, which was lower than those in rat kidney (32 U/mg protein, Adem and Ciftci 2012), rainbow trout liver (36.25 U/mg protein, Cankaya et al. 2011), similar to chicken erythrocytes (20.86 U/mg protein, Yilmaz et al. 2002), but higher than in bivon lens (2.64 U/mg, Ulusu et al. 1999) and sheep lens (0.15 U/mg, Charlton and Heyningen 1971). The observation of different specific activities for G6PD from different sources was not uncommon, depending on several factors such as enzyme, NADP, salt, Mg2+ (or Mn2+) concentrations and pH (Yoshida 1966; Holten 1972; Aksoy et al. 2001).
In the present study, with SDS-PAGE, a molecular weight of G6PD was 71.85 kDa, which was similar to that reported in rat kidney (68 kDa, Adem and Ciftci 2012), bovine lens (69.2 kDa, Ulusu et al. 1999), buffalo erythrocyte (67.6 kDa, Ciftci et al. 2003), chicken erythrocytes (73.2 kDa, Yilmaz et al. 2002), but was higher than those in dog liver (52.5 kDa, Ozer et al. 2002), human placenta (54 kDa, Ozer et al. 2001), rainbow trout (60 kDa, Erdogan et al. 2005), rainbow trout liver (48.5 kDa; Cankaya et al. 2011).
Determining optimum pH conditions of enzyme had an important role for kinetic studies because each enzyme in different tissues could have a specific optimum pH for enzyme activity. In the present study, the optimum temperature was found to be 42 °C from the graph, which was similar to that in lamb kidney cortex (45 °C, Tandogan and Ulusu 2005), lower than that in rat liver and kidney (55 °C, Corpas et al. 1995), chicken erythrocytes (60 °C, Yilmaz et al. 2002) and buffalo erythrocyte (60 °C, Ciftci et al. 2003), and higher than that in lens (25 °C, Ulusu et al. 1999). In this study, the optimum pH of the grass carp hepatopancreas G6PD had more than one maximum value (pH 7.5 and 9.0). This type of curve was seen for diprotic systems and indicated that the active site of the enzyme contained several ionizable groups (Ulusu and Tandogan 2006). The pH versus velocity curve had two peaks at pH 7.7 and 9.6 (Ulusu et al. 1999). Ciftci et al. (2004) also reported that the activity versus pH curve had more than one maximum value (pH 7.0 and 8.0). In the present study, although there was no significant difference between pH 8.0 and pH 9.0, the enzyme activities were higher at pH 9.0 than pH 8.0, in agreement with the report by Yilmaz et al. (2002).
In the present study, K m values were found as 0.026 and 0.0068 mM, and Vmax values were calculated as 2.20 and 2.27 μM min−1 mg protein−1 for G6-P and NADP+, respectively. According to these values, the K m value for NADP+ was lower than that for G6-P, suggesting the higher affinity of G6PD to NADP+ when compared with G6-P, similar to those in other studies (Ciftci et al. 2004, 2007; Ozer et al. 2002; Yilmaz et al. 2002; Ulusu and Tandogan 2005, 2006). The catalytic efficiency for G6-P and NADP was 0.085 and 0.33 × 10−6 min−1 mg protein−1, respectively, suggesting that the enzyme was catalytically more efficient with NADP as the substrate.
