Abstract
An embryonic stem (ES)-like cell culture system RESC from a commercially important freshwater carp, Labeo rohita, was developed using blastula stage embryos. The cells were cultured in Leibovitz-15 (L-15) medium in gelatin-coated cell culture flask supplemented with 15 % fetal bovine serum along with 10 ng ml−1 basic fibroblast growth factor at 28 °C under feeder-free conditions. The ES-like cells were characterized by their unique morphology, alkaline phosphatase activity, embryoid body formation tendency, expression of transcription factor Oct4, and consistent chromosome count. The RESC cells when treated with retinoic acid differentiated into cells of different lineages. The RESC developed from mid-blastula embryos of L. rohita would be a useful tool for cellular differentiation and gene expression studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The detailed study of the biology of mouse stem cells led to the discovery of fish embryonic stem cells in 1996 (Hong et al. 1996) and then human embryonic stem cells in 1998 (Thomson et al. 1998). Embryonic stem cells can participate in normal development and contribute to several tissues of the host, including cells of the germ line (Bradley et al. 1984). These cells have the potential to produce any type of cell of the body and can be propagated in unlimited quantities for clinical applications. These characteristics make embryonic stem cells (ES) an ideal experimental system for in vitro studies of embryonic cell development and differentiation, and a vector for the efficient transfer of foreign DNA into the germ line of an organism (Gossler et al. 1986). Hong et al. (1996) developed a feeder cell-free culture condition under which mid-blastula embryos (MBE) cells were grown on gelatin-coated surface. ES-like cell culture systems developed from mid-blastula stage have been characterized for different applications in few fish species (Chen et al. 2003a, b; Dash et al. 2010). In recent years, a number of embryonic stem-like cell lines have been established by various workers from fish species (Sun et al. 1995; Hong et al. 1996; Chen et al. 2003a, b; Parameswaran et al. 2006, 2007; Lakra 2010).
Rohu, Labeo rohita (Hamilton), is an important Indian major carp species belonging to the family Cyprinidae of order Cypriniformes. The species occurs widely in rivers and freshwater lakes in and around northern and central India, Bangladesh, Nepal, Myanmar, and Pakistan (Talwar and Jhingran 1991). L. rohita is one of the three Indian major carps used extensively in carp polyculture systems in the region. There have been several incidences of mass mortality of carps in culture systems, suspected to be caused by microbial diseases, particularly of viral etiology (Mohan and Shankar 1994; Roberts et al. 1993). Therefore, development of cell culture systems from important cultivable carp species is of immense importance for in vitro research in carps. Development of embryonic stem cell culture systems from mid-blastula embryos of Labeo rohita carps will be instrumental in gene expression and cell differentiation studies in carps. The present study aimed at developing embryonic stem (ES)-like cell culture system (RESC) from blastula stage embryos of a commercially important freshwater carp, L. rohita.
Materials and methods
Primary culture and subculture
Fertilized eggs of L. rohita were collected from the carp hatchery of NBFGR, Lucknow, and were brought to laboratory for the collection of blastula stage embryos. Blastula stage embryos were observed under microscope, and embryos were immediately transferred to 35-mm small petridish. A batch of 25–30 embryos were first disinfected with 70 % ethanol and then washed five times with sterile phosphate-buffered saline (PBS, pH7.2) in the petridish. The cell mass was harvested by tearing the chorion with fine forceps, and the chorion and egg shells were removed. Single cells were platted through gentle pipetting, and cells were transferred to a new gelatin-coated cell culture flask (25 cm2) containing Leibovitz-15 (L-15) medium along with antibiotic and antimycotic solution (1,000 U penicillin, 1,000 μg streptomycin, and 25 μg amphotericin B ml−1) (Invitrogen). The L-15 medium was supplemented with 15 % fetal bovine serum (FBS) and 10 ng ml−1 basic fibroblast growth factor (bFGF). The cells were cultured at 28 °C in a CO2 incubator, and the medium was changed at an interval of 2 days with 50 % fresh medium. After reaching 60–70 % confluency, the cells were subcultured using TPVG solution (0.1 % trypsin, 0.2 % ethylene diamine tetra-acetic acid (EDTA), and 2 % glucose in 1× PBS) following 1:2 split ratio. In the initial subcultures, 50 % of the culture medium was replaced with the fresh medium.
Morphological observation
The plates were observed daily for radiation, spreading and proliferation of cells, and other morphological details using an inverted microscope (Olympus Optical Co., Ltd., Tokyo, Japan).
Alkaline phosphatase activity
Activity of alkaline phosphatase was assessed by using BCIP/NBT substrate system. In order to assess the ALP activity, RESC cells grown in 12-well plates were washed with PBS and then fixed with glutaraldehyde solution (1 %). After 10 min, the glutaraldehyde solution was removed, and cells were washed twice with PBS. These cells were then stained in dark with bromochloroindolyl phosphate/nitroblue tetra-zolium (BCIP/NBT, Sigma-Aldrich) as substrate.
Chromosomal analysis
Chromosomal counts were established at different passages. Cells were seeded in duplicate 75 cm2 tissue culture flasks in L-15 medium with 20 % FBS. After 24-h incubation, medium was replaced with 10 ml of fresh medium containing 0.1 ml colcemid solution (1 μg ml−1),(Sigma, St Louis, MO, USA) and incubated for 2 h at 28 °C. After harvesting by centrifugation (700 g, 5 min), the cells were suspended in a hypotonic solution consisting of 0.56 % KCl for 10 min and fixed in methanol:acetic acid (3:1). Slides were prepared following the conventional drop-splash technique (Freshney 1994). The chromosomes were counted under a microscope, after staining with 5 % Giemsa for 10 min.
Embryoid body formation and in vitro differentiation
Embryoid bodies were induced to form in RESC cells when the cells were grown by hanging drop method. At first, the RESC cells were detached by trypsinization and suspended in L-15 media supplemented with 20 % FBS at a density of 1 × 105 cells/ml. Drops of 20 μl cell suspension were placed on the inner side of the lid of 60-mm tissue culture dishes. To avoid loss of nutrients through evaporation, the dishes were filled with PBS and incubated at 28 °C for 48 h in hanging drop state in CO2 incubator. After an interval of 2–3 days, the three-dimensional embryoid bodies were formed, which were transferred into culture flasks having growth media. In order to study the effect of retinoic acid (RA) on differentiation, all-trans RA dissolved in dimethyl sulfoxide was added to cell cultures at a final concentration of 2 μM.
Immunocytochemistry
The expression of the transcription factor Oct4 and differentiation of RESC cells in neuronal lineage after treatment with RA were confirmed by immunocytochemistry with monoclonal antibodies Oct4 and Map2, respectively. In brief, cells were grown to confluency in 12-well plates (Nunc). Upon reaching 70–80 % confluency, cells were washed with PBS and were fixed in 4 % paraformaldehyde (PFA). After fixation, the cells were washed in PBS two times and were blocked with 5 % sheep serum and 0.1 % Triton X in PBS, further the cells were incubated for 40 min at 37 °C. Block was removed, and 100 μl of monoclonal antibody Oct4 and Map2 (Gentix) in dilution of 1:40 was added to the fixed cells. Slides were incubated for overnight at 4 °C. Next day, cells were washed with PBS and were incubated for 30 min with 100 μl of 1:300 dilution of FITC-labeled anti-mouse IgG. Then the cells were washed in PBS, covered with 50 % glycerol under coverslip, and were observed under fluorescence microscope. A cell line derived from fin of L. Rohita (RF) was used as appropriate control.