In the present study, IC50 values of Zn+2, Mn+2, Al+3, Cu+2, and Cd+2 were 0.42, 0.54, 0.94, 1.20, and 4.17 mM, respectively, which indicated, of all the divalent metal ions tested, Zn2+ was the most potent inhibitor. In another study, Zang et al. (1991) reported that 96-h median lethal concentration (LC50) of Zn for grass carp was 4.6 mg/l. In China, the concentration of waterborne Zn, Cu, and Cd in freshery water was limited to less than 0.1, 0.01, and 0.005 mg/l, respectively, and there was no limit for Mn and Al (CEBP 1989). The K i constants of Zn+2, Al+3, Cu+2, and Cd+2 were 0.52, 1.12, 0.26, and 4.8 mM, respectively. Zn+2, Al+3, and Cd+2 showed competitive inhibition, while Cu+2 inhibited the G6PD in a noncompetitive inhibition manner. Similarly, Cankaya et al. (2011) reported that IC50 value of Fe, Pb, Hg, Cu, Zn, and Cd on the purified G6PD activity of trout was 0.39, 0.78, 0.87, 1.19, 1.97, 2.16 and the K i constants 0.197, 0.213, 0.542, 1.721, 2.034, 2.770, respectively. The inhibitory potentials are in the following sequence: Fe+2 > Pb+2 > Hg+2 > Cu+2 > Zn+2 > Cd+2 (Cankaya et al. 2011). Ibraheem et al. (2005) reported that the inhibitions by Zn2+ and Co2+ ions were competitive with respect to G6-P with K i values of 6.6 and 4.7 μM, respectively. Ciltas et al. (2003) reported that Ki and IC50 values were 3.967 and 2.156 mM for CuSO4. These inhibitions may cause some important physiological changes, that is, reducing production of NADPH, which plays important role in the regeneration of reduced glutathione (GSH), and therefore overwhelming antioxidant defense mechanisms. Other studies also indicated that excessive metal ions caused deleterious effect on grass carp. For example, Fernandez-Davila et al. (2012) reported that waterborne Al exposure (0.1 mg l−1) induced oxidative stress and caused important damages to grass carp. Yang et al. (2010) reported that Zn, Cu, and Cd inhibited an acid phosphatase extracted from liver of grass carp. Wang et al. (2007) reported that Cd affected the activity of glutamate pyruvate transaminase of grass carp, resulting in the damage of gills, hepatopancreas, intestine, and spleen. On the other hand, the fish meat is a valuable foodstuff of animal sources for human consumption. Under certain environmental conditions, metal ions accumulated in fish up to a toxic concentration will be dangerous and harmful for human health. Thus, it was impending to reduce the concentration of metal ion in contaminated lakes and rivers in China, for fishery and also human health.
References
Adem S, Ciftci M (2012) Purification of rat kidney glucose 6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase, and glutathione reductase enzymes using 2′, 5′-ADP Sepharose 4B affinity in a single chromatography step. Protein Expr Purif 81:1–4
Aksoy Y, Ogus IH, Ozer N (2001) Purification and some properties of human placental glucose—6-phosphate dehydrogenases. Protein Exp Purif 21:286–292
Alici HA, Ekinci D, Beydemir S (2008) Intravenous anesthetics inhibit human paraoxonase-1 (PON1) activity in vitro and in vivo. Clin Biochem 41:1384–1390
Askar M, Sumathy K, Baquer NZ (1996) Regulation and properties of purified glucose-6-phosphate dehydrogenase from rat brain. Ind J Biochem Biophys 33:512–518
Bautista JM, Garrido-Pertierra A, Soler G (1988) Glucose-6-phosphate dehydrogenase from Dicentrarchus labrax liver: kinetic mechanism and kinetics of NADPH inhibition. Biochim Biophys Acta 967:354–363
Beutler E (1984) Red cell metabolism: a manual of biochemical methods. Academic Press, London, 1971, pp 68–71
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
CEPB (Chinese Environment Protection Bureau) (1989) Water quality standards for fisheries (GB11607-89).