Transfection with GFP reporter gene
Subconfluent monolayers (60–70 % confluency) of RESC cells at 20th passage were transected with pEGFP-C1 plasmid using lipofectamine LTX and Plus reagents (Invitrogen). In brief, the cells of RESC were seeded at a density of 1 × 105 in 12-well plates individually and incubated for 18 h at 28 °C in normal atmospheric incubator. Before transfection, cells were rinsed with PBS and supplemented with 500 μl of fresh L-15 medium devoid of serum and antibiotics at pH 7.4. The plasmid (200 ng of pEGFP-C1) was dissolved in 100 μl of optimum, and then 0.5 μl of plus reagent was added. The mixture was incubated for 5 min at 30 °C, and then 2 μl of LTX was added and incubated for 30 min. Then the mixture was added dropwise on 60–70 % confluent RESC cells in 12-well plates. The medium was changed with fresh medium after 6h. The green fluorescence signals were observed after 18 h under a fluorescence microscope.
Results
Morphological observation
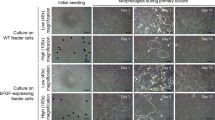
An embryonic stem cell–like cell culture system (RESC) was developed from the blastula stage embryos of L. rohita after 2 h of fertilization. The photomicrographs of the blastula stage embryo and blastomeres are given in Fig. 1a, b. The RESC cells were morphologically round or polygonal. The cells reached full confluency in the gelatin-coated flask after 4–5 days. RESC cells posses high nucleo-cytoplasmic ratio, which reveals its proliferative state.
Subculturing
The cells were subcultured after an interval of 3 days, and RESC cells have been maintained more than 33 passages (Fig. 1c). The cells were quite healthier during initial subcultures. When the RESC cells were seeded in low density, the cells were able to form dense colonies in 9–12 days (Fig. 2a). Cells of these colonies were homogenous in nature.
Alkaline phosphatase activity
RESC cells exhibited a strong alkaline phosphatase (AP) activity in the present study. Only a small percentage of cells about 7–8 % did not show AP activity (Fig. 2b).
Pluripotency and in vitro differentiation potential
Pluripotency of RESC cells was confirmed by immunohistochemistry with monoclonal antibody against transcription factor Oct4 (Fig. 3). Expression of transcription factor Oct4 was found to be high in nucleus region of RESC cells as compared to cytoplasm region, which is in relevance with its usual location in nucleus. After treatment with all-trans RA, the RESC cells were found to be differentiated into cells of different lineages (Fig. 4a, b). Further, differentiation of RESC into neural lineage was confirmed by immunocytochemistry with Map2 marker (Fig. 4c).
Embryoid body formation
RESC cells aggregated to form spherical three-dimensional embryoid bodies after 4–5 days, when the trypsinized RESC cells were grown by hanging drop method in a 60-mm cell culture plate (Fig. 5a).
Chromosomal analysis
Chromosomal analysis of 79 metaphase plates revealed that number of diploid chromosomes in RESC ranged from 35–52 with a model value of 2n = 50, which is identical with the modal chromosome number of L. rohita. (Fig. 6a–d).
Transfection
The RESC cells were successfully transfected with pEGFP-C1 plasmid using lipofectamine LTX and Plus reagents (Invitrogen). The expression of GFP in the RESC cells was detected after 18 h of transfection (Fig. 7).
Discussion
The totipotent ES cells have been recognized as powerful experimental tool in developmental biology. In the field of biomedical research, ES cells posses high potential in treatment of various disease. ES cells also provide an important tool to study the molecular events of developmental and functional genomics especially in the field of gene targeting (Muller 1999; Alvarez et al. 2007). Similarly, ES cells derived from fish have been used in germline transmission and posses potential for the production of superior stocks in aquaculture (Gong et al. 2001). Hong et al. (1996) used pluripotent stem cells from early embryos of medaka, Oryzias latipes, to develop ES cell technology. Subsequently, in 2006, Hong and Schartl (2006) derived embryonic stem cells from mid-blastula embryos (MBEs) of medaka for studying differentiation and stem cell biology in vitro. The ES-like cell culture systems have also been developed from mid-blastula stage embryos of Sparus aurata (Bejar et al. 1999), Lateolabrax japonicas, and Chrysophrys major (Chen et al. 2003a, b), Catla catla (Dash et al. 2010). The present study reported the development and characterization of a RESC cell culture system from mid-blastula stage embryos of L. rohita. Since RESC was developed from MBEs, germline transmission of RESC cells would not be possible. However, the RESC would be a useful tool for cellular differentiation and gene expression studies to produce superior stocks in aquaculture and viral disease studies affecting the production of this important aquaculture species.
The RESC cells derived from blastula stage embryos of L. rohita have important morphological characteristic similar to ES cell, including stable growth, a typical ES cell phenotype, that is, having a round/polygonal shape, a small size, high nucleo-cytoplasmic ratio. Similar morphology of ES cells was reported by Hong et al. (1996), Chen et al. (2003a, b), Alvarez et al. (2007). The major challenge for the establishment of embryonic stem cell lines is to inhibit their spontaneous differentiation (Chen et al. 2002). As highly confluent state of cells exhibits differentiation, efforts were made to maintain confluency of RESC cells around 60–70 % by seeding cells at low density. During the process of subsequent passaging, RESC cells formed colonies, which showed an important feature of ES-like cells as similar with previous studies (Chen et al. 2003a, b). Further, strong alkaline phosphatase activity was present in RESC cells with only few cells about 7–8 % without AP activity, which suggests that few RESC cells underwent spontaneous differentiation and lost pluripotency during culture. Activity of alkaline phosphatase has been used as an indicator of pluripotency in ES or ES-like cells by many researchers (Wobus et al. 1984; Pease and Williams 1990; Wakamatsu et al. 1994; Hong et al. 1996; Parameswaran et al. 2007).
Transcription factor Oct4 was found to be expressed in the nuclei of RESC cells, which indicates the pluripotent nature of cells. Expression of Oct4 was reduced and even lost when RESC cells were induced by RA for differentiation. Similar pattern of Oct4 expression in Medaka haploid ES cells was also reported by Yi et al. (2009). Map2, which is a marker for matured neuron, was also found to be expressed in RESC cells that were treated with all-trans RA, which indicated in vitro differentiation potential. RESC cells also formed spherical three-dimensional embryoid bodies. Formation of embryoid body and differentiation under controlled condition and specific media are another important feature of ESC in vertebrates (Familari and Selwood 2006). This could be the result of intimate cell-to-cell contact and appropriate exchange of autocrine/paracrine factors permit by the microdrop niche consisting of growth/differentiation factors. The spontaneous development of neurite-like structures for communication between embryonic stem cell colonies was seen, when the 18- to 20-day-old embryoid bodies were transferred to culture flask and were grown in culture media (Fig. 5b, c).
Chromosomal analysis of different metaphase plates revealed that number of diploid state of chromosomes in RESC with a model value of 2n = 50. Similarly, dipoloid state of chromosomes in ES-like cells has been reported in previous studies (Sun et al. 1995; Hong et al. 1996). This diploid karyotype stability is of high importance for ES or ES-like cells to enable them to form functional germline chimeras (Bradley et al. 1984).
In order to determine applicability of RESC cells in the field of transgenics, RESC cells were successfully transfected with pEGFP-C1 vector which showed its potential application in transgenisis. ES-like cells expressing GFP can be used for studying in vivo differentiation of cells into various tissues in host embryo (Chen et al. 2003a, b).
In conclusion, this study reports the development of an embryonic stem cell culture system from the blastula stage embryo in in vitro culture condition. The developed RESC cell culture system expressed the common features of embryonic stem-like cells as reported earlier in other fish species. The development of RESC cell culture system from an important carp species, L. rohita, would facilitate as a useful tool for cellular differentiation and gene expression studies.