Cankaya M, Sisecioglu M, cifitic M, Ozdemir H (2011) Effect of some metals on metal ions on trout liver G6PD. Res J Environ Toxicol 5(6): 385–391
Charlton JM, Heyningen R (1971) Glucose-6-phosphate dehydrogenase in the mammalian lens. Exp Eye Res 11:147–160
Chen QL, Luo Z, Zheng JL, Li XD, Liu CX, Zhao YH, Gong Y (2012) Protective effects of calcium on copper toxicity in Pelteobagrus fulvidraco; copper accumulation, enzymatic activities and histology. Ecotoxicol Environ Saf 76:126–134
Ciardiello MA, Camardella L, Carratore V, di Prisco G (1997) Enzymes in Antarctic fish: glucose-6-phosphate dehydrogenase and glutamate dehydrogenase. Comp Biochem Physiol 118A:1031–1036
Ciftci M, Beydemir S, Yılmaz H, Altıkat S (2003) Purification of glucose 6-phosphate dehydrogenase from Buffalo (Bubalus bubalis) erythrocytes and investigation of some kinetic properties. Protein Expr Purif 29:304–310
Ciftci M, Ciltas A, Erdogan O (2004) Purification and characterization of glucose 6-phosphate dehydrogenase from rainbow trout (Oncorhynchus mykiss) erythrocytes. Vet Med Czech 49:327–333
Ciftci M, Turkoglu V, Coban TA (2007) Effects of some drugs on hepatic glucose 6-phosphate dehydrogenase activity in Lake Van Fish (Chalcalburnus Tarischii Pallas, 1811). J Hazard Mater 143:415–418
Ciltas A, Erdogan O, Hisar O, Çiftçi M (2003) Effects of Chloramine-T and CuSO4 on enzyme activity of glucose 6-phosphate dehydrogenase from rainbow trout (Oncorhynchus mykiss) erythrocytes in vitro and in vivo. Isr J Aquac-Bamidgeh 55:187–196
Corpas FJ, Salguero LG, Peragon J, Lupianez JA (1995) Kinetic properties of hexose monophosphate dehydrogenase. I. Isolation and partial purification of glucose-6-phosphate dehydrogenase from rat liver and kidney cortex. Life Sci 56:179–189
Erdogan O, Hisar O, Köroglu G, Ciltas A (2005) Sublethal ammonia and urea concentrations inhibit rainbow trout (Oncorhynchus mykiss) erythrocyte glucose-6-phosphate dehydrogenase. Comp Biochem Physiol 141C:145–150
FAO (Food and Agriculture Organization of the United Nations) (1999) The FAO Yearbook of Fishery Statistics: Aquaculture Production, vol. 88/2. FAO, Rome
Fernandez-Davila ML, Razo-Estrada AC, Garcia-Medina S, Gomez-Olivan LM, Pinon-Lopez MJ, Ibarra RG, Galar-Martinez M (2012) Aluminum-induced oxidative stress and neurotoxicity in grass carp (Cyprinidae-Ctenopharyngodon idella). Ecotoxicol Environ Saf 76:87–92
Heath AG (1987) Water pollution and fish physiology. CRC Press, Florida, p 245
Holten D (1972) Relationships among the multiple molecular forms of rat liver glucose-6-phosphate dehydrogenase forms. Biochim Biophys Acta 268:4–12
Ibraheem O, Adewale IO, Afolayan A (2005) Purification and properties of glucose-6-phosphate dehydrogenase from Aspergillus aculeatus. J Biochem Mol Biol 38:584–590
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Levy HR (1979) Glucose-6-phosphate dehydrogenases. In: Meister A (ed) Advan. Enzymol, 48. John Wiley and Sons, New York, pp 97–192
Levy HR, Cook C (1991) Purification and properties of NADP-linked glucose-6-phosphate dehydrogenase from Acetobacter hansenii (Acetobacter xylinum). Arch Biochem Biophys 291:161–167
Li S, Zhang Q (2010) Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. J Hazard Mater 176:579–588
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Liu XJ, Luo Z, Li CH, Xiong BX, Zhao YH, Li XD (2011) Antioxidant responses, hepatic intermediary metabolism, histology and ultrastructure in Synechogobius hasta exposed to waterborne cadmium. Ecotoxicol Environ Saf 74:1156–1163