References
Alvarez MC, Bejar J, Chen S, Hong Y (2007) Fish ES cells and application to biotechnology. Mar Biotechnol 6:117–127
Bejar J, Hong Y, Alvarez MC (1999) Towards obtaining ES cells in the marine fish species Sparus aurata; multipassage maintenance, characterization and transfection. Genet Anal Biomolecular Eng 15:125–129
Bradley A, Evans M, Kaufman MH, Robertson E (1984) Formation of germ-line chimeras from embryo-derived teratocarcinoma cell lines. Nature 309:255–256
Chen SL, Hong Y, Schartl M (2002) Development of a positive-negative selection procedure for gene targeting in fish cells. Aquaculture 214:67–79
Chen SL, Sha ZX, Ye HQ (2003a) Establishment of a pluripotent embryonic cell line from sea perch (Lateolabrax japonicus) embryos. Aquaculture 218:141–151
Chen SL, Ye HQ, Sha ZX, Hong Y (2003b) Derivation of a pluripotent embryonic cell line from red sea bream blastulas. J Fish Biol 63:795–805
Dash C, Routray P, Tripathy S, Verma DK, Guru BC, Meher PK, Nandi S, Eknath E (2010) Derivation and characterization of embryonic stem-like cells of Indian major carp Catla catla. J Fish Biol 77:1096–1113
Familari M, Selwood L (2006) The potential for derivation of embryonic stem cell in vertebrates. Mol Reprod Dev 73:123–131
Freshney RI (1994) Culture of animal cells: a manual of basic technique. Wiley-Liss, New York
Gong Z, Ju B, Wan H (2001) Green fluorescent protein (GFP) transgenic fish and their applications. Genetica 111:213–225
Gossler A, Doetschman T, Korn R, Serfling E, Kembler R (1986) Transgenesis by means of blastocyst-derived embryonic stem cell lines. Proc Natl Acad Sci USA 83:9065–9069
Hong Y, Schartl M (2006) Isolation and differentiation of medaka embryonic stem cells. Methods Mol Biol 329:3–16
Hong Y, Winkler C, Schartl M (1996) Pluripotency and differentiation of embryonic stem cell lines from the medaka fish (Oryzias latipes). Mech Dev 60:33–44
Lakra WS (2010) Pluripotent embryonic stem cell line from catfish. ICAR News 16(1):3
Mohan CV, Shankar KM (1994) Role of fungus in epizootic ulcerative syndrome of fresh and brackishwater fishes of Karnataka, India. Curr Sci 66:656–658
Muller U (1999) Ten years of gene targeting: targeted mouse mutant, from vector design to phenotype analysis. Mech Dev 82:3–21
Parameswaran V, Shukla R, Bhonde RR, Hameed ASS (2006) Establishment of embryonic cell line from sea bass (Lates calcarifer) for virus isolation. J Virolog Methods 137:309–316
Parameswaran V, Shukla R, Bhonde RR, Hameed ASS (2007) Development of a pluripotent ES-like cell line from Asian seabass (Lates calcarifer)-an oviparous stem cell line mimickingviviparous ES cells. Mar Biotechnol 9:766–775
Pease S, Williams RI (1990) Formation of germ-line chimeras from embryonic stem cells maintained with recombinant leukemia inhibitory factor. Exp Cell Res 190:209–211
Roberts RJ, Willoughby LG, Chinabut S (1993) Mycotic aspect of epizootic ulcerative syndrome (EUS) of Asian fishes. J Fish Dis 16:169–183
Sun L, Bradford CS, Ghosh C, Collodi P, Barnes DW (1995) ES-like cell cultures derived from early zebrafish embryos. Mol Mar Biol Biotechnol 4:193–199
Talwar PK, Jhingran AG (1991) Inland fishes of india and adjacent countries, vol 1. A. A. Balkema, Rotterdam
Thomson JA, Itskovitz-Eldor J, Sharpiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282:1145–1147
Wakamatsu Y, Ozato K, Sasado T (1994) Establishment of a pluripotent cell line derived from a Medaka (Oryzias latipes) blastula embryo. Mol Mar Biol Biotechnol 3:185–191
Wobus AM, Holzhausen H, Jakel P, Schoneich J (1984) Characterization of a pluripotent stem cell line derived from a mouse embryo. Exp Cell Res 152:212–219
Yi M, Hong N, Hong Y (2009) Generation of medaka fish haploid embryonic stem cells. Science 326:430–433
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Goswami, M., Lakra, W.S., Yadav, K. et al. Development of an ES-like cell culture system (RESC) from rohu, Labeo rohita (Ham.). Fish Physiol Biochem 38, 1775–1783 (2012). https://doi.org/10.1007/s10695-012-9674-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-012-9674-5