Opuxzynski K, Shireman JV (1995) Herbivorous fishes: culture and use for weed management. CRC Press, Boca Raton
Ozer N, Aksoy Y, Ögüs IH (2001) Kinetic properties of human placental glucose-6-phosphate dehydrogenase. Int J Biochem Cell Biol 33:221–226
Ozer N, Bilgi C, Ogus H (2002) Dog liver glucose-6-phosphate dehydrogenase: purification and kinetic properties. Int J Biochem Cell Biol 34:253–262
Qiao SY, Jiang JY, Xiang W, Tang JH (2007) Heavy metals pollution in lakes of Wuhan city. Water Resour Prot 23:45–48 (in Chinese with English Abstract)
Rosemeyer MA (1987) The biochemistry of glucose-6-phosphate dehydrogenase, 6-phosphogluconate dehydrogenase and glutathione reductase. Cell Biochem Funct 5:79–95
Sahin A, Senturk M, Ciftci M, Varoglu E, Kufrevioglu OI (2010) The effects of chemical and radioactive properties of Tl-201 on human erythrocyte glucose 6-phosphate dehydrogenase activity. Nucl Med Biol 37:389–394
Segel IH (1975) Enzyme kinetics. Wiley, Toronto
Senturk M, Ceyhun SB, Erdoğan O, Küfrevioğlu Öİ (2009) In vitro and in vivo effects of some pesticides on glucose-6-phosphate dehydrogenase enzyme activity from rainbow trout (Oncorhynchus mykiss) erythrocytes. Pest Biochem Physiol 95:95–99
Tandogan B, Ulusu NN (2005) Characterization of glucose-6-phosphate dehydrogenase purified from lamb kidney cortex. Turk J Biochem 30(2):178–182
Ulusu N, Tandogan B (2005) Characterization of glucose-6-phosphate dehydrogenase purified from lamb kidney cortex. Turk J Biochem 30:178–182
Ulusu NN, Tandogan B (2006) Purification and kinetics of sheep kidney cortex glucose-6-phosphate dehydrogenase. Comp Biochem Physiol 143B:249–255
Ulusu NN, Kus MS, Acan NL, Tezcan EF (1999) A rapid method for the purification of glucose-6-phosphate dehydrogenase from bovine lens. Int J Biochem Cell Biol 31:787–796
Wang S, Wang W, Guo Y, Guo Y, Wei J, Zhang Y (2007) Acute and chronic toxicity of chromium and cadmium on grass carp fries (Ctenopharyngodon idellus). J Lanzhou Univ (Nat Sci) 43(4):60–64 (in Chinese with English Abstract)
Yang L, Xiao B, Wang X, Hou Y, Wang J, Sun J (2010) Characterization of acid phosphatase from Ctenopharyngodon idellus and effects of metal ions on the enzyme activity. J Fish Sci China 17:969–976 (in Chinese with English Abstract)
Yi YJ, Wang ZY, Yu GA (2008) Sediment pollution and its effect on fish through food chain in the Yangtze River. Int J Sed Res 23:338–347
Yilmaz H, Ciftci M, Beydemir S, Bakan E (2002) Purification of glucose 6-phosphate dehydrogenase from chicken erythrocytes. Investigation of some kinetic properties. Prep Biochem Biotechnol 32:287–301
Yoshida A (1966) Glucose-6-phosphate dehydrogenase of human erythrocytes. J Biol Chem 241:4966–4976
Yoshida A, Huang IY (1986) Structure of human G6PD. Academic Press Inc. Ltd., London
Zang W, Ye L, Xu X, Gong S (1991) Toxic effects of zinc on four species of freshwater fish. Chin J Oceanol Limnol 9:64–70
Acknowledgments
This work was funded by “973” project, China (grant no. 2009CB118706). Thanks were also extended to two anonymous reviewers for their invaluable suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hu, W., Zhi, L., Zhuo, MQ. et al. Purification and characterization of glucose 6-phosphate dehydrogenase (G6PD) from grass carp (Ctenopharyngodon idella) and inhibition effects of several metal ions on G6PD activity in vitro. Fish Physiol Biochem 39, 637–647 (2013). https://doi.org/10.1007/s10695-012-9726-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-012-9726-